Introduction
Prostate volume (PV) plays an important role in
planning for radiation therapy (RT). Smaller PV implies smaller
areas of organs-at-risk in Planning Target Volume (PTV) before
start of RT, thus minimizing side effects from normal surrounding
tissues. Larger PV, on the other hand, demands radiation of larger
areas, thus increasing the risk of side effects. One theoretical
rationale for offering endocrine therapy in the neoadjuvant setting
before RT is to reduce the PV. There is convincing evidence from
several previous studies that a short period of ADT prior to the
radiation therapy may reduce PV by 25–40% (1–6). ADT
used in these studies varied among luteinizing hormone release
hormone (LHRH)-analogue alone, or in combination with anti-androgen
or surgical castration only, or a combination of anti-androgen and
5-alfa reductase inhibitor. Whittington et al (7) showed, by using LHRH-analogue, that the
greatest decrease of PV occurred in those with the largest PV at
baseline. Thus, it remains as physicians' options to use ADT to get
maximum volume reduction.
There are different methods for volume measurement
of prostate gland. Minimally invasive surgery has shown that
ultrasound is the ideal imaging system for targeting treatments
because of its ease of use and the absence of adverse effects
(8). Computed tomography (CT)
derived estimations of PVs are generally larger than PV assessed by
Magnetic Resonance, especially towards the seminal vesicles and the
apex of the prostate (9). In
addition, a PV evaluation in ten patients before prostate
brachytherapy showed that the CT-based prostate volumes ranged from
31.1 cubic centimetres (cc) to 48.1 cc, whereas corresponding
figures for transrectally ultrasound (TRUS)-based volumes were 26.6
to 46.4 cc (10). Furuya et
al (11) showed prospectively,
by using TRUS, that ADT significantly decreased prostate- and
seminal vesicles volumes. Thus, TRUS assessed PV is expected to
generate PTV's including minimum volumes of organs-at-risk.
There is, to our knowledge, no published randomised
study addressing differences in PV reduction following treatment
with an androgen receptor inhibitor monotherapy vs castration plus
an androgen receptor inhibitor. Thus, the aim of the present study
was to compare changes in PV in the randomised ADT study (12). The hypothesis was that PV reduction
would be larger in the combined group compared to androgen receptor
inhibitor monotherapy, and that PTV subsequently would be smaller
in the castration plus androgen receptor inhibitor group.
Patients and methods
Patients
Consecutive patients with localised prostate cancer
intended for curative treatment with radiotherapy were included in
the randomised ADT study (12). The
primary aim of the ADT study was to compare health-related
quality-of-life (HRQoL) between the two groups over time. All
patients were treated at a single institution, the Department of
Oncology, Karolinska University Hospital, Sweden. Included patients
signed an informed consent form before randomisation to an androgen
receptor inhibitor monotherapy or to castration plus an androgen
receptor inhibitor.
Methods
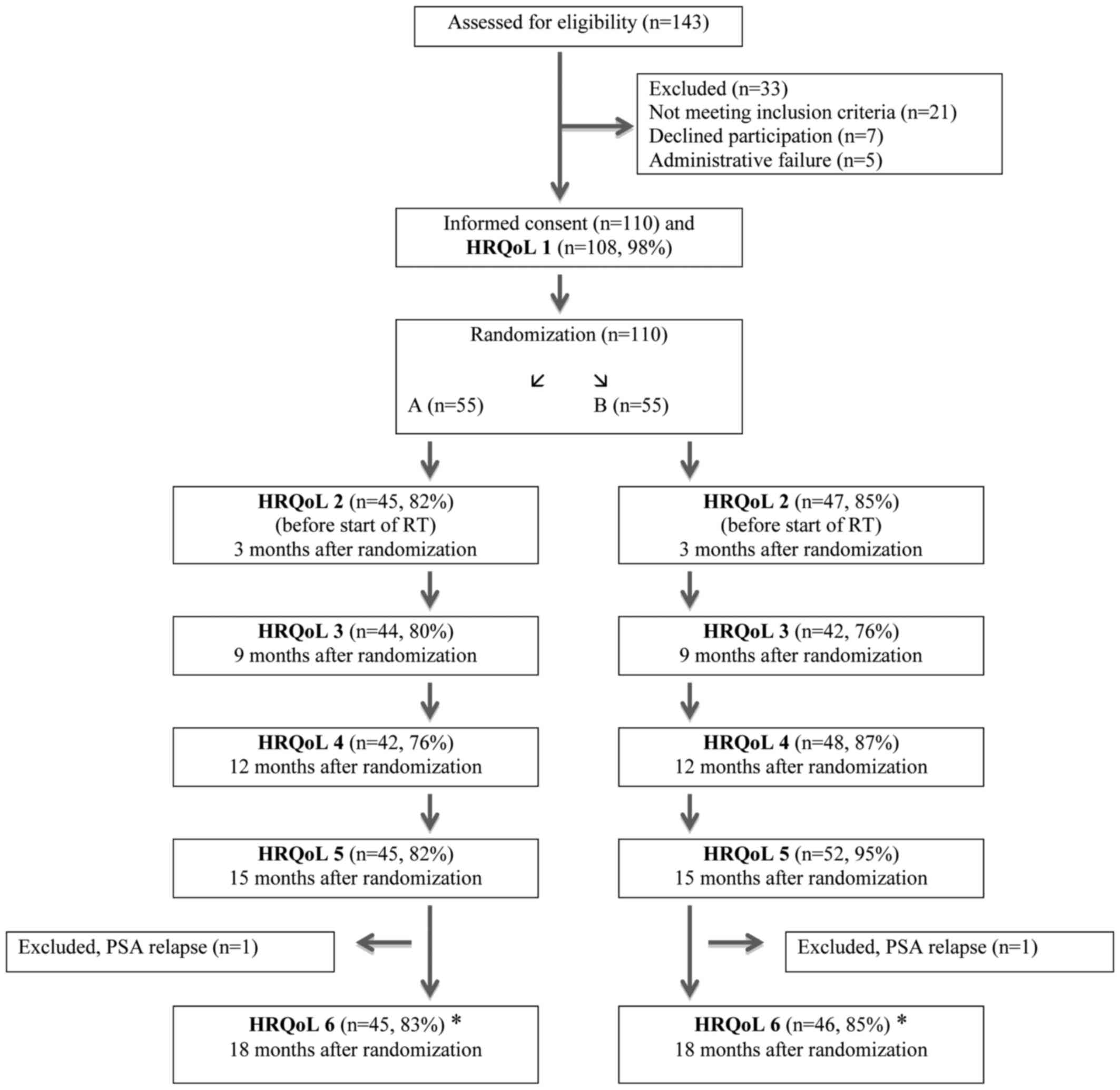
Between 2005 and 2011 a total of 110 patients were
included in the ADT study (12).
Before 2008, the referring urologist measured PV (Volume 1) before
referral to the Department of Oncology. Between 2008 and 2011 PV
measurement were routinely performed at the Department of Oncology
to ensure homogeneity. Second PV measurement (Volume 2) was
performed before start of HDR brachytherapy, about three months
after randomization. Planning target volume was decided upon by
computerized tomography.
Randomization
Eligible patients were randomly allocated between
the treatment arms in a 1:1 ratio (Fig.
1). A total of 55 patients were randomised to Group A
(Bicalutamide 150 mg orally daily) vs. 55 patients who were
randomised to Group B (Bicalutamide 50 mg orally daily + Implant
Goserelin 3,6 mg sub-cutaneous every 28±2 days). Patients in both
groups were offered the option to use anti-oestrogen orally if
needed against breast-tenderness or gynecomastia. The Clinical
Trials Unit, located at the Karolinska University Hospital,
performed the randomization per pre-constructed randomization
lists. By use of permuted block technique, randomization lists were
generated per standard procedures. Stratification was done for age
(≤65, >65 years) and lymph node dissection (yes or no).
PV assessment
TRUS (BK Medical endocavity biplane Transducer
8848.12-4 MHz) was used to measure PV at the Department of
Oncology. The procedure was performed in an operating room with the
patient in the dorsal lithotomy position. The TRUS transducer was
positioned in a stepping device that allowed the prostate to be
scanned systematically in both axial and sagittal planes. The
ultrasound system permitted very accurate volume and surface
outline calculations of the prostate and seminal vesicles. The
height and width were measured in the transverse plane and length
in the sagittal plane. Ultrasound apparatus then generated the
volume automatically. The results were recorded in the patients'
medical chart, providing the physicians access to individual
results during patient consultations.
Assessment of HRQoL
The patients completed HRQoL questionnaires after
informed consent and before randomisation, and again before start
of RT, about three months after randomisation. The European
Organization for Research and Treatment of Cancer Quality of Life
Core Questionnaire (EORTC QLQ-C30) and the EORTC Prostate Cancer
Specific Module (EORTC PR-25) were used (13,14).
Statistical methods
Absolute differences in PV between the baseline and
the follow-up assessment were tested for each group by the paired
t-test. The mean paired change is presented together with 95%
confidence intervals. Differences between the study groups at
baseline and at follow-up were estimated and tested using linear
regression models. At the follow-up visit differences were
estimated both by not including Volume 1 in the model (univariate
analysis), and by including the Volume 1 in the model (multivariate
analysis). P-values from these models refer to Wald tests. All
statistical analyses were based on the ‘intention-to-treat’
principle.
The cut-off 20% for decrease in PV was set as
minimum decrease, based on the observation from similar studies
(1–5). The cut-off was set to 10% for increase,
based on one study done by Henderson and co-workers (5) where 8% increase in PV was noted in
patients without hormonal therapy. The between group comparison of
PTV was performed by unpaired t-test.
The HRQoL results are presented as mean differences
and 95% confidence intervals (CIs). The reported P-values are
two-sided and refer to Wald tests. A P-value of ≤0.05 was
considered statistically significant.
The present study was approved by the Ethics
Committee at the Karolinska Institutet, Stockholm, Sweden (Dnr.
2008/1222-32).
Results
The baseline clinical characteristics by
randomisation groups are presented in Table I. A total of 110 patients were
included in the neoadjuvant study (12). Eleven patients were, however, not
included in the present analyses as information of PV at baseline
was lacking. Another 11 patients were excluded because they were
treated with EBRT only, where volume measurement was not performed
routinely at RT start. Reasons for not being subjected for combined
EBRT-brachytherapy (11 patients) were the following: ‘No decrease
in PV after AA’ (1 patient), ‘PV>65 cc’ (3 patients), ‘Earlier
transurethral resection of prostate’ (3 patients), ‘Co-morbidity’
(3 patients), ‘Lobus tertius’ (1 patient). Thus, 88 patients (80%)
remained to be analysed, 45 patients (51%) in Group A and 43
patients (49%) in Group B. Two patients switched over from Group A
to the Group B (however treated with LHRH analogue only) due to
liver toxicity, but were included per the intention-to-treat
principle.
 | Table I.Patient characteristics according to
randomization arms. |
Table I.
Patient characteristics according to
randomization arms.
|
| Arm A n=45 | Arm B n=43 | Total n=88 |
|---|
| Age (year) mean
(range) | 67 (54–76) | 66 (53–78) |
|
| T-stage, n (%) |
|
|
|
| T1C | 12 (27) | 14 (33) | 26 |
| T2 | 26 (58) | 22 (51) | 48 |
| T2-3 | 3 (7) | 2 (5) | 5 |
| T3 | 4 (9) | 5 (12) | 9 |
| Gleason score, n
(%) |
|
|
|
| 6 | 12 (27) | 8 (19) | 20 |
| 7 | 31 (69) | 34 (79) | 65 |
| 8 | 0 (0) | 1 (2) | 1 |
| 9 | 2 (4) | 0 (0) | 2 |
| PSA at inclusion |
|
|
|
| Mean
(range) | 10.0 (2.7–38.0) | 8.8 (2.9–24.0) |
|
| Order of RT, n
(%) |
|
|
|
|
BTx2-Ext | 33 (73) | 33 (77) | 66 |
|
Ext-BTx2-Ext | 5 (11) | 3 (7) | 8 |
|
Ext-BTx2 | 5 (11) | 7 (16) | 12 |
|
BT-Ext-BT | 2 (4) | 0 (0) | 2 |
| Volume 1 before |
|
|
|
| Randomization
(cc) |
|
|
|
| Mean
(range) | 33 (11–50) | 30 (18–50) | 31.5 |
| Volume 2 before RT
(cc) |
|
|
|
| Mean
(range) | 27,2 (15–42) | 21,6 (11,8–30,4) |
|
| Time between volume 1
and volume 2 (number of weeks) |
|
|
|
| Mean
(range) | 13 (9–22.5) | 13.5 (8–23) |
|
Castration plus an androgen receptor inhibitor was
more effective in PV reduction as compared to androgen receptor
inhibitor monotherapy (P<0.001) (Table II). Mean volume reduction was 28%
(30 to 21.6 cc) and 17.5% (33 to 27.2 cc) respectively. In Group A,
PV was reduced by ≥20% in 23 patients (51%). Corresponding fig. for
Group B was 34 patients (79%). PV was increased by ≥10% in 4
patients (8%) in Group A and in 1 patient (2%) in Group B. The time
between the assessments was similar in both groups. There was no
statistically significant difference in duration of neoadjuvant
treatment or in clinical and demographic variables between the two
groups.
 | Table II.Volume measured at baseline, measured
at follow-up and change in volume between the occassions by
allocated treatments. |
Table II.
Volume measured at baseline, measured
at follow-up and change in volume between the occassions by
allocated treatments.
|
|
|
| Between treatment
comparisonsa |
|---|
|
|
|
|
|
|---|
|
| Allocated treatment
(SD) | Univariate
analysis | Multivariate
analysisb |
|---|
|
|
|
|
|
|---|
| Volume (CC) | Group A (n=45) | Group B (n=43) | Mean difference (95%
CI) | P-value | Mean difference (95%
CI) | P-value |
|---|
| Cross-sectional
analysis: |
|
|
|
|
|
|
| At baseline,
mean | 33.6 (9.8) | 31.1 (8.0) | −2.6 (−6.4 to
1.2) | 0.18 |
|
|
| At follow-up,
mean | 27.2 (6.9) | 21.6 (5.4) | −5.6
(−8.2 to - 2.9) | <0.001 | −4.2 (−5.9 to
−2.5) | <0.001 |
| Within treatment
changec: |
|
|
|
|
|
|
| Absolute mean change,
(95% CI) | −6.4 (−8.1 to
−4.8) | −9.4 (−11.3 to
−7.6) |
|
|
|
|
| P-Value: | <0.001 | <0.001 |
|
|
|
|
A comparison of prostate target volume (PTV) for the
planning of radiotherapy revealed a statistically significant
difference (P>0.001) between the two groups in mean volume, 47.4
cc (SD=12.8) in Group A vs. 37.9 cc (SD=7.6) in Group B.
At the assessment after the first 3 months
statistically significant differences between the groups in
‘overall quality of life’, ‘fatigue’, and ‘sexual interest’,
favouring Group A (Table III). No
other between group differences was found for HRQoL.
 | Table III.EORTC QLQ-30 and QLQ-PR25 mean values
and SD at the three months' assessment and mean scales differences
(CI) corrected for baseline between Group A and Group B. |
Table III.
EORTC QLQ-30 and QLQ-PR25 mean values
and SD at the three months' assessment and mean scales differences
(CI) corrected for baseline between Group A and Group B.
| Variable | Group A mean
(SD) | Group B mean
(SD) | Difference (CI) | P-value |
|---|
| EORTC QLQ-C30 |
|
|
|
|
| Overall
quality of lifea | 80 (22) | 74 (19) | −9 (−15 to −3) | 0.006 |
|
Physical
functioninga | 92 (11) | 92 (12) | −1 (−4 to 3) | NS |
| Role
functioninga | 94 (14) | 90 (18) | −5 (−12 to 2) | NS |
|
Emotional
functioninga | 84 (23) | 84 (18) | −3 (−9 to 4) | NS |
|
Cognitive
functioninga | 91 (15) | 87 (17) | −5 (−11 to 1) | NS |
| Social
functioninga | 89 (19) | 89 (18) | −2 (−9 to 5) | NS |
|
Fatigueb | 18 (20) | 23 (18) | 8 (1 to 15) | 0.023 |
| Nausea
and vomiting | 4 (9) | 3 (7) | −1 (−5 to 2) | NS |
|
Pain | 10 (18) | 7 (16) | −5 (−11 to 1) | NS |
|
Dyspnoea | 15 (23) | 18 (24) | 3 (−5 to 12) | NS |
|
Insomnia | 21 (27) | 27 (29) | 8 (−3 to 18) | NS |
|
Appetite loss | 2 (8) | 3 (9) | 0 (−3 to 2) | NS |
|
Constipation | 6 (15) | 6 (19) | 2 (−5 to 9) | NS |
|
Diarrhoea | 7 (15) | 5 (12) | −2 (−8 to 4) | NS |
| EORTC PR-25 |
|
|
|
|
| Sexual
interesta | 31 (29) | 10 (14) | −21 (−30 to
−13) | <0.001 |
| Sexual
functioninga | 67 (19) | 59 (12) | −15 (−30 to 1) | NS |
| Urinary
problemsb | 14 (12) | 16 (17) | 3 (−3 to 10) | NS |
| Bowel
problemsb | 3 (7) | 5 (7) | 3 (−1 to 6) | NS |
| Use of
padsb | 16 (11) | 16 (10) | 0 (−4 to 4) | NS |
Discussion
PV plays an important role when planning irradiation
with curative intention in prostate cancer, since large PTV may
affect organs at risk and subsequent radiation related side
effects. PV reduction is one of the rationales for using
neo-adjuvant ADT to minimize the radiation field, and thus the side
effects. In the present, prospective study, castration plus an
androgen receptor inhibitor significantly decreased PV more than
androgen receptor inhibitor monotherapy and subsequently PTV was
smaller in Group B than in Group A. One retrospective study showed,
in 22 patients, that the median percentage of volume reduction
after combination group was 25% (4).
Another non-randomised prospective study showed an 8% volume
reduction in the bicalutamide group compared to a 26% reduction in
the goserelin group after final analysis of 81 patients (5). Thus, our hypothesis was in concordance
with results from other studies.
In the present study PV was increased by ≥10% in 4
patients (8%) in Group A and in 1 patient (2%) in Group B. This
finding is surprising, as no other study has, to our knowledge,
reported similar findings. The increase in PV during ADT treatment
might be explained by the fact that the same physician did not
assess PV at the first point of assessment and at the second
measurement three months later. Thus, the absolute figures for PV
should be considered with caution. Ideally, the same physician
should have performed both assessments. Kucway et al
(15) mentioned in their study that
one of the sources of error in measurement of PV was
inter-physician variability, and pointed out that variability in PV
measurement is unavoidable. Patients in both randomised groups in
our study suffered this variability to the same extent. Thus, we do
not consider this to hamper our results.
RT has many side effects that are expected to
negatively influence patients' quality of life. A cross sectional
study of 989 prostate cancer patients treated with RT showed that
defecation urgency was the most common symptom among survivors
after 2–14 years' follow-up, followed by faecal leakage and loose
stools (16). Similar results have
been presented in patients treated with pelvic irradiation, both
men and women, where defecation urgency and faecal leakage has been
identified as the most disturbing of all radiation-induced symptoms
(17–21). In the neoadjuvant study (12), differences between the groups at the
three months' assessment, before the start of RT, were found for
‘overall quality of life’, ‘fatigue’ and ‘sexual interest’, all in
favour of monotherapy. These differences were expected, as
castration obtained in Group B might cause these problems. About 18
months after randomization (around nine months after termination of
RT), statistically significant differences were found for
‘cognitive functioning’ and ‘sexual interest’ (12). There were, however, no differences in
urinary or bowel symptoms at this assessment point. These findings
were surprising, as the smaller volumes irradiated in the
combination group were expected to result in lower levels of
urinary and bowel symptoms. One possible explanation may be that
combined EBRT BT irradiated both groups, and that the small
putative differences in side-effects caused by volume differences
of the external RT were outweighed by side effects from the
brachytherapy.
The randomized prospective single-centre design is
the strength of our study. In addition, TRUS was used to perform
assessment of PV, which is one of the most reliable methods for
this kind of assessment. Two urologic-oncologists, knowledgeable of
radiation planning in prostate cancer, screened all patients'
medical charts carefully. One weakness of the study is that the
results would be more reliable if PV measurement had been confined
to the same physician at both points of assessment. Sample size is
another weakness of the study.
In summary, a significantly less prominent PV
reduction was achieved following neoadjuvant ADT using an androgen
receptor inhibitor monotherapy compared to castration plus an
androgen receptor inhibitor. This PTV reduction, however, appeared
not to translate into a more favourable quality of life profile
during the subsequently given curative combined EBRT-brachytherapy.
Potential differences regarding anti-tumoral effects on micro
metastatic disease and radiation potentiating remains to be
addressed in future prospective trials.
References
|
1
|
Lee WR: The role of androgen deprivation
therapy combined with prostate brachytherapy. Urology. 60(3 Suppl
1): S39–S44. 2002. View Article : Google Scholar
|
|
2
|
Merrick GS, Butler WM, Wallner KE,
Galbreath RW, Allen ZA and Kurko B: Efficacy of neoadjuvant
bicalutamide and dutasteride as a cytoreductive regimen before
prostate brachytherapy. Urology. 68:116–120. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Nishiyama T, Tomita Y and Takahashi K:
Influence of androgen deprivation therapy on volume of anatomic
zones of prostate in patients with prostate cancer using magnetic
resonance imaging. Urology. 63:917–921. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Zelefsky MJ, Leibel SA, Burman CM, Kutcher
GJ, Harrison A, Happersett L and Fuks Z: Neoadjuvant hormonal
therapy improves the therapeutic ratio in patients with bulky
prostate cancer treated with three-dimensional conformal radiation
therapy. Int J Radiat Oncol Biol Phys. 29:755–761. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Henderson A, Langley SM and Laing RW: Is
bicalutamide equivalent to goserelin for prostate volume reduction
before radiation therapy? A prospective, observational study. Clin
Oncol (R Coll Radiol). 15:318–321. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Shearer RJ, Davies JH, Gelister JS and
Dearnaley DP: Hormonal cytoreduction and radiotherapy for carcinoma
of the prostate. Br J Urol. 69:521–524. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Whittington R, Broderick GA, Arger P,
Malkowicz SB, Epperson RD, Arjomandy B and Kassaee A: The effect of
androgen deprivation on the early changes in prostate volume
following transperineal ultrasound guided interstitial therapy for
localized carcinoma of the prostate. Int J Radiat Oncol Biol Phys.
44:1107–1110. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Carter SS, Torp-Pedersen ST and Holm HH:
Ultrasound-guided implantation techniques in treatment of prostate
cancer. Urol Clin North Am. 16:751–762. 1989.PubMed/NCBI
|
|
9
|
Rasch C, Barillot I, Remeijer P, Touw A,
van Herk M and Lebesque JV: Definition of the prostate in CT and
MRI: A multi-observer study. Int J Radiat Oncol Biol Phys.
43:57–66. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Badiozamani KR, Wallner K, Cavanagh W and
Blasko J: Camparability of CT-based and TRUS-based prostate
volumes. Int J Radiat Oncol Biol Phys. 43:375–378. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Furuya R, Hisasue S, Furuya S, Saitoh N,
Ogura H, Takahashi S and Tsukamoto T: Fate of seminal vesicles and
prostate after medical castration: How long is the optimal duration
of neoadjuvant treatment for prostate cancer before radiation?
Urology. 72:417–421. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Majumder K, Nilsson S, Johansson H, Ullén
A, Lennernäs B, Bergenmar M and Brandberg Y: Higher sexual interest
with androgen receptor inhibitor monotherapy than with castration
plus an androgen receptor inhibitor in prostate cancer patients
treated with curative radiotherapy, but otherwise small
health-related quality of life differences: A randomised
prospective 18-month follow-up study. Eur J Cancer. 65:43–51. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Aaronson NK, Ahmedzai S, Bergman B,
Bullinger M, Cull A, Duez NJ, Filiberti A, Flechtner H, Fleishman
SB, de Haes JC, et al: The European organization for research and
treatment of cancer QLQ-C30: A quality-of-life instrument for use
in international clinical trials in oncology. J Natl Cancer Inst.
85:365–376. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
van Andel G, Bottomley A, Fosså SD,
Efficace F, Coens C, Guerif S, Kynaston H, Gontero P, Thalmann G,
Akdas A, et al: An international field study of the EORTC QLQPR25:
A questionnaire for assessing the health-related quality of life of
patients with prostate cancer. Eur J Cancer. 44:2418–2424. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Kucway R, Vicini F, Huang R, Stromberg J,
Gonzalez J and Martinez A: Prostate volume reduction with androgen
deprivation therapy before interstitial brachytherapy. J Urol.
167:2443–2447. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Alsadius D, Olsson C, Pettersson N, Tucker
SL, Wilderäng U and Steineck G: Patient-reported gastrointestinal
symptoms among long-term survivors after radiation therapy for
prostate cancer. Radiother Oncol. 112:237–243. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Gillespie C, Goode C, Hackett C and
Andreyev HJ: The clinical needs of patients with chronic
gastrointestinal symptoms after pelvic radiotherapy. Aliment
Pharmacol Ther. 26:555–563. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Hopfgarten T, Adolfsson J, Henningsohn L,
Onelöv E and Steineck G: The choice between a therapy-induced
long-term symptom and shortened survival due to prostate cancer.
Eur Urol. 50:280–289. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Andreyev HJ, Vlavianos P, Blake P,
Dearnaley D, Norman AR and Tait D: Gastrointestinal symptoms after
pelvic radiotherapy: Role for the gastroenterologist? Int J Radiat
Oncol Biol Phys. 62:1464–1471. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Lilleby W, Fosså SD, Waehre HR and Olsen
DR: Long-term morbidity and quality of life in patients with
localized prostate cancer undergoing definitive radiotherapy or
radical prostatectomy. Int J Radiat Oncol Biol Phys. 43:735–743.
1999. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Al-Abany M, Helgason AR, Cronqvist AK,
Svensson C, Wersäll P and Steineck G: Long-term symptoms after
external beam radiation therapy for prostate cancer with three or
four fields. Acta Oncol. 41:532–542. 2002. View Article : Google Scholar : PubMed/NCBI
|