Introduction
Over the past 50 years, the worldwide incidence of
lung cancer has significantly increased. In developed countries and
large cities, lung cancer has become the most common type of cancer
among males. In many countries, the number of mortalities due to
lung cancer has surpassed the combined numbers from prostate
cancer, breast cancer and gastrointestinal tumors (1,2). The
majority of patients with lung cancer are males over the age of 40;
however, the number of female patients has also increased
significantly in recent years. Typically, lung cancer is divided
into two categories: Small cell lung cancer (SCLC) and non-SCLC
(NSCLC). As SCLC is distinct from NSCLC with respect to its
biological behavior, treatment, prognosis and other features, the
present study focused on the associations between NSCLC prognosis
and the uPA system. Although there has been considerable progress
in the diagnosis and treatment of NSCLC in recent years, the
majority of cases are diagnosed only at an advanced stage.
Consequently, the 5-year survival rate for NSCLC is ~15% (3). The invasion and distant metastasis of
NSCLC cells are the most important biological characteristics of
malignant lung tumors; these phenomena result in the associated low
survival rates of patients with NSCLC (4). Thus, a biomarker that facilitates the
recognition of NSCLC invasion, metastasis and prognosis would aid
thoracic surgeons in treatment regimen selection.
The uPA system includes urokinase plasminogen
activator (uPA), uPA receptor (uPAR), and two inhibitors, PA
inhibitor type 1 (PAI-1) and PAI type 2 (PAI-2). uPA can be
produced by fibroblasts, monocytes, neutrophils, epithelial cells
and tumor cells, among others. The specific receptor for uPA, uPAR,
is a multifunctional receptor able to mediate the activation of PA,
signaling pathways, cell adhesion and metastasis following the
binding of uPA (5–8). uPAR is composed of 283 amino acid
residues and has a molecular weight of 55–60 kDa. The receptor is a
glycoprotein that is anchored to the membrane by a glycosylated
phosphatidylinositol. This glycoprotein regulates proteolysis at
the invading edge of tumors (9).
uPAR serves a critical function in cancer progression through its
interaction with integrins and vitronectin, and its role as a
regulator of angiogenesis. PAI-1 and PAI-2 are specific inhibitors
of uPA; PAI-1 exhibits increased activity in comparison with PAI-2,
and is the primary inhibitor of uPA. PAI-1 is predominantly
produced by blood platelets, epithelial cells, granulosa cells and
tumor cells. PAI-2 is produced by tumor cells, trophoblasts and
monocytes, and exhibits increased expression levels in the plasma
of pregnant females. PAI combines with uPA (1:1 ratio) to generate
a stable complex that inhibits uPA-mediated activities (10).
Numerous studies have indicated that the expression
levels of uPA, uPAR, PAI-1 and PAI-2 significantly correlate with
poor prognoses (11–13). In the present study, recent and
relevant literature regarding associations between the uPA system
and NSCLC were analyzed, and inconsistencies in the conclusions
were revealed. Certain articles reported that high uPAR expression
levels correlate with poor overall survival (OS), while other
articles revealed no such association. Thus, a meta-analysis on the
current and available research was conducted, in order to evaluate
the associations between protein expression in the uPA system and
the prognosis of patients with NSCLC.
Data collection methods
Publication search
The PubMed (https://www.ncbi.nlm.nih.gov/pubmed), EMBASE
(www.embase.com), Web of Science (http://isiknowledge.com) and Cochrane Library
(http://www.cochranelibrary.com)
databases were used to search for academic articles written in the
English language, with the most recent search conducted on June 30,
2016. The search criteria consisted of combinations of medical
subheadings and keywords, including ‘lung cancer’, ‘pulmonary
neoplasm’, ‘uPA’, ‘uPAR’, ‘PAI-1’, ‘PAI-2’ and ‘uPA system’. Using
Endnote X7 software (Clarivate Analytics, London, UK) the two
authors independently removed and filtered the duplicates.
Inclusion and exclusion criteria
Regarding the meta-analysis, the inclusion criteria
were as follows: 1) The diagnosis histological subtype was either
squamous cell carcinoma (SCC), adenocarcinoma (AC), large cell lung
cancer (LCC), bronchioalveolar carcinoma (BAC) or another rare
NSCLC subtypes (NSCLC mix, nos); 2) the patients were >18 years
of age; 3) the research focused on the expression of uPA, uPAR,
PAI-1 and PAI-2 and the associated patient prognosis. The following
exclusion criteria were applied: 1) Literature regarding the
expression of proteins in the uPA system in SCLC; 2) the detection
of uPA system proteins in the patient's circulation, effusion or
cerebrospinal fluid; 3) reports that lacked relevant outcomes or
Kaplan-Meier survival curve data, or studies without available or
extractable data; 4) abstracts only and reviews; 5) literature not
published in the English language; 6) duplicate articles; 7) animal
and cell research. In total, 2,403 papers were searched, of which
11 articles (937 patients) were included in this meta-analysis.
Data extraction
The two authors independently retrieved the relevant
data from the literature. The data included the following: the name
of the first author; the date of publication, the country and
region; the total number of patients included in the literature and
their pathological classifications; the hazard ratios (HRs) and 95%
confidence intervals (CIs) pertaining to the OS and the
differential expression of uPA, uPAR, PAI-1 and PAI-2 in patients
with NSCLC. For articles that only provided the Kaplan-Meier
survival curve (K-M survival curve), the data were gathered from
the K-M survival curve, which was obtained using the graph data
extraction software Engauge Digitizer (version 4.1; http://markummitchell.github.io/engauge-digitizer)
through continuous point gathering. The HR values were calculated
using the Microsoft Office Excel (version 2013) (Microsoft
Corporation; Redmond, WA, USA) program provided by Tierney et
al (14), and finally the data
were converted from the HR values to Ln (HR) and se [ln (HR)] for
statistical analysis using RevMan (version 5.3; http://community.cochrane.org/tools/review-production-tools)
(15). Any discrepancies were
resolved following discussion or through consultation with a third
party.
Statistical analysis
A chi-squared-based Q-test was used to examine the
assumption of heterogeneity. An I2 value was used to
describe the heterogeneity between multiple studies. When the
I2 value was ≥50%, it indicated that there was a
relatively large degree of inter-study heterogeneity, and thus, a
random effects model was required to calculate the combined HR
value pertaining to each study. Otherwise, a fixed effects model
was used. OS was evaluated by using pooled Cox proportional HRs and
95% CIs. Statistical analyses, forest plots and funnel plots were
performed and created using RevMan software (version 5.3).
P<0.05 was considered to indicate a statistically significant
difference.
Results
Nature of the literature used in the
meta-analysis
The current meta-analysis follows the relevant
criteria of the Preferred Reporting Items for Systemic Reviews and
Meta-Analyses statement (16).
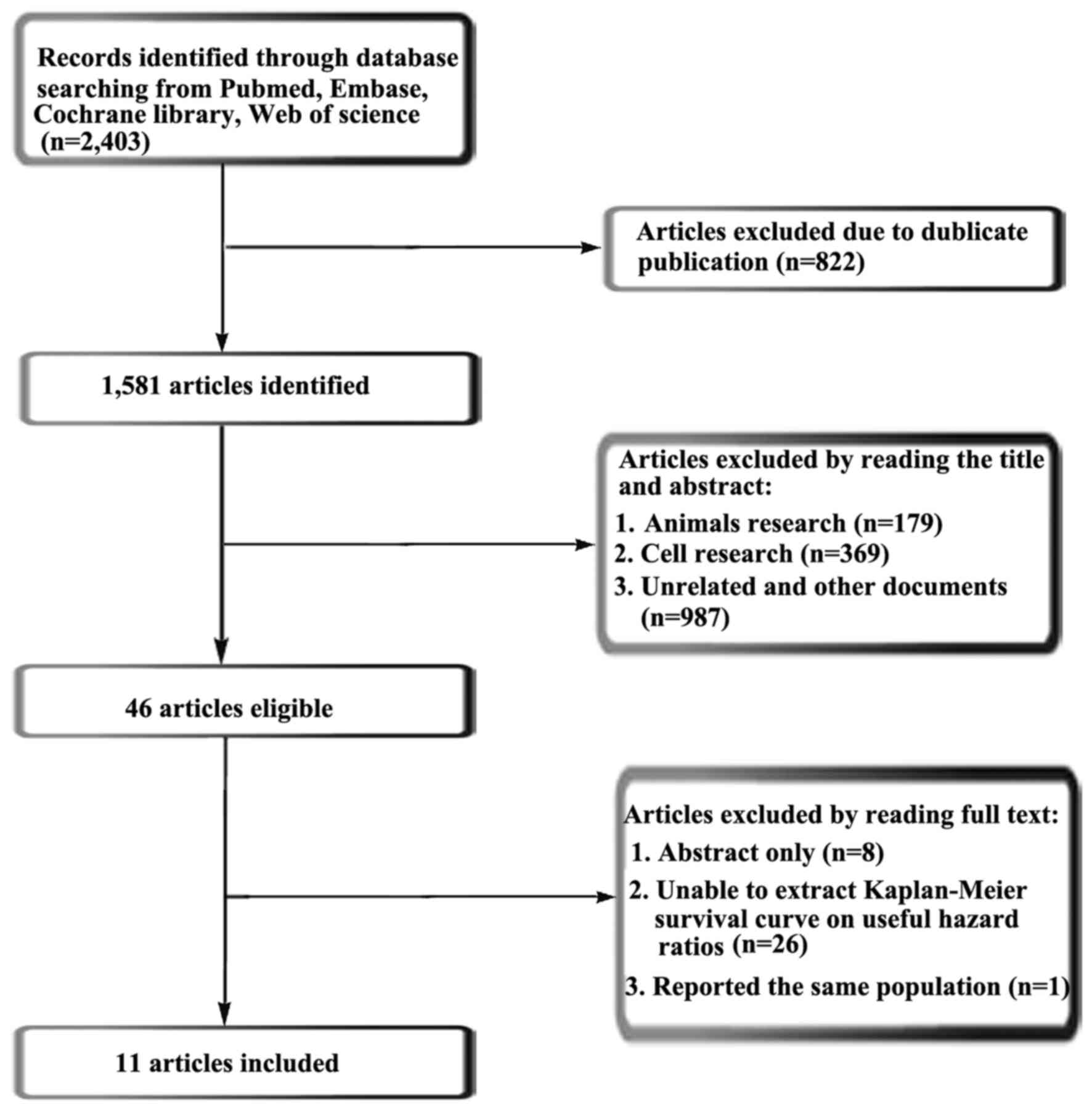
According to retrieval strategies, the present meta-analysis
retrieved a total of 2,403 articles. Using Endnote X7 software, 821
duplicate articles were removed. A further 179 articles regarding
animal research, 369 articles regarding cell research and 987
irrelevant articles were removed once the respective titles and
abstracts were read and analyzed. Following reading of the full
text of the remaining 47 articles, 36 were removed in accordance
with the selection criteria, leaving 11 articles (17–27) (937
patients) to be included in the meta-analysis. The retrieval
process is presented in Fig. 1. Of
the 11 articles selected, there were 8 associated with SCC, 10
associated with AC, 6 associated with LCC and 2 associated with
mixed-type NSCLC and NSCLC not otherwise specified. All of the
relevant details of the articles included are listed in Table I. In the meta-analysis, OS was set as
the outcome of the final observation.
 | Table I.Features of the included studies. |
Table I.
Features of the included studies.
| First author | Country | Publication time | N | Histological
type | Indicators | Survival
analysis | (Refs.) |
|---|
| Almasi | Denmark | 2005 | 63 | SCC | uPAR | OS | (17) |
| Almasi | Denmark | 2009 | 32 | SCC, AC, LCC | uPAR | OS | (18) |
| Almasi | Denmark | 2011 | 171 | SCC, AC, LCC, BAC
NSCLC mix, nos | uPAR | OS | (19) |
| Blumenschein | USA | 2011 | 54 | SCC, AC | uPA, PAI-1, uPAR | OS, PFS | (20) |
| Su | Taiwan | 2015 | 98 | SCC, AC, LCC | uPA, PAI-1, PAI-2,
uPAR | OS, DFS | (21) |
| Pappot | Denmark | 1999 | 54 | AC | PAI-1, uPAR | OS | (22) |
| Offersen | Denmark | 2007 | 118 | SCC, AC, LCC | uPA, PAI-1 | OS | (23) |
| Pappot | Denmark | 2006 | 99 | AC | uPA, PAI-1 | OS | (24) |
| Pedersen | Denmark | 1994 | 54 | AC | uPA, PAI-1 | OS | (25) |
| Salden | Netherlands | 2000 | 88 | SCC, AC, LCC, NSCLC
mix | uPA, PAI-1, PAI-2,
uPAR | OS | (26) |
| Werle | Germany | 2004 | 106 | SCC, AC, LCC | PAI-1 | OS | (27) |
Indicator expression discrepancies in
the uPA system, and their association with OS
In this meta-analysis, a cohort that exhibited
relatively high heterogeneity was evaluated for associations
between PAI-1, PAI-2 and uPAR expression and the associated OS
values. Thus, the random effects model was used to analyze the
association between expression of PAI-1, PAI-2, and uPAR and OS,
while a fixed effects model was used for uPA. Among the 11 articles
that were used in this meta-analysis, eight investigated the
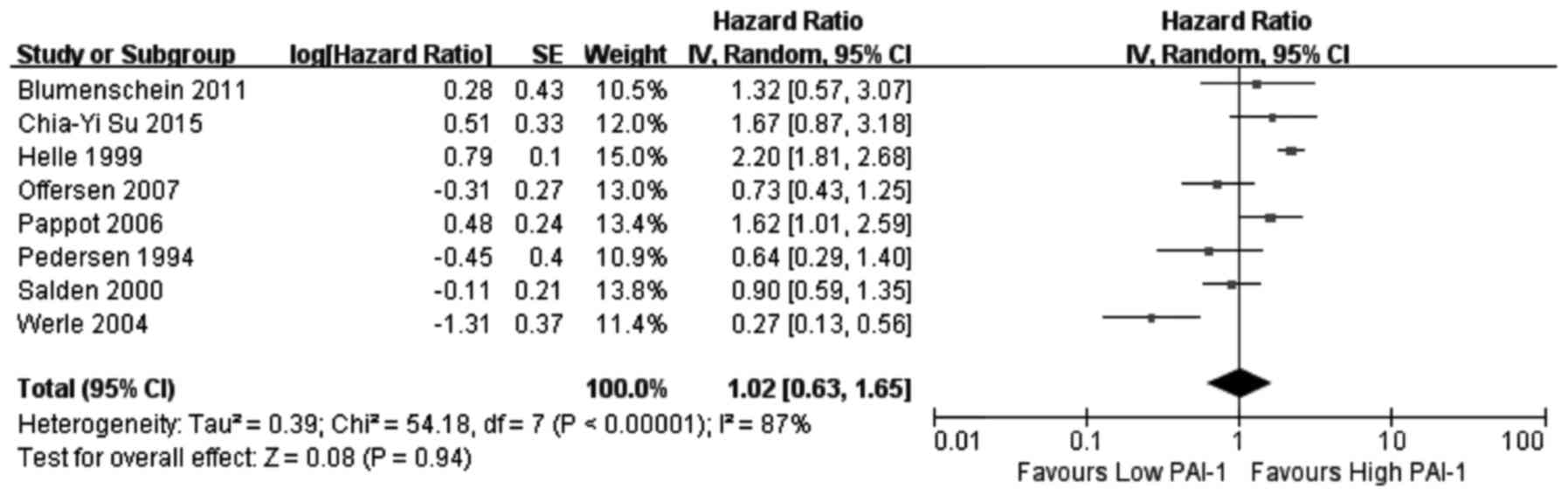
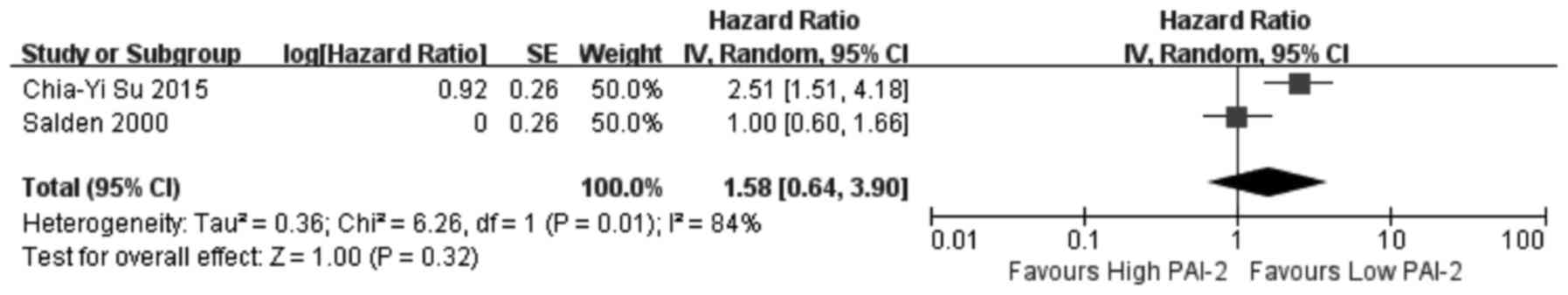
association between PAI-1 expression and OS, two investigated
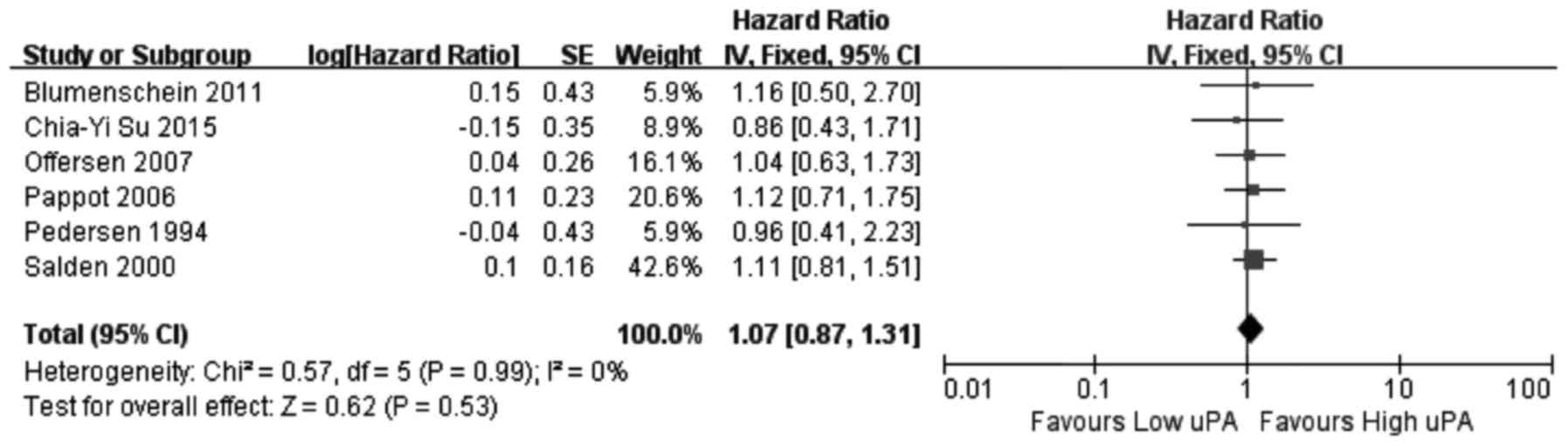
PAI-2, six investigated uPA and seven investigated uPAR. The
results of the meta-analysis revealed that increased expression of
uPA (Fig. 2) and PAI-1 (Fig. 3) and low expression of PAI-2
(Fig. 4) did not significantly
correlate with OS (uPA: HR=1.07, 95% CI=0.87–1.31, P=0.53; PAI-1:
HR=1.02, 95% CI=0.63–1.65, P=0.94; PAI-2: HR=1.58, 95%
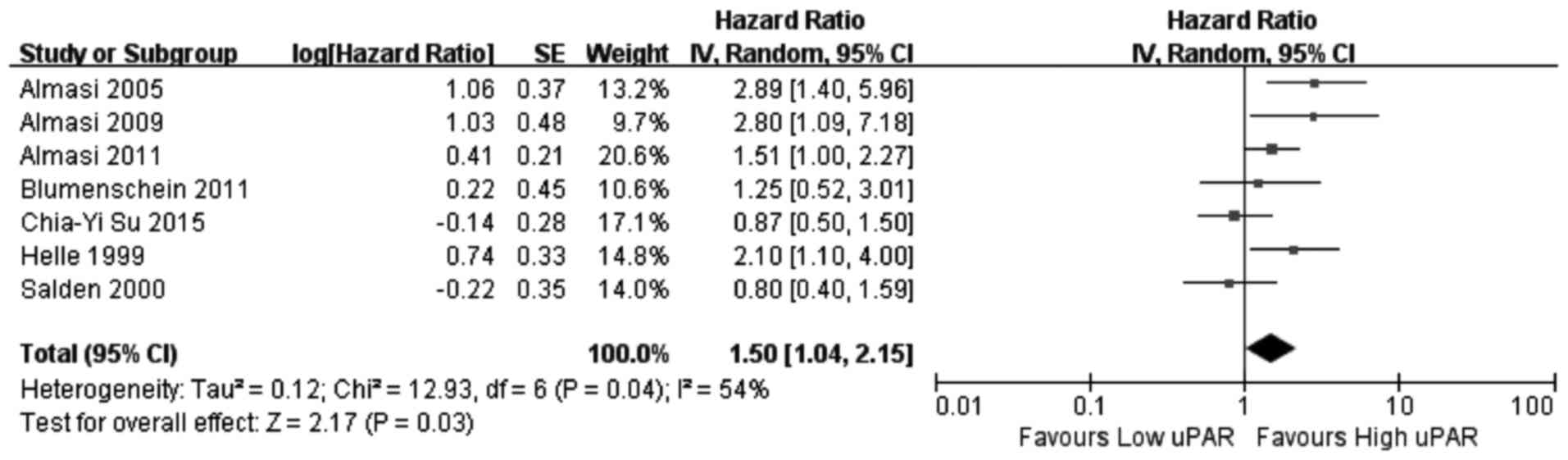
CI=0.64–3.90, P=0.32). Increased uPAR expression was associated
with poor OS (HR=1.50, 95% CI=1.04–2.15, P=0.03; Fig. 5), indicating that uPAR is an
independent prognostic factor in NSCLC. No significant publication
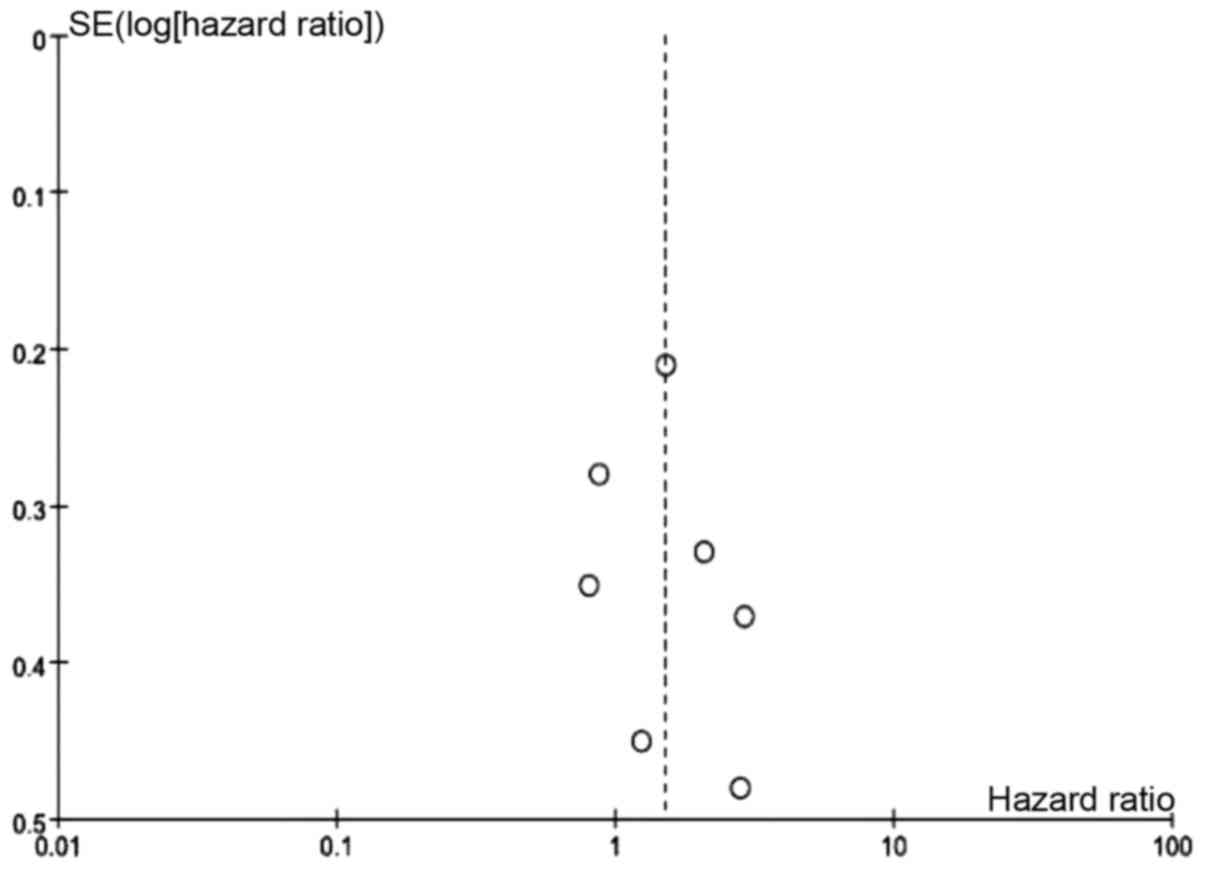
bias of OS (uPAR) was detected using the funnel plot (Fig. 6).
Discussion
The present meta-analysis investigated the
associations between OS and uPA, uPAR, PAI-1 and PAI-2 expression
in patients with NSCLC using English language peer-reviewed
scientific articles. Following reading of the full journal articles
selected from the databases, certain discrepancies were identified
in the conclusions. Thus, to more accurately evaluate the
associations between the expression of proteins in the uPA system
and the prognosis of patients with NSCLC, a total of 11 articles
were selected to conduct a meta-analysis of the included 937
patients. Results of the meta-analysis indicated that the
expression levels of uPA, PAI-1 and PAI-2 had no significant
association with poor OS in patients with NSCLC. Conversely, high
expression of uPAR did significantly correlate with poor OS for
patients with NSCLC.
uPAR is a 55–60 kDa glycoprotein that belongs to the
Ly-6 family (28). The gene encoding
for this receptor is located on chromosome 19q13.2, and is composed
of seven exons separated by six introns (29). The binding of uPA to uPAR
orchestrates various signaling molecules that mediate a number of
biological responses, including proliferation, migration, invasion,
angiogenesis and metastasis (30).
Cancer cells can produce uPA and uPAR; uPA binds to uPAR on the
surface of these cells, activating the proteolytic domain of uPA,
as well as stimulating intracellular signals leading to the
upregulation of uPAR and uPA itself. The uPA-uPAR complex can
subsequently facilitate the transformation of plasminogen to
plasmin, which can then cause extracellular matrix degradation
(31). These events confer tumor
cells with the ability to degrade the components of the surrounding
extracellular matrix, thus contributing to tumor cell invasion and
metastasis, resulting in poor OS.
The interaction between uPA and uPAR also results in
the release of signaling molecules that stimulate cell
proliferation/survival and the expression of tumor-promoting genes
(32). Consequently, the binding of
uPAR by uPA assists in the process of tumor development. Indeed, an
inhibitory peptide that prevents uPA-uPAR interaction has
demonstrated promise in prolonging patient survival during the
early stages of a clinical trial (33). Furthermore, recent studies have
identified significant differences in the expression of uPAR
between cancer tissues and normal tissues (34). In general, the importance of the uPAR
co-receptor in facilitating the tumor-promoting effects of uPAR
suggests that disruption of the interactions between uPAR and its
functional partners may be a potentially crucial strategy for the
development of anticancer therapeutic agents.
Some limitations need to be acknowledged for the
current meta-analysis. First, the sample size included was
relatively small (937 patients with NSCLC). Second, the various
histological types of NSCLC, including squamous cell carcinoma and
adenocarcinoma, exhibit stereotypical biological characteristics,
which create a certain level of bias. Third, the uPA expression
profiles pertaining to numerous rare types of NSCLC, including
squamous carcinoma, sarcomatoid carcinoma, carcinoid, and
unclassified cancer types, are currently unknown. Thus, further
clinical trials are required before these forms of cancer can be
optimally evaluated. Fourth, the potential confounding variables in
each individual study were unable to be assessed. For example,
certain studies may have evaluated a combination of various
histological types of NSCLC; alternatively, the methods used for
the evaluation of uPAR expression and differences pertaining to the
cutoff (detailed data are not listed) may have differed between
studies. Fifth, certain studies could not be included in the
meta-analysis owing to the lack of detailed data; sixth, the varied
locations and ethnicities may have also contributed to hidden
bias.
In conclusion, the present meta-analysis
demonstrated that uPAR is associated with the poor prognosis of
NSCLC. Thus, uPAR can be used as a biomarker for determining the
prognosis of NSCLC, and may assist clinicians in selecting and
applying more effective strategies for the treatment of patients
with NSCLC.
References
|
1
|
Torre LA, Bray F, Siegel RL, Ferlay J,
Lortet-Tieulent J and Jemal A: Global cancer statistics, 2012. CA
Cancer J Clin. 65:87–108. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Chen W, Zheng R, Baade PD, Zhang S, Zeng
H, Bray F, Jemal A, Yu XQ and He J: Cancer statistics in China,
2015. CA Cancer J Clin. 66:115–132. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Ben Amar J, Ben Safta B, Zaibi H, Dhahri
B, Baccar MA and Azzabi S: Prognostic factors of advanced stage
non-small-cell lung cancer. Tunis Med. 94:360–367. 2016.PubMed/NCBI
|
|
4
|
Zhang T, Yu TT, Zhang DM, Hou XM, Liu XJ,
Zhao D and Shan L: Vasohibin-1 expression detected by
immunohistochemistry correlates with prognosis in non-small cell
lung cancer. Med Oncol. 31:9632014. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Bice T, Li G, Malinchoc M, Lee AS and
Gajic O: Incidence and risk factors of recurrent acute lung injury.
Crit Care Med. 39:1069–1073. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Rubenfeld GD, Caldwell E, Peabody E,
Weaver J, Martin DP, Neff M, Stern EJ and Hudson LD: Incidence and
outcomes of acute lung injury. N Engl J Med. 353:1685–1693. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Matthay MA, Robriquet L and Fang X:
Alveolar epithelium: Role in lung fluid balance and acute lung
injury. Proc Am Thorac Soc. 2:pp. 206–213. 2005; View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Smith HW and Marshall CJ: Regulation of
cell signalling by uPAR. Nat Rev Mol Cell Biol. 11:23–36. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Feng MY, Liao QJ and Su Q: Advance in
research on uPAR and tumor. Int J Pathol Clin Med. 31:49–53.
2011.(In Chinese).
|
|
10
|
Andres SA, Edwards AB and Wittliff JL:
Expression of urokinase-type plasminogen activator (uPA), its
receptor (uPAR), and inhibitor (PAI-1) in human breast carcinomas
and their clinical relevance. J Clin Lab Anal. 26:93–103. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Thummarati P, Wijitburaphat S, Prasopthum
A, Menakongka A, Sripa B, Tohtong R and Suthiphongchai T: High
level of urokinase plasminogen activator contributes to
cholangiocarcinoma invasion and metastasis. World J Gastroenterol.
18:244–250. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Urban P, Vuaroqueaux V, Labuhn M,
Delorenzi M, Wirapati P, Wight E, Senn HJ, Benz C, Eppenberger U
and Eppenberger-Castori S: Increased expression of urokinase-type
plasminogen activator mRNA determines adverse prognosis in
ErbB2-positive primary breast cancer. J Clin Oncol. 24:4245–4253.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Mekkawy AH, Morris DL and Pourgholami MH:
HAX1 augments cell proliferation, migration, adhesion, and invasion
induced by urokinase-type plasminogen activator receptor. J Oncol.
2012:9507492012. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Tierney JF, Stewart LA, Ghersi D, Burdett
S and Sydes MR: Practical methods for incorporating summary
time-to-event data into meta-analysis. Trials. 8:162007. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Guyot P, Ades AE, Ouwens MJ and Welton NJ:
Enhanced secondary analysis of survival data: Reconstructing the
data from published Kaplan-Meier survival curves. BMC Med Res
Methodol. 12:92012. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Panic N, Leoncini E, de Belvis G,
Ricciardi W and Boccia S: Evaluation of the endorsement of the
preferred reporting items for systematic reviews and meta-analysis
(PRISMA) statement on the quality of published systematic review
and meta-analyses. PLoS One. 8:e831382013. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Almasi CE, Høyer-Hansen G, Christensen IJ,
Danø K and Pappot H: Prognostic impact of liberated domain I of the
urokinase plasminogen activator receptor in squamous cell lung
cancer tissue. Lung Cancer. 48:349–355. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Almasi CE, Høyer-Hansen G, Christensen IJ
and Pappot H: Prognostic significance of urokinase plasminogen
activator receptor and its cleaved forms in blood from patients
with non-small cell lung cancer. APMIS. 117:755–761. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Almasi CE, Christensen IJ, Høyer-Hansen G,
Danø K, Pappot H, Dienemann H and Muley T: Urokinase receptor forms
in serum from non-small cell lung cancer patients: Relation to
prognosis. Lung Cancer. 74:510–515. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Blumenschein GR Jr, Reck M, Fossella F,
Stewart DJ, Lathia C and Peña C: Plasma biomarkers correlating with
clinical outcome in a phase II study of sorafenib in advanced
NSCLC. Cancer Biomark. 10:287–298. 2011–2012. View Article : Google Scholar
|
|
21
|
Su CY, Liu YP, Yang CJ, Lin YF, Chiou J,
Chi LH, Lee JJ, Wu AT, Lu PJ, Huang MS and Hsiao M: Plasminogen
activator inhibitor-2 plays a leading prognostic role among
protease families in non-small cell lung cancer. PLoS One.
10:e01334112015. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Pappot H: The plasminogen activation
system in lung cancer-with special reference to the prognostic role
in ‘non-small cell lung cancer’. APMIS Suppl. 92:1–29.
1999.PubMed/NCBI
|
|
23
|
Offersen BV, Pfeiffer P, Andreasen P and
Overgaard J: Urokinase plasminogen activator and plasminogen
activator inhibitor type-1 in nonsmall-cell lung cancer: Relation
to prognosis and angiogenesis. Lung Cancer. 56:43–50. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Pappot H, Pedersen AN, Brünner N and
Christensen IJ: The complex between urokinase (uPA) and its type-1
inhibitor (PAI-1) in pulmonary adenocarcinoma: Relation to
prognosis. Lung Cancer. 51:193–200. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Pedersen H, Grøndahl-Hansen J, Francis D,
Osterlind K, Hansen HH, Danø K and Brünner N: Urokinase and
plasminogen activator inhibitor type 1 in pulmonary adenocarcinoma.
Cancer Res. 54:120–123. 1994.PubMed/NCBI
|
|
26
|
Salden M, Splinter TA, Peters HA, Look MP,
Timmermans M, van Meerbeeck JP and Foekens JA: The urokinase-type
plasminogen activator system in resected non-small-cell lung
cancer. Rotterdam Oncology Thoracic Study Group. Ann Oncol.
11:327–332. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Werle B, Kotzsch M, Lah TT, Kos J,
Gabrijelcic-Geiger D, Spiess E, Schirren J, Ebert W, Fiehn W,
Luther T, et al: Cathepsin B, plasminogenactivator-inhibitor
(PAI-1) and plasminogenactivator-receptor (uPAR) are prognostic
factors for patients with non-small cell lung cancer. Anticancer
Res. 24:4147–4161. 2004.PubMed/NCBI
|
|
28
|
Li Y, Shen Y, Miao Y, Luan Y, Sun B and
Qiu X: Co-expression of uPAR and CXCR4 promotes tumor growth and
metastasis in small cell lung cancer. Int J Clin Exp Pathol.
7:3771–3780. 2014.PubMed/NCBI
|
|
29
|
Thielemann A, Baszczuk A, Kopczyński P and
Kopczyński Z: High concentration of urokinase-type plasminogen
activator receptor in the serum of women with primary breast
cancer. Contemp Oncol (Pozn). 17:440–445. 2013.PubMed/NCBI
|
|
30
|
Pulukuri SM, Gorantla B, Dasari VR, Gondi
CS and Rao JS: Epigenetic upregulation of urokinase plasminogen
activator promotes the tropism of mesenchymal stem cells for tumor
cells. Mol Cancer Res. 8:1074–1083. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
de Bock CE and Wang Y: Clinical
significance of urokinase-type plasminogen activator receptor
(uPAR) expression in cancer. Med Res Rev. 24:13–39. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Chiang SP, Cabrera RM and Segall JE: Tumor
cell intravasation. Am J Physiol Cell Physiol. 311:C1–C14. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Noh H, Hong S and Huang S: Role of
urokinase receptor in tumor progression and development.
Theranostics. 3:487–495. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Montuori N, Pesapane A, Rossi FW, Giudice
V, De Paulis A, Selleri C and Ragno P: Urokinase type plasminogen
activator receptor (uPAR) as a new therapeutic target in cancer.
Transl Med UniSa. 15:15–21. 2016.PubMed/NCBI
|