Introduction
The mortality rates of patients with non-small-cell
lung cancer (NSCLC) with stage II or IIIA disease remain high. Even
when complete surgical resection is performed, the 5-year survival
rate is only 54.1% in Japanese patients with pathological (P) stage
IIIA disease, 47.4% for P-stage IIB and 32.8% for P-stage IIIA
(1). The most frequently observed
recurrence is distant metastasis. Adjuvant chemotherapy has been
administered to patients with completely resected NSCLC in order to
control the cancer cells and to improve patient survival. The
efficacy of platinum-based adjuvant chemotherapy has been confirmed
in large clinical trials (LACE) (2–4).
However, the absolute improvement in the 5-year survival rate was
only 5% (5,6). Regimens including cisplatin
occasionally cause severe side effects, including renal failure,
deafness and gastrointestinal disorders. Furthermore, the ratio of
patients who complete the treatment is insufficient, and only a
~11% reduction in mortality has been achieved thus far (7–9).
Carboplatin plus paclitaxel chemotherapy has been one of the most
frequently used chemotherapy regimens for advanced and recurrent
NSCLC (10–12), and is occasionally used as an
adjuvant regimen for completely resected NSCLC (13,14).
Carboplatin is considered to cause milder side effects compared
with cisplatin. Side effects such as neuropathy, neutropenia and
thrombocytopenia prevent patients from completing a full 3-week
regimen cycle with cisplatin. Bi-weekly paclitaxel plus carboplatin
has been identified as a method of reducing such side effects,
while maintaining similar efficacy to the 3-week regimen (15). This is mainly due to the fact that
carboplatin rarely causes nephrotoxicity, neurotoxicity or
ototoxicity, and rarely triggers emesis and thrombocytopenia,
unlike cisplatin (16). In the
present study, the bi-weekly carboplatin plus paclitaxel regimen
was selected for adjuvant chemotherapy. The 2-year DFS with
bi-weekly carboplatin plus paclitaxel in patients with stage
IB-IIIB completely resected NSCLC was previously reported to be
89.0% (17).
S-1 (Taiho Pharmaceutical Co., Ltd., Tokyo, Japan)
is an oral anticancer agent comprising tegafur, 5-chloro-2,
4-dihydroxypyridine and potassium oxonate, in a molar ratio of
1:0.4:1 (18), which has achieved
the highest response rate among several oral anticancer agents
against unresectable advanced carcinomas in phase II studies
(19). Postoperative S-1
chemotherapy is one of the standard therapies for gastric cancer in
the US, Europe and Japan (20,21).
Several clinical trials for NSCLC using S-1 chemotherapy and S-1 +
platinum-based chemotherapy have been conducted, with largely
favorable findings (22–24). The mean relative dose intensity was
64.6%, and grade 3/4 toxicities occurred at the rate of only 15.4%
over a 2-week oral administration of S-1 followed by a 1-week
interval (24).
In the present study, the feasibility and
tolerability of S-1 as adjuvant therapy for advanced lung cancer
was examined.
Patients and methods
Patients
A multicenter randomized feasibility study of
paclitaxel plus carboplatin vs. S-1 in patients with locally
advanced completely resected NSCLC was conducted. A total of 40
patients underwent complete resection and were diagnosed with
pathological stage II or IIIA NSCLC according to the 7th edition of
the Tumor-Node-Metastasis classification (25) at the Nagoya City University Hospital
(Nagoya, Japan) and its affiliated hospitals, between January 2008
and December 2013. Written informed consent was obtained from all
the patients, and the study protocol was approved by the
Institutional Review Board of each participating institution. This
study was registered on the UMIN Clinical Trial database
(ID:000001510).
The eligibility criteria were as follows:
Histologically confirmed NSCLC, completely resected, pathological
stage II or IIIA disease, no previous chemotherapy or radiotherapy,
age 20–75 years, Eastern Cooperative Oncology Group performance
status of 0 or 1, white blood cell count
3,500–12,000/mm3 (normal, 3,000–8,500/mm3),
absolute neutrophil count ≥2,000/mm3 (normal,
38.3–74.7%), platelet count ≥100,000/mm3 (normal,
150,000–361,000/mm3), hemoglobin level ≥10 g/dl (normal,
10.8–14.9 g/dl), aspartate aminotransferase and creatinine level
<upper limit of normal (ULN), creatinine clearance rate >60
ml/min, percutaneous oxygen saturation concentration by room air
≥95%, and aspartate aminotransferase, alanine aminotransferase and
total bilirubin levels <2 times the ULN; the patients also had
to have started chemotherapy within 8 weeks following surgery and
been able to receive oral intake. The exclusion criteria were
patients with previous chemotherapy or radiotherapy, concomitant
malignancy within 5 years, interstitial pneumonia with clinical
symptoms, and significant cardiac arrhythmia or heart failure.
Treatment schedule
The randomization was performed centrally at the
Department of Oncology, Immunology and Surgery of the Nagoya City
University Graduate School of Medical Sciences (Nagoya, Japan). The
patients were randomly assigned either to arm A (18 cases)
receiving paclitaxel plus carboplatin bi-weekly, or to arm B (19
cases) receiving S-1. The treatments performed in the present study
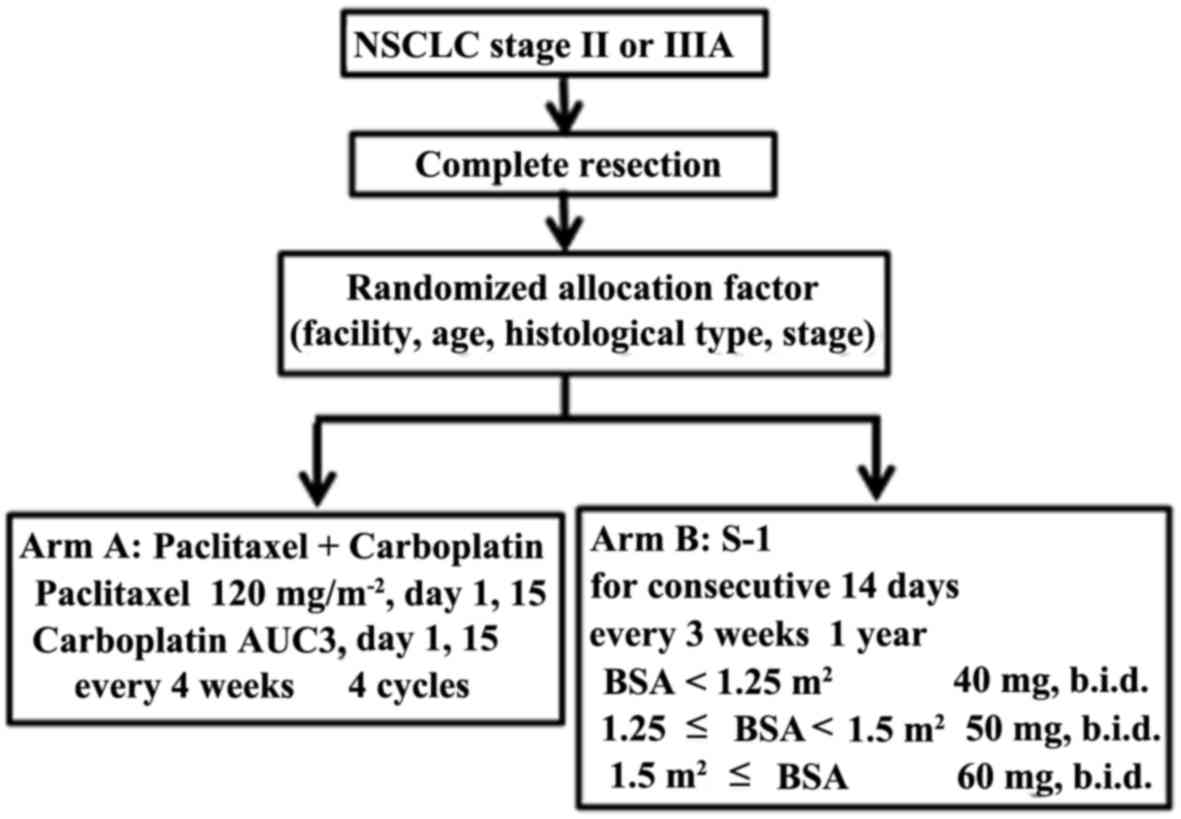
are schematically summarized in Fig.
1. Randomized allocation factors included facility, age,
histological type and stage.
The infusing dosage of paclitaxel was 120
mg/m2 on days 1 and 15. Carboplatin at an area under the
curve (AUC) dose of 3 was also administered on days 1 and 15. The
patients received adjuvant chemotherapy with paclitaxel plus
carboplatin every 4 weeks for up to 4 cycles. The Calvert's formula
was used to calculate the dose of the AUC for carboplatin (26), while the creatinine clearance was
determined with the Jelliffe formula (27).
The dosage of S-1 was established as follows:
Patients with a body surface area (BSA) <1.25 m2
received 40 mg twice daily (80 mg/day); those with BSA ≥1.25
m2 but <1.5 m2 received 50 mg twice daily
(100 mg/day); and those with a BSA ≥1.5 m2 received 60
mg twice daily (120 mg/day). S-1 was administered for 2 weeks
followed by a 1-week rest period for up to 1 year. Both arms A and
B continued on the above prescription unless there was any evidence
of relapse, other malignancies, or severe adverse events.
Throughout the study, the dosage of paclitaxel plus
carboplatin was adjusted according to the presence and severity of
hematological and non-hematological toxicities. For patients
exhibiting evidence of hematological or non-hematological toxicity,
the treatment on day 15 was omitted and the dosage for the next
course was reduced by one level (from paclitaxel 90 mg
m2 and carboplatin AUC 2, to paclitaxel 60 mg
m2 and carboplatin AUC 2). The dosage of S-1 was also
planned to be reduced by 1 level (15 mg/m2) up to 2
times for patients exhibiting evidence of grade ≥3 hematological or
non-hematological toxicities. All dose reductions were limited to
two levels.
Recurrence was diagnosed on the basis of imaging
study findings. Chest and abdominal computed tomography and
positron emission tomography plus head magnetic resonance imaging
were performed at 6- and 12-month intervals, respectively. In
addition, when the patients complained of any symptoms or exhibited
elevated tumor markers on blood tests, imaging studies were
performed.
Evaluation of feasibility and
toxicity
All the eligible patients who had received any
definitive treatment were considered as assessable for feasibility
and toxicity. The feasibility was evaluated based on the rate of
treatment completion (4 cycles completed for carboplatin plus
paclitaxel, and 1 year completed for S-1) and safety (rate of grade
≥3 toxicities). Adverse events were graded according to the
National Cancer Institute Common Toxicity Criteria (Common
Terminology Criteria for Adverse Events) version 3.0 (28).
Statistical analysis
The sample size was determined based on a phase II
study reported by Kawamura et al applying docetaxel plus
gemcitabine as an adjuvant chemotherapy in 35 patients (29). This previous study reported a 2-year
DFS rate of ~52%, with a 95% confidence interval (CI) of 35–69%.
Based on this result, the expected and threshold values of the
2-year DFS were 40 and 65%, respectively. The number of patients
required was determined with an α risk of 0.05 and a β risk of 0.1.
The number of patients in each arm was calculated using the Fleming
method and found to be 32 per arm. Sufficient data for patients in
the present study could not be gathered within the study period.
The primary endpoint was the 2-year DFS rate, and the secondary
endpoints were the feasibility and toxicity. The characteristics,
feasibility, adverse events, DFS and overall survival of 37
patients were analyzed. The cumulative total administration cycles,
2 and 5-year DFS and overall survival (OS) were examined by the
Kaplan-Meier method and the difference between the two arms was
calculated by the log-rank test. The differences in the rate of
adverse events were evaluated by the χ2 test. All the
data were analyzed with EZR software (30). P≤0.05 was considered to indicate a
statistically significant difference.
Results
Patient enrollment
A total of 40 patients with stage II or IIIA NSCLC
who had received surgically complete resection were enrolled. Of
the 40 patients, 3 were excluded in accordance with the exclusion
criteria: 1 patient refused to continue participating in this study
after registering, 1 patient used another chemotherapy regimen
during the follow-up period (under recurrence-free conditions) and
1 patient had a history of multiple cancers. The remaining 37
patients were randomized to either arm (18 cases in arm A and 19 in
arm B). The patient characteristics are summarized in Table I. Briefly, the patients included 6
women and 31 men, with a mean age of 62.8 years (range, 39–75
years). In arm A, 2 patients with pneumonectomy (2/37, 5%) were
included. These 2 patients completed four cycles of carboplatin
plus paclitaxel, but were confirmed to have recurrence within 1
year following surgery. When interpreting the results of the
present study, this point should be kept in mind.
 | Table I.Characteristics of 37 eligible
patients. |
Table I.
Characteristics of 37 eligible
patients.
| Characteristics | All patients | PTX + CBDCA | S-1 | P-value |
|---|
| Number of
patients | 37 | 18 | 19 |
|
| Observation period,
months |
|
Range | 13–79 | 17–75 | 13–79 | 0.62 |
|
Median | 47 | 39 | 48 |
|
| Sex |
|
Male | 31 | 14 | 17 | 0.405 |
|
Female | 6 | 4 | 2 |
|
| Age, years |
|
Range | 39–75 | 47–73 | 39–75 | 0.471 |
|
Mean | 62.8 | 62.8 | 62.9 |
|
| Histological
type |
|
Adenocarcinoma | 23 | 10 | 13 | 0.379 |
|
Squamous cell carcinoma | 12 | 6 | 6 |
|
|
Others | 2 | 2 | 0 |
|
| Pathological
stage |
|
IIA | 17 | 9 | 8 | 0.98 |
|
IIB | 11 | 5 | 6 |
|
|
IIIA | 9 | 4 | 5 |
|
| Surgery |
|
RUL | 13 | 6 | 7 | 0.702 |
|
RMLL | 2 | 0 | 2 |
|
|
RLL | 9 | 4 | 5 |
|
|
LUL | 6 | 4 | 2 |
|
|
LLL | 5 | 2 | 3 |
|
| Left
pneumonectomy | 2 | 2 | 0 |
|
Treatment delivery
In total, 50% of the patients in arm A received
paclitaxel plus carboplatin and 52.6% of the patients in arm B
received S-1, along with the planned schedule and at the planned
dose (Table II). In arm B, 1 of the
2 patients refused to continue the treatment due to financial
difficulties, and the other interrupted the treatment due to
continued grade 1 anorexia.
 | Table II.Administration of treatment. |
Table II.
Administration of treatment.
|
| Carboplatin +
paclitaxel | S-1 |
|---|
|
|
|
|
|---|
| Patients | No. | % | No. | % |
|---|
| Patients following
planned schedule and dose | 9 | 50 | 10 | 52.6 |
| Patients
discontinuing treatment | 3 | 16.7 | 6 | 31.6 |
| Patients developing
toxicity | 3 | 16.7 | 4 | 21.1 |
| Patient
refusal | 0 | 0 | 2 | 10.5 |
| Patients receiving
dose reduction | 6 | 33.3 | 3 | 15.8 |
Feasibility and toxicity
The drug-related adverse events are listed in
Table III. The main adverse events
in arm A were anaphylaxis, hematological toxicity, neuropathy and
alopecia. Two patients developed a grade 4 allergic reaction
(anaphylactic shock); however, immediately after cessation of the
infusion of paclitaxel, and following treatment with steroid
therapy, the patients recovered without sequelae. Both patients
discontinued adjuvant chemotherapy with paclitaxel plus
carboplatin: 1 patient went on to receive 4 cycles of gemcitabine
plus carboplatin as adjuvant therapy, and the other patient
received no further adjuvant chemotherapy. There were no grade 3 or
4 adverse events in arm B. Adverse events occurred in 15 patients
(83.3%) in arm A, and in 11 patients (57.9%) in arm B; the
difference was non-significant (P=0.151). In total, 3 patients
(16.7%) in arm A and 6 patients (31.6%) in arm B discontinued drug
administration due to adverse events caused by the agent and the
patients' wishes. In addition, 6 patients (33.3%) in arm A and 3
(15.8%) in arm B required a dose reduction due to adverse events.
No treatment-associated deaths occurred.
 | Table III.Toxicity. |
Table III.
Toxicity.
|
| Carboplatin +
paclitaxel (n=19) | S-1 (n=18) |
|---|
|
|
|
|
|---|
| Adverse events | G1/2 No. (%) | G3 No. (%) | G4 No. (%) | G1/2 No. (%) | G3 No. (%) | G4 No. (%) |
|---|
| Neutropenia | 2
(11) | 2 (11) |
| 1 (6) |
|
|
| Leukopenia | 4
(21) |
|
|
|
|
|
|
Thrombocytopenia | 1 (5) |
|
|
|
|
|
| Anorexia |
|
|
| 2
(11) |
|
|
| Nausea |
|
|
| 1 (6) |
|
|
| Vomiting | 1 (5) |
|
|
|
|
|
| Elevation of ALT,
AST |
|
|
| 3
(17) |
|
|
| Elevation of
bilirubin |
|
|
| 1 (6) |
|
|
| Neuropathy
(sensory) | 6
(32) |
|
|
|
|
|
| Fatigue | 1 (5) |
|
|
|
|
|
| Alopecia | 4
(21) |
|
|
|
|
|
| Urticaria |
|
|
| 2
(11) |
|
|
| Anaphylaxis |
|
| 2 (11) |
|
|
|
| Stomatitis |
|
|
| 1 (6) |
|
|
| Weight loss |
|
|
| 1 (6) |
|
|
| Others | 3
(16) |
|
| 5
(28) |
|
|
Survival
Ultimately, 9 patients (50%) in arm A and 8 (42.1%)
in arm B relapsed. In arm A, the relapse sites were the brain (4
cases), the brain and mediastinal lymph nodes (1 case), the bone (1
case), the adrenal glands (1 case), the mediastinum (1 case), and
the mediastinum and supraclavicular lymph nodes (1 case). In arm B,
the relapse sites were the brain and mediastinal lymph nodes (1
case), the trachea (1 case), the supraclavicular lymph nodes (1
case), the mediastinal lymph nodes (2 cases), and the intrathoracic
cavity (3 cases). Two patients were excluded from the analysis of
DFS and OS as they were unable to continue paclitaxel plus
carboplatin chemotherapy due to an anaphylactic reaction following
infusion of paclitaxel. The median follow-up time was 47 months
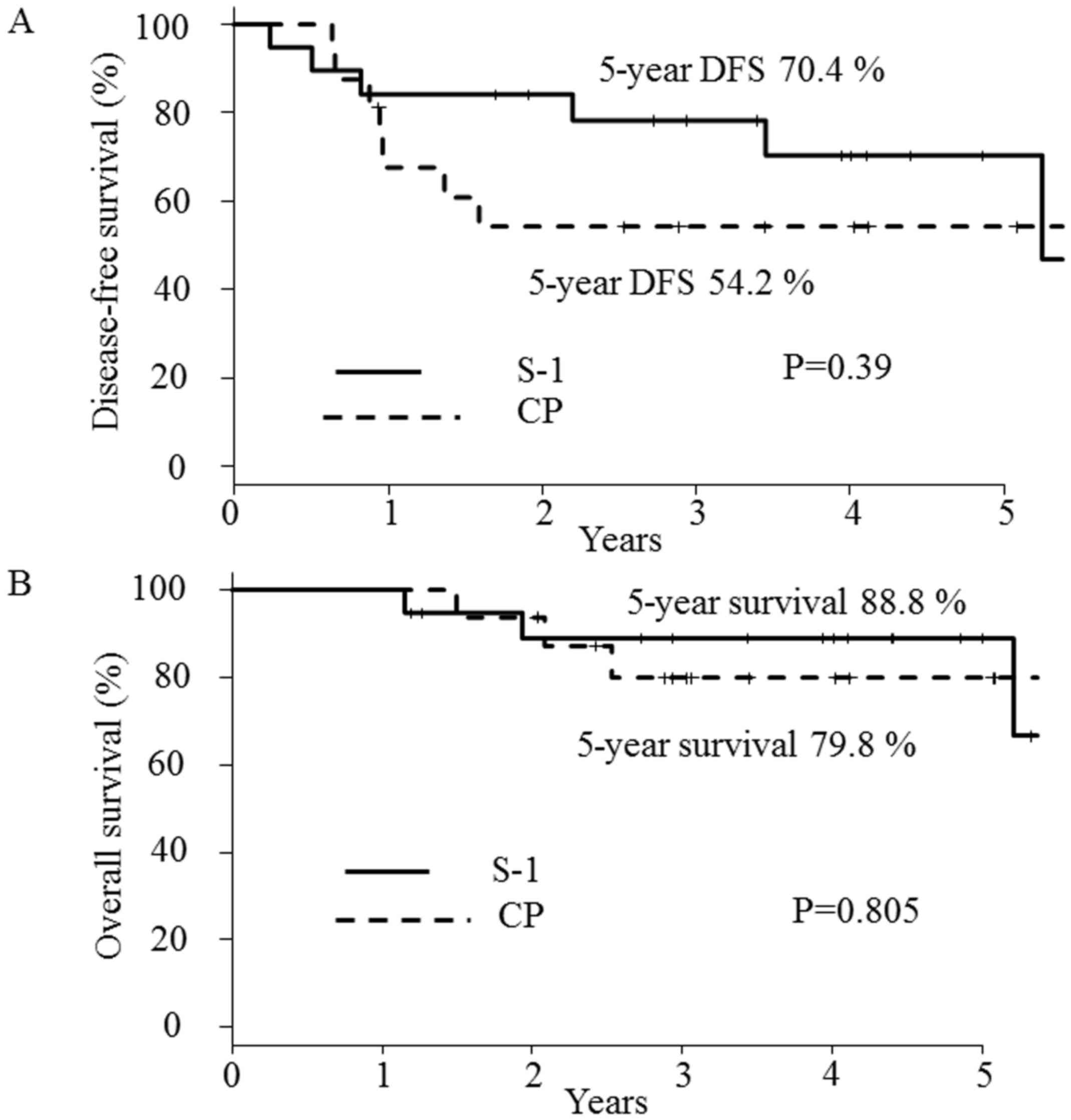
(range, 13–79 months). The 2-year DFS rates were 54.2% (95% CI:
27.1–75.0%) in arm A and 84.2% (95% CI: 58.7–94.6%) in arm B
(Fig. 2A). No statistically
significant difference in the 2-year DFS was noted between the two
arms, although there was a weak tendency toward an improved rate in
arm B. For further detailed breakdowns of the effects on survival,
the 5-year DFS (Fig. 2A) and OS
(Fig. 2B) was also investigated.
Discussion
The survival of patients with advanced lung cancer
is unfavorable compared with malignant tumors of other organs
(31). However, adjuvant
chemotherapy may improve the outcomes of advanced lung cancer
patients who have undergone surgically complete resection.
The result of clinical trials in Japan regarding the
oral administration of UFT (tegafur and uracil at a 1:4 molar
ratio) demonstrated significant survival benefits for stage I
patients who have undergone complete surgical resection (32). Platinum-based chemotherapy is used as
standard adjuvant chemotherapy for patients with locally advanced
(stage II or IIIA) disease (3,4), but its
treatment outcomes have been controversial (5,6). Several
challenges have been associated with the use of platinum-doublet
adjuvant chemotherapy (3,4), such as severe adverse effects,
including renal failure, deafness and gastrointestinal toxicity. To
reduce the rate and severity of such side effects, several adjuvant
chemotherapy regimens have been proposed and evaluated in a series
of clinical trials (7–9). However, no optimal adjuvant
chemotherapy has yet been established for advanced lung cancer.
At present, carboplatin doublet chemotherapy has
been found to have almost the same effects as cisplatin doublet
chemotherapy in the treatment of patients with recurrent and
advanced lung cancer (10–12). Paclitaxel plus carboplatin is also
considered a standard chemotherapy regimen for recurrent and
advanced lung cancer, although several specific side effects,
including anaphylactic reaction and neuropathy, may occur (11–13).
In the present study, the efficacy of orally
administered S-1 was evaluated as adjuvant chemotherapy for stage
II or IIIA patients who had undergone complete surgical resection
of their tumors. S-1 is a fluorouracil chemotherapeutic agent used
for recurrent and advanced lung cancer as second- or third-line
chemotherapy. As S-1 is considered more effective compared with
UFT, long-term S-1 administration may be promising as an adjuvant
chemotherapy for advanced lung cancer (22). Similar to the results reported by
Iwamoto et al (22), the
present study found adjuvant chemotherapy with long-term S-1 in
completely resected stage II–IIIA NSCLC to be safe and effective.
In the present study, S-1 therapy was compared with a carboplatin
plus paclitaxel regimen, which is associated with fewer and less
severe adverse events compared with cisplatin doublet treatment.
The efficacy and safety of S-1 adjuvant therapy were similar or
better compared with those of carboplatin plus paclitaxel. Indeed,
several studies reported that S-1 administration as adjuvant
chemotherapy is associated with significant survival benefits
following surgically complete resection for gastric cancer,
squamous cell carcinoma of head and neck, breast cancer and NSCLC
(19,22–24,33,34). The
5-year DFS and OS were almost the same between the S-1 group and
the paclitaxel plus carboplatin group. As the side effects of S-1
were tolerable, S-1 chemotherapy may be considered to be a
promising adjuvant chemotherapy for patients with advanced disease
who have undergone complete surgical resection.
Several studies have investigated the optimal
regimen for S-1 administration. One previous study reported that a
treatment schedule of a 2-week administration followed by a 1-week
interval appears more feasible and safer compared with the
conventional 4-week administration followed by a 2-week interval
(35). Considering this report, a
treatment schedule of 2-week administration followed by a 1-week
interval was selected for the present study; although the adverse
events associated with S-1 treatment were of grade 1 or 2, and the
majority were controllable, 6 patients discontinued treatment,
resulting in an unsatisfactory completion rate of S-1 adjuvant
therapy. Therefore, a more feasible administration schedule for S-1
as adjuvant therapy for advanced lung cancer must be developed. The
main limitation of the present study was the small patient
population. Further large-scale clinical trials with a longer
administration period for S-1 are required. Overall, the results of
the present study demonstrated that S-1 treatment for 1 year with a
2-week administration followed by a 1-week interval appeared to be
tolerable and safe as an adjuvant chemotherapy regimen.
In conclusion, the 2-year DFS rate as the primary
endpoint was found to be acceptable. S-1 chemotherapy for patients
with completely resected stage II or IIIA NSCLC was feasible and
safe, and it may therefore be considered as an option for adjuvant
chemotherapy in advanced NSCLC.
References
|
1
|
Asamura H, Goya T, Koshiishi Y, Sohara Y,
Eguchi K, Mori M, Nakanishi Y, Tsuchiya R, Shimokata K, Inoue H, et
al: A Japanese lung cancer registry study: Prognosis of 13,010
resected lung cancers. J Thorac Oncol. 3:46–52. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Pignon JP, Tribodet H, Scagliotti GV,
Douillard JY, Shepherd FA, Stephens RJ, Dunant A, Torri V, Rosell
R, Seymour L, et al: Lung adjuvant cisplatin evaluation: A pooled
analysis by the LACE collaborative group. J Clin Oncol.
26:3552–3559. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Pisters KM, Evans WK, Azzoli CG, Kris MG,
Smith CA, Desch CE, Somefield MR, Brouwers MC, Darling G, Ellis PM,
et al: Cancer care onario and American society of clinical oncology
adjuvant chemotherapy and adjuvant radiation therapy for stages
I–IIIA resectable non-small cell lung cancer guideline. J Clin
Oncol. 25:5506–5518. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Crinò L, Weder W, van Meerbeeck J and
Felip E; ESMO Guidelines Working Group, : Early stage and locally
acvanced (non-metastatic) non-small-cell lung cancer: ESMO clinical
practice guidelines for diagnosis, treatment and follow-up. Ann
Oncol. 21 Suppl 5:v103–v115. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
NSCLC Meta-analyses Collaborative Group, ;
Arriagada R, Auperin A, Burdett S, Higgins JP, Johnson DH, Le
Chevalier T, LePecoux C, Parmar MK, Pignon JP, et al: Adjuvant
chemotherapy, with or without postoperative radiotherapy, in
operable non-small-cell lung cancer: Two meta-analyses of
individual patient data. Lancet. 375:1267–1277. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Fruh M, Rolland E, Pignon JP, Seymour L,
Ding K, Tribodet H, Winton T, Le Chevalier T, Scagliotti GV,
Douillard JY, et al: Pooled analysis of the effect of age on
adjuvant cisplatin-based chemotherapy for completely resected
non-small-cell lung cancer. J Clin Oncol. 26:3573–3581. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Hotta K, Matsuo K, Ueoka H, Kiura K,
Tabata M and Tanimoto M: Role of adjuvant chemotherapy in patients
with resected non-small-cell lung cancer: Reappraisal with
meta-analysis of randomized controlled trials. J Clin Oncol.
22:3860–3867. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Sedrakyan A, van der Meullen J, O'Byrne K,
Prendiville J, Hill J and Treasure T: Postoperative chemotherapy
for non-small cell lung cancer: A systematic review and
meta-analysis. J Thorac Cardiovasc Surg. 128:414–419. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Berghmans T, Paesmans M, Meert AP, Mascaux
C, Lothaire P, Lafitte JJ and Sculier JP: Survival improvement in
resectable non-small cell lung cancer with (neo)adjuvant
chemotherapy: Results of a meta-analysis of the literature. Lung
Cancer. 49:13–23. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Kelly K, Crowley J, Bunn PA Jr, Presant
CA, Grevstad PK, Moinpour CM, Ramsey SD, Wozniak AJ, Weiss GR,
Moore DF, et al: Randomized phase III trial of paclitaxel plus
carboplatin versus vinorelbine plus cisplatin in the treatment of
patients with advanced non-small-cell lung cancer: A southwest
oncology group trial. J Clin Oncol. 19:3210–3218. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Ohe Y, Ohashi Y, Kubota K, Tamura T,
Nkagawa K, Negoro S, Nishiwaki Y, Saijo N, Ariyoshi Y and Fukuoka
M: Randomized phase III study of cisplatin plus irinotecan versus
carboplatin plus paclitaxel, cisplatin plus gemcitabine, and
cisplatin plus vinorelbine for advanced non-small-cell lung cancer:
Four-Arm cooperative study in Japan. Ann Oncol. 18:317–323. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Schiller JH, Harrington D, Belani CP,
Langer C, Sandler A, Krook J, Zhu J and Johnson DH; Eastern
Cooperative Oncology Group, : Comparison of four chemotherapy
regimens for advanced non-small-cell lung cancer. N Engl J Med.
346:92–98. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Yamashita Y, Kataoka K, Ishida T, Matsuura
M, Seno N, Mukaida H, Miyahara E, Miyata Y, Okita R, Shimizu K, et
al: A feasibility study of postoperative adjuvant therapy of
carboplatin and weekly paclitaxel for completely resected non-small
cell lung cancer. J Thorac Oncol. 3:612–616. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Maruyama R, Yoshino I, Tokunaga S, Ohta M,
Kato M, Yoshimine H, Yamazaki K, Nakanishi Y and Ichinose Y:
Feasibility trial of adjuvant chemotherapy with paclitaxel and
carboplatin after surgical resection in Japanese patients with
non-small cell lung cancer: Report of the lung oncology group in
kyushu (LOGIK) protocol 0501. Gen Thorac Cardiovasc Surg. 56:68–73.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Ichiki M, Kawasaki M, Takayama K, Ninomiya
K, Kuba M, Iwami F, Miyazaki N, Oishi K, Takeo S, Aizawa H and
Nakanishi Y: A multicenter phase II study of carboplatin and
paclitaxel with a biweekly schedule in patients with advanced
non-small-cell lung cancer: Kyushu thoracic oncology group trial.
Cancer Chemother Pharmacol. 58:368–373. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Strauss GM, Herndon JE II, Maddaus MA,
Johnstone DW, Johnson EA, Harpole DH, Gilenwater HH, Watson DM,
Sugarbaker DJ, Schilsky RL, et al: Adjuvant paclitaxel plus
carboplatin compared with observation in stage IB non-small-cell
lung cancer: CALGB 9633 with the cancer and leukemia group B,
radiation therapy oncology group and north central cancer treatment
group study groups. J Clin Oncol. 26:5043–5051. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Sugaya M, Uramoto H, Uchiyama A, Nagashima
A, Nakanishi R, Sakata H, Nakanishi K, Hanagiri T and Uasumoto K.:
PPhase II trial of adjuvant chemotherapy with bi-weekly carboplatin
plus paclitaxel in patients with completely resected non-small cell
lung cancer. Anticancer Res. 30:3039–3044. 2010.PubMed/NCBI
|
|
18
|
Shirasaka T, Nakano K, Takechi T, Satake
H, Uchida J, Fujioka A, Saito H, Okabe H, Oyama K, Takeda S, et al:
Antitumor activity of 1 M tegafur-0.4 M
5-chloro-2,4-dihydroxypyridine-1 M potassium oxonate (S-1) against
human colon carcinoma orthotopically implanted into nude rats.
Cancer Res. 56:2602–2606. 1996.PubMed/NCBI
|
|
19
|
Sakuramoto S, Ssako M, Yamaguchi T,
Kinoshita T, Fujii M, Nashimoto A, Furukawa H, Nakajima T, Ohashi
Y, Imamura H, et al: Adjuvant chemotherapy for gastric cancer with
S-1, an oral fluoropyrimidine. N Engl J Med. 357:1810–1820. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Foukakis T, Lundell L, Gubanski M and Lind
PA: Advances in the treatment of patients with gastric
adenocarcinoma. Acta Oncol. 46:277–285. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Sasako M: Surgery and adjuvant
chemotherapy. Int J Clin Oncol. 13:193–195. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Iwamoto Y, Mitsudomi T, Sakai K, Ymanaka
T, Yoshioka H, Takahama M, Yoshimura M, Yoshino I, Takeda M,
Sugawara S, et al: Randomized phase II study of adjuvant
chemotherapy with long-term S-1 versus Cisplatin+S-1 in completely
resected stage II–IIIA non-small cell lung cancer. Clin Cancer Res.
21:5245–5252. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Okumura S, Sasaki T, Satoh K, Kitada M,
Nagase A, Yatsuyanagi E and Ohsaki Y: Feasibility of adjuvant
chemotherapy with S-1 consisting of a 4-week administration and a
two-week rest period in patients with completely resected non-small
cell lung cancer. Mol Clin Oncol. 1:124–130. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Soh J, Okumura N, Nakata M, Nakamura H,
Fukuda M, Kataoka M, Kajiwara S, Sano Y, Aoe M, Kataoka K, et al:
Randomized feasibility study of S-1 for adjuvant chemotherapy in
completely resected Stage IA non-small-cell lung cancer: Results of
the setouchi lng cancer group study 0701. Jpn J Clin Oncol.
46:741–747. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
TNM Classification of Malignant Tumours.
Wiley-Blackwell; Oxford: 2009
|
|
26
|
Calvert AH, Newell DR, Gumbrell LA,
O'Reilly S, Burnell M, Boxall FE, Siddik ZH, Judson IR, Gore ME and
Wiltshaw E: Carboplatin dosage: Prospective evaluation of a simple
formula based on renal function. J Clin Oncol. 7:1748–1756. 1989.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Jellifie RW and Jelliffe SM: A computer
program for estimation of creatine clearance from unstable serum
creatine levels, age, sex and weight. Math Biosci. 14:17–24. 1972.
View Article : Google Scholar
|
|
28
|
Cancer Therapy Evaluation Program (CTEP),
. Common Terminology Criteria for Adverse Events, version 3.0,
DCTD, NCI, NIH, DHHS. March 31–2003, http://ctep.cancer.govAugust 9–2006
|
|
29
|
Kawamura M, Eguchi K, Izumi Y, Ymato Y,
Koike T, Sakaguchi H, Hada E and Kobayashi K: Phase II trial of
gemcitabine and docetaxel in patients with completely resected
stage IIA-IIIA non-small-cell lung cancer. Cancer Chemother
Pharmacol. 60:495–501. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Kanda Y: Investigation of the freely
available easy-to-use software ‘EZR’ for medical statistics. Bone
Marrow Transplant. 48:452–458. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Vallières E, Shepherd FA, Crowley J, Van
Houtte P, Postmus PE, Carney D, Chansky K, Shaikh Z and Goldstraw
P; International Association for the Study of Lung Cancer
International Staging Committee and Participating Institutions, :
The IASLC lung cancer staging project: Proposals regarding the
relevance of TNM in the pathologic staging of small cell lung
cancer in the forthcoming (seventh) edition of the TNM
classification for lung cancer. J Thorac Oncol. 4:1049–1059. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Kato H, Ichinose Y, Ohta M, Hata E,
Tsubota N, Tada H, Watanabe Y, Wada H, Tsuboi M, Hamajima N, et al:
A randomized trial of adjuvant chemotherapy with uracil-tegafur for
adenocarcinoma of the lung. N Engl J Med. 350:1713–1721. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Tsukuda M, Kida A, Fujii M, Kono N,
Yoshihara T, Hasegawa Y and Sugita M; Chemotherapy Study Group of
Head and Neck Cancer, : Randomized scheduling feasibility study of
S-1 for adjuvant chemotherapy in advanced head and neck cancer. Br
J Cancer. 93:884–889. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Shigekawa T, Osaki A, Sekine H, Sato N,
Kanbayashi C, Sano H, Takeuchi H, Ueda S, Nakamiya N, Sugitani I,
et al: SSafety and feasibility of adjuvant chemotherapy with S-1 in
Japanese breast cancer patients after primary systemic
chemotherapy: A feasibility study. BMC Cancer. 15:2532015.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Yano T, Yamazaki K, Maruyama R, Tokunaga
S, Shoji F, Higashi H, Takeo S, Ichinose Y and Maehara Y; Lung
Oncology Group in Kyushu (LOGIK), : Feasibility study of
postoperative adjuvant chemotherapy with S-1 (tegaful, gimeracil,
oteracil potassium) for non-small cell lung cancer-LOGIK 0601
study. Lung Cancer. 67:184–187. 2010. View Article : Google Scholar : PubMed/NCBI
|