Introduction
Breast cancer has been confirmed by gene expression
analysis to be a heterogeneous disease (1,2) that may
be divided into biologically distinct subtypes. In clinical
practice, the cancer subtype is determined using
immunohistochemistry (3,4) and a therapeutic plan is designed
according to the subtype (5). Lung
metastasis is commonly observed in breast cancer patients at the
time of relapse. Patients with metastatic disease have median
survival times of 12–24 months (6,7). The
majority of patients with breast cancer metastases to the lungs are
treated with systemic therapy. However, lung nodules that develop
in breast cancer patients during follow-up after curative breast
surgery may not always represent metastatic lesions. Therefore, the
final treatment strategy for breast cancer patients with lung
nodules depends on the pathological diagnosis of the nodules.
However, it is difficult to obtain an accurate diagnosis only by
using imaging modalities, such as computed tomography (CT),
particularly in patients with solitary nodules. For a definitive
diagnosis of the lung lesions, tumor biopsy, such as transbronchial
or CT-guided biopsy, may be performed. Video-assisted thoracoscopic
surgery (VATS) with intraoperative inspection is an optimal
procedure for the complete removal of the lesions and for reaching
a definitive diagnosis, but the survival benefit of total biopsy of
lung nodules by VATS remains to be established.
The aim of the present study was to evaluate the
significance of lung biopsy, including total biopsy by VATS, in
breast cancer patients who develop lung nodules during follow-up
after curative breast surgery.
Patients and methods
Patients
In total, 53 consecutive patients who underwent lung
biopsy following curative surgery for breast cancer in two
institutions (Hiroshima University Hospital and Hiroshima
Prefectural Hospital, Hiroshima, Japan) between 1995 and 2014 were
enrolled in this retrospective study. The age at lung biopsy was
27–84 years (mean, 63 years). A total of 30 patients (57%) had a
solitary lung nodule, 9 (17%) had 2 nodules, and 14 (26%) had ≥3
nodules. VATS was performed in 45 patients, transbronchial lung
biopsy in 7 patients, and CT-guided biopsy in 1 patient. The
indications for lung biopsy included lung nodules that were
difficult to diagnose clinically, and those for which the treatment
strategy would depend on the pathological diagnosis.
In the event of malignant lung tumors, a
non-curative lung biopsy was defined as a macroscopically visible
incomplete resection. When lung biopsy was performed in patients
with metastatic disease at extrapulmonary sites, the procedure was
also non-curative.
The majority of the patients who were included in
the present study underwent curative surgery for primary breast
cancer at one of the two aforementioned institutions and they were
subsequently followed up. The clinical records and pathological
reports of patients who underwent surgery for primary breast cancer
at other hospitals and presented to our hospital with lung nodules
were obtained from the respective hospitals. Use of these data was
approved by the Institutional Review Board (H27-073).
Patients were divided into the metastasis (patients
with metastatic lung cancer from breast cancer; n=25) and others
(patients with other pathologies; n=28) groups. In the present
study, we focused on the survival benefits of lung biopsy in the
metastasis group compared with the others group with variable
backgrounds, as the number of patients with primary lung cancer or
benign lung tumors was limited. Various clinicopathological factors
recorded at the time of primary breast cancer surgery were compared
between the groups, and a subgroup analysis was performed in the
metastasis group to compare their outcomes after lung biopsy.
Histological assessment
Routine hematoxylin and eosin staining was performed
on sections from tumor specimens in order to determine the
histological tumor type. Nuclear grade was determined according to
the 17th edition of the general rules for clinical and pathological
recording of breast cancer of the Japanese Breast Cancer Society
(8). Immunohistochemical staining
was performed to evaluate the expression status of estrogen
receptor (ER), progesterone receptor (PR), human epidermal growth
factor receptor 2 (HER2) and Ki-67, as previously described
(9). The following monoclonal
antibodies were used in the analysis: ER (SP1; 790–4324,
prediluted, Ventana Medical Systems, Tucson, AZ, USA); PR (1E2;
790–2223, prediluted, Ventana Medical Systems); Ki-67 (MIB-1;
M7240, 1:80 dilution, DAKO, Glostrup, Denmark); and HER2 (4B5;
790–2991, prediluted, Ventana Medical Systems). Based on the
expression of ER, PR, HER2 and Ki-67, patients were classified as
having one of the following subtypes, as previously described
(9): Luminal A, luminal B
HER2-negative, luminal B HER2-positive, HER2-positive and
triple-negative types.
Statistical analysis
Data are presented as number (%) or as mean, unless
otherwise stated. Categorical variables in both groups were
compared using Pearson's Chi-squared test, and small samples were
assessed using Fisher's exact test.
The overall survival (OS) in the metastasis group
was calculated from the date of lung biopsy to the date of death
from any cause, or the date of the last follow-up. The Kaplan-Meier
method was used to calculate OS, and patient subgroups were
compared using the log-rank test. All statistical analyses were
performed using EZR (Saitama Medical Center, Jichi Medical
University: http://www.jichi.ac.jp/saitama-sct/SaitamaHP.files/statmedEN.html;
Kanda, 2012), which is a graphical user interface for R statistical
software, version 2.13.0 (The R Foundation for Statistical
Computing, Vienna, Austria). More precisely, it is a modified
version of the R commander (version 1.6–3) that was designed to
include statistical functions frequently used in biostatistics
(10).
Results
Characteristics of primary breast
cancer patients with lung nodules
All patients who underwent lung biopsy had no major
fatal complications, although there were a few minor complications,
such as air leakage caused by the biopsy of peripheral lung
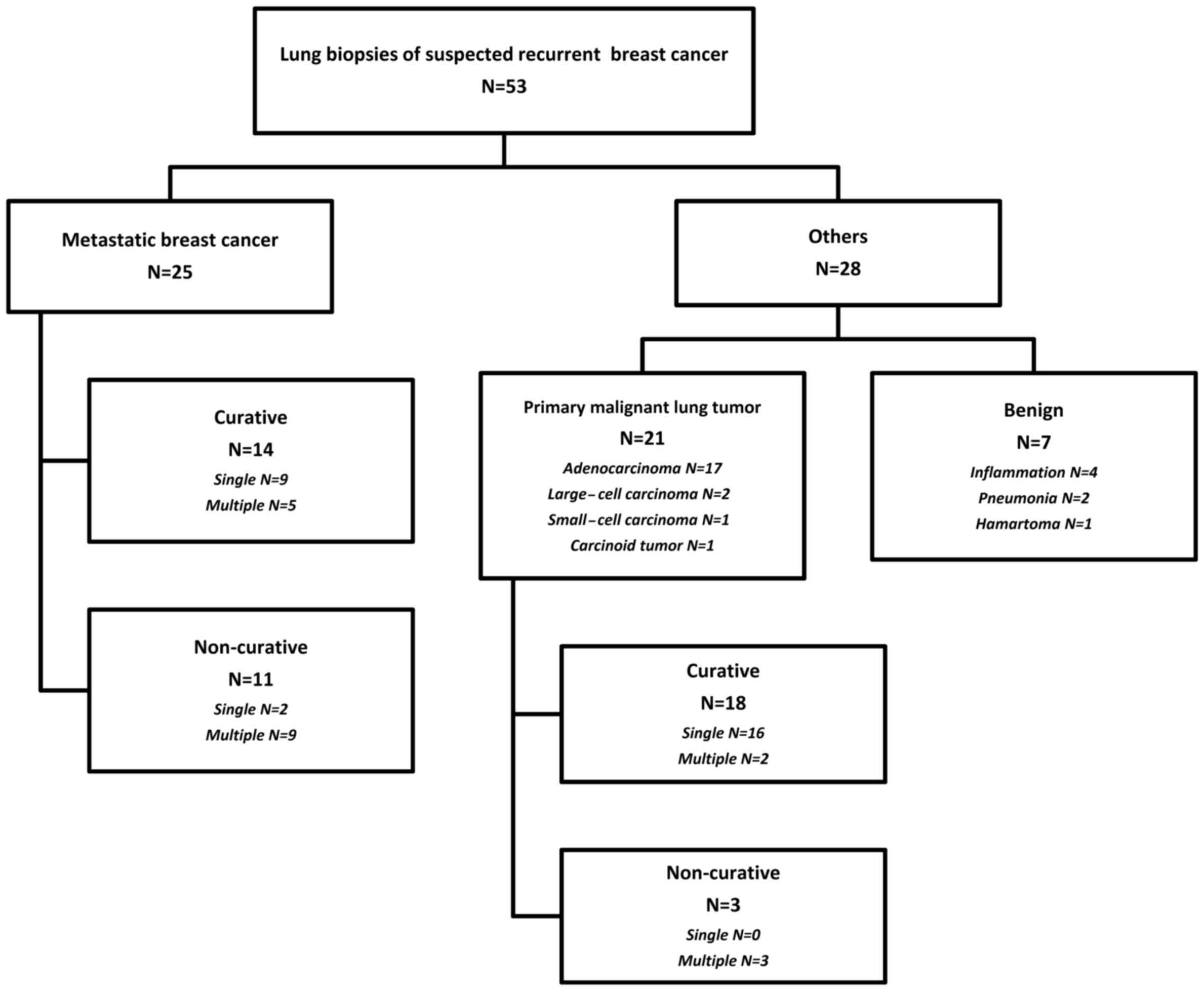
nodules. The pathological diagnoses of the lung nodules included
breast cancer metastases to the lungs in 25 (47%), primary
malignant lung tumor in 21 (40%) and benign disease in 7 patients
(13%). Of the 21 patients with primary malignant lung tumors, 17
had adenocarcinoma, 2 had large-cell carcinoma, 1 had small-cell
carcinoma and 1 had a carcinoid tumor. Of the 7 patients with
benign disease, 4 had inflammation, 2 had organizing pneumonia and
1 had a hamartoma. Of the 25 patients with breast cancer metastases
to the lungs, curative lung biopsy was performed in 14 patients
(56%). Of the 21 patients with primary malignant lung tumors, 18
(86%) had stage I disease and 19 (90%) underwent curative lung
biopsy (Fig. 1).
The comparison of the clinical factors between the
metastasis and the others groups at the time of primary breast
surgery by univariate analysis revealed that the patients in the
metastasis group were significantly younger compared with those in
the others group (median age, 59 vs. 65 years, respectively;
P<0.001). The rate of premenopausal status was significantly
higher in the metastasis group compared with that in the others
group (56 vs. 14%, respectively; P=0.003). The patients in the
metastasis group had higher node-positive rates compared with those
in the others group (56 vs. 11%, respectively; P=0.001). The
clinical stage of the patients in the metastasis group was also
higher compared with that in the others group (P=0.027). Therefore,
mastectomy (64 vs. 18%, respectively; P<0.001) and axillary
lymph node dissection (96 vs. 32%, respectively; P<0.001) were
performed more frequently in the metastasis group compared with the
others group. Postoperative radiotherapy and primary systemic
chemotherapy were administered more frequently in the metastasis
group compared with the others group (56 vs. 18%, P=0.005 and 80
vs. 32%, P<0.001, respectively;). The clinicopathological
characteristics of the patients are summarized in Tables I and II.
 | Table I.Clinical characteristics of primary
breast cancer. |
Table I.
Clinical characteristics of primary
breast cancer.
| Variables | Metastasis
(n=25) | Others (n=28) | P-value | Multivariate
P-value |
|---|
| Median age, years
(range) | 59 (25–63) | 65 (41–78) | <0.001 |
|
| Menopausal
status |
|
| 0.003 | – |
|
Premenopausal/postmenopausal | 14/11 | 4/24 |
|
|
| BMI, kg/m2
(range) | 22.4 (16.6–30.6) | 23.6 (17.4–34.2) | 0.232 |
|
| Patients with other
cancers |
|
| 0.183 |
|
|
Yes/no | 22/3b | 20/8c |
|
|
| Contralateral breast
cancer |
|
| 0.113 |
|
|
Yes/no | 0/25 | 4/24 |
|
|
| Breast/ovarian cancer
family history |
|
| 0.404 |
|
|
Yes/no | 4/21 | 2/26 |
|
|
| Clinical tumor
stage |
|
| 0.417 |
|
|
Tis/T1,2/T3,4 | 0/22/3 | 3/23/2 |
|
|
| Clinical node
stage |
|
| 0.001 | – |
|
Negative/positive | 11/14 | 25/3 |
|
|
| Clinical stage |
|
| 0.027 |
|
|
0/I/II/III | 0/8/12/5 | 3/16/8/1 |
|
|
| Type of breast
surgery |
|
| <0.001 | 0.045 |
|
Mastectomy/partial
mastectomy | 16/9 | 5/23 |
|
|
| Axillary LN
dissection |
|
| <0.001 | 0.001 |
| None or
SLNB/Ax | 1/24 | 19/9 |
|
|
| Radiation
therapy |
|
| 0.005 | – |
|
Yes/no | 11/14 | 23/5 |
|
|
|
Chemotherapya |
|
| <0.001 | – |
|
Yes/no/unknown | 20/4/1 | 9/19/0 |
|
|
| Hormonal therapy |
|
| 0.321 |
|
|
Yes/no/unknown | 13/11/1 | 19/9/0 |
|
|
 | Table II.Pathological characteristics of
primary breast cancer. |
Table II.
Pathological characteristics of
primary breast cancer.
| Variables | Metastasis
(n=25) | Others (n=28) | P-value | Multivariate
P-value |
|---|
| Pathological tumor
stage; (y)pT |
|
| 0.229 |
|
|
Tis/T1,2/T3,4 | 0/21/4 | 4/23/1 |
|
|
| Pathological node
stage; (y)pN |
|
| 0.0492 | – |
|
Negative/positive | 13/12 | 22/6 |
|
|
| Pathological
stage |
|
| 0.101 |
|
|
0/I/II/III | 0/8/10/7 | 4/13/7/4 |
|
|
| Lymphovascular
invasion |
|
| 0.004 | – |
|
Negative/positive/unknown | 4/13/8 | 17/7/4 |
|
|
| Nuclear grade |
|
| 0.0552 |
|
|
1/2/3/unknown | 3/3/8/11 | 6/11/4/7 |
|
|
| ER status |
|
|
|
|
|
Negative/positive/unknown | 8/15/2 | 5/23/0 | 0.207 |
|
| PR status |
|
| 0.167 |
|
|
Negative/positive/unknown | 12/11/2 | 9/19/0 |
|
|
| HER2 status, n
(%) |
|
| 0.092 |
|
|
0/1+/2+/3+/unknown | 7/4/5/4/5 | 5/11/3/1/7 |
|
|
| Ki-67 |
|
|
|
|
|
<20%/>20%/unknown | 7/11/7 | 7/6/15 | 0.481 |
|
| Tumor subtype, n
(%) |
|
| 0.168 |
|
|
Luminal/non-luminal | 16/7 | 21/3 |
|
|
The comparison of the pathological factors between
the groups at the time of primary breast surgery by univariate
analyses revealed that the patients in the metastasis group had
higher node-positive rates compared with those in the others group
(79 vs. 52%, respectively; P=0.0492). The rate of positive
lymphovascular invasion was significantly higher in the metastasis
group compared with that in the others group (52 vs. 25%,
respectively; P=0.004).
The mean disease-free interval from the surgery for
primary breast cancer to lung biopsy, multiplicity of lung nodules,
and curability by lung biopsy did not differ significantly between
the two groups (Table III).
 | Table III.Characteristics of patients with lung
nodules. |
Table III.
Characteristics of patients with lung
nodules.
| Variables | Metastases
(n=25) | Others (n=28) | P-value | Multivariate
P-value |
|---|
| Disease-free
interval, months |
|
| 0.325 |
|
| Mean
(SD) | 66.3 (45.5) | 52.7 (53.8) |
|
|
| No. of lung
nodules |
|
| 0.101 | – |
|
Solitary/multiple | 11/14 | 19/9 |
|
|
| Resection of
metastases |
|
|
|
|
|
Curative/non-curative | 14/11 | 22/6 | 0.139 | – |
Multivariate analysis revealed that mastectomy
(P<0.001) and axillary resection (P<0.001) were independent
factors predicting whether the lung nodules would be metastases
from breast cancer (Table I).
Survival analysis
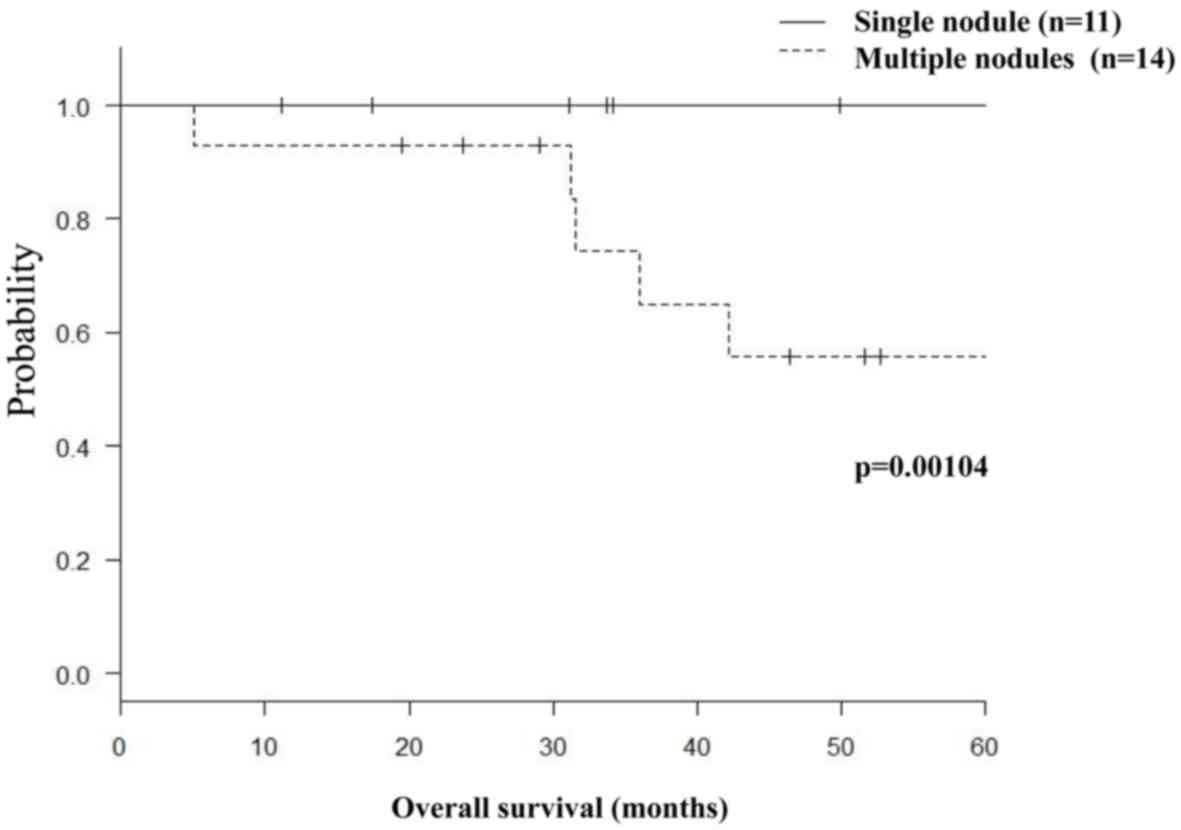
In the metastasis group, 11 patients with solitary
nodules had significantly better survival rates compared with 14
patients with multiple nodules (3-year survival rates, 100 vs.
74.3%, respectively; P=0.00104; Fig.
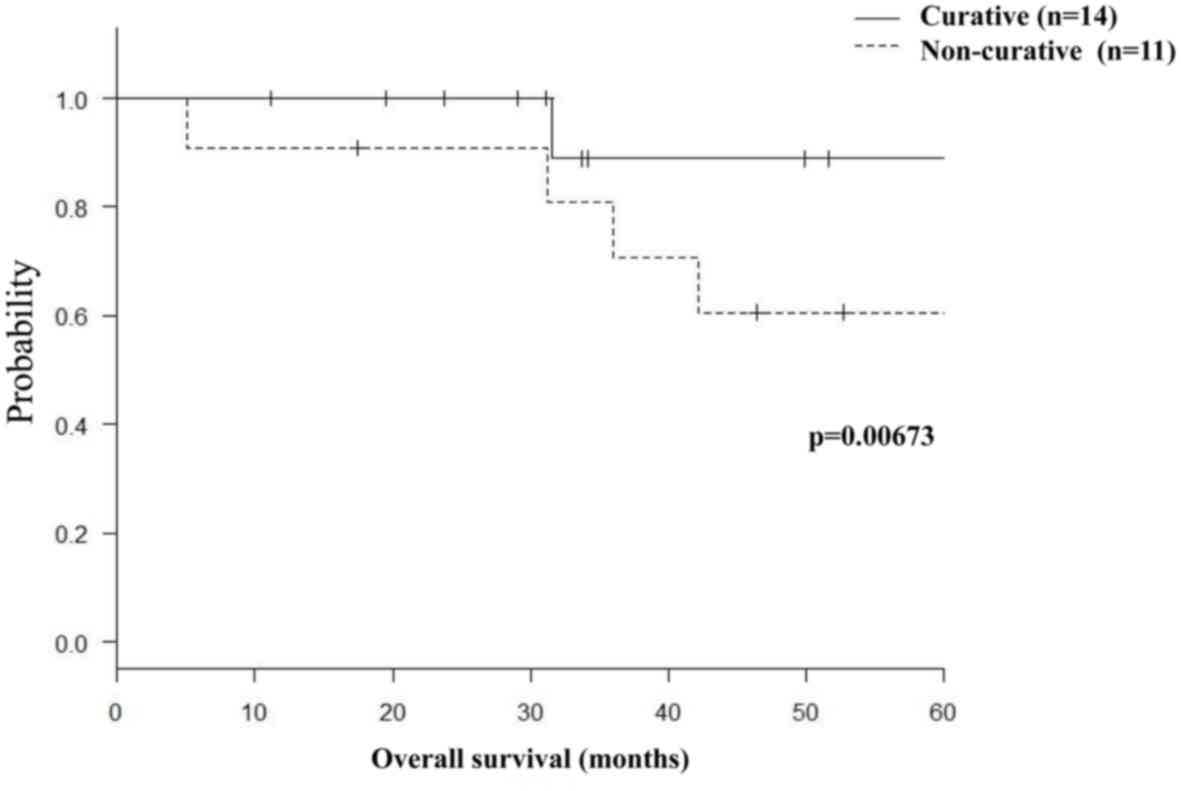
2). Moreover, 14 patients who underwent curative lung biopsy
had significantly higher survival rates compared with 11 patients
who underwent non-curative biopsy (3-year survival rates, 89 vs.
81%, respectively; P=0.00673; Fig.
3).
Discordance in subtype between the
primary tumor and metastases
A total of 17 patients with metastases maintained
the same tumor phenotype as the primary tumor, whereas discordance
of the ER, PR, HER2, or Ki-67 expression status was observed
between the primary and metastatic tumors in 6 patients (24%). A
total of 3 patients with ER or PR upregulation received endocrine
therapy instead of chemotherapy following identification of
discordance (Table IV).
 | Table IV.Discordance in subtype between the
primary tumor and metastases. |
Table IV.
Discordance in subtype between the
primary tumor and metastases.
| Variables |
| n=25 (%) | Patient status
(n) |
|---|
| Concordant
phenotype |
| 17 (68) | Alive (7) |
|
|
|
| Deceased (10) |
| Discordant
phenotype | Change of HR, HER2
and Ki-67 | 6 (24) | Alive (4) |
|
|
|
| Deceased (2) |
| Luminal
A → luminal B | Upregulation of
Ki-67 | 1 (17) | Alive |
| Luminal
HER2 → luminal B | Loss of HER2 | 2 (33) | Deceased |
|
Triple-negative → luminal
B | Upregulation of ER
and/or PR | 3 (50) | Alive |
| N/A phenotype |
| 2 (8) | Alive (1) |
|
|
|
| Deceased (1) |
Discussion
Although lung nodules may develop during the
follow-up period in breast cancer patients, they are not
necessarily metastases from the primary breast cancer but may
correspond to other pathologies, such as primary lung cancer or
benign lesions. The choice of treatment strategy largely depends on
the pathology. In the event of pathologically confirmed lung
metastasis, the phenotype of the tumor may be identified from the
analysis of the specimens, in order to determine whether the
phenotype of the metastatic lesion is in concordance or discordance
with that of the primary lesion. If discordance is detected, the
systemic therapy regimen must be selected accordingly. Thus, based
on the advantages of a definitive pathological diagnosis for
recurrent breast cancer, the international guidelines for breast
cancer recommend a biopsy for recurrent lesions whenever possible
(11).
Previous studies have demonstrated that patients
with breast cancer are at high risk of developing secondary
malignancies (12–15). In the present study, over half of the
lung nodules that developed during the follow-up period after
breast cancer surgery were not lung metastases from breast cancer,
but comprised a variety of histological diagnoses, including 21
primary lung cancers. Jensen et al also reported that the
biopsies from the suspicious metastatic lesions revealed benign
disease or other malignancies in 14% of the patients (16), whereas Tanaka et al (17) demonstrated that 75% of the 52
patients who underwent surgery for lung nodules that developed
during the follow-up period after breast cancer surgery had
pathologically confirmed metastases from the primary breast cancer.
In the present study, of the 53 patients with lung nodules, 47% of
those who underwent lung biopsy had metastasis from the primary
breast cancer. However, this proportion was lower compared with
that reported in previous studies (16,17).
This may be attributed to the fact that the patients included in
our cohort were highly selected, i.e., the indications for lung
biopsy were restricted to only lung nodules with difficult clinical
diagnoses. When the lung nodules were proven to be metastatic based
on the clinical course and imaging findings, it was decided that
there was no indication for lung biopsy, although this policy was
not in accordance with the recommendations of the international
guidelines published in 2015 (11).
Rena et al (18) demonstrated that there were no
statistically significant differences between the radiological
characteristics of lung nodules and the pathological profiles of
the metastases from primary breast cancer, primary lung cancer, or
benign tumors. The present study demonstrated that the patients in
the metastasis group were younger compared with those in the others
group, and the clinicopathological characteristics of the primary
cancer in the metastasis group indicated more advanced disease
compared with the others group. However, a definitive diagnosis of
the lung nodules that develop during the follow-up period as
metastases from the primary breast cancer is difficult to make
based on the clinicopathological characteristics of the primary
cancer alone.
The lack of evidence regarding any survival benefits
conferred by surgical treatment of breast cancer metastases to the
lung renders the procedure controversial. Fan et al
(19) demonstrated in a
meta-analysis that the pooled 5-year survival rate after lung
metastasectomy in breast cancer patients was 46%. They also
demonstrated the prognostic value of complete resection of
metastases and solitary lung metastasis. The present study
investigated the outcomes in patients with solitary metastases when
surgical resection was performed as a total biopsy of the lung
nodule. The survival benefits of metastasectomy should be further
discussed, as there are no long-term data available and the number
of cases in the present retrospective study was limited, with
variable backgrounds.
Changes in the HER2 and hormone receptor status of
metastatic foci from primary cancer may lead to the modification of
treatment strategies based on the indications for HER2-targeted or
endocrine therapies. Previous studies reported discordance rates of
10–40% for the hormone receptor status and 5–20% for the HER2
status (20–22). In the present study, the discordance
rates for hormone receptor and HER2 status were 12 and 8%,
respectively. Welter et al (23) demonstrated that the number of
metastases, tumor stage at initial presentation, complete
resection, and pleural/chest wall involvement were not prognostic
factors of survival. Instead, ER expression predicted prolonged
survival, with a 5-year survival rate of 76% for ER-positive and
12% for ER-negative tumors, whereas similar statistically
significant differences were identified according to the HER2
expression status (23). The
appropriate introduction of endocrine therapies may contribute to
longer survival in the future.
Patients with other lung pathologies identified by
biopsy may also benefit from lung biopsy. The majority of patients
with primary malignant lung tumors confirmed by biopsy had
early-stage disease and were able to undergo curative resection.
This allows the circumvention of unnecessary systemic therapies for
metastatic breast cancer. Patients with benign pathologies
confirmed by biopsy may also benefit from lung biopsy.
In conclusion, the clinical diagnosis of lung
nodules that developed during follow-up after curative breast
surgery remains difficult, despite the current developments in
imaging techniques. Although the clinicopathological profiles of
primary breast cancers provide useful information regarding the
differential diagnosis of lung nodules, lung biopsy is key to
reaching a definitive diagnosis and designing the subsequent
treatment strategy. Moreover, it may improve the prognosis when
metastatic lung nodules from primary breast cancer are resected
with curative intent.
References
|
1
|
Sørlie T, Perou CM, Tibshirani R, Aas T,
Geisler S, Johnsen H, Hastie T, Eisen MB, van de Rijn M, Jeffrey
SS, et al: Gene expression patterns of breast carcinomas
distinguish tumor subclasses with clinical implications. Proc Natl
Acad Sci USA. 98:pp. 10869–10874. 2001; View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Perou CM, Sørlie T, Eisen MB, van de Rijn
M, Jeffrey SS, Rees CA, Pollack JR, Ross DT, Johnsen H, Akslen LA,
et al: Molecular portraits of human breast tumours. Nature.
406:747–752. 2000. View
Article : Google Scholar : PubMed/NCBI
|
|
3
|
Cheang MC, Chia SK, Voduc D, Gao D, Leung
S, Snider J, Watson M, Davies S, Bernard PS, Parker JS, et al: Ki67
index, HER2 status and prognosis of patients with luminal B breast
cancer. J Natl Cancer Inst. 101:736–750. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Prat A, Cheang MC, Martín M, Parker JS,
Carrasco E, Caballero R, Tyldesley S, Gelmon K, Bernard PS, Nielsen
TO and Perou CM: Prognostic significance of progesterone
receptor-positive tumor cells within immunohistochemically defined
luminal A breast cancer. J Clin Oncol. 31:203–209. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Coates AS, Winer EP, Goldhirsch A, Gelber
RD, Gnant M, Piccart-Gebhart M, Thürlimann B and Senn HJ: Panel
Members: Tailoring therapies-improving the management of early
breast cancer: St Gallen International Expert Consensus on the
Primary Therapy of Early Breast Cancer 2015. Ann Oncol.
25:1533–1546. 2015. View Article : Google Scholar
|
|
6
|
Rashid OM and Takabe K: The evolution of
the role of surgery in the management of breast cancer lung
metastasis. J Thorac Dis. 4:420–424. 2012.PubMed/NCBI
|
|
7
|
Siegel RL, Miller KD and Jemal A: Cancer
statistics, 2015. CA Cancer J Clin. 65:5–29. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Society TJBC, . General rules for clinical
and pathological recording of breast cancer. 2012.
|
|
9
|
Ohara M, Akimoto E, Noma M, Matsuura K,
Doi M, Kagawa N and Itamoto T: Prognostic impact of progesterone
receptor status combined with body mass index in breast cancer
patients treated with adjuvant aromatase inhibitor. Oncol Lett.
10:3286–3292. 2015.PubMed/NCBI
|
|
10
|
Kanda Y: Investigation of the freely
available easy-to-use software ‘EZR’ for medical statistics. Bone
Marrow Transplant. 48:452–458. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Gradishar WJ, Anderson BO, Balassanian R,
Blair SL, Burstein HJ, Cyr A, Elias AD, Farrar WB, Forero A,
Giordano SH, et al: Breast Cancer Version 2.2015. J Natl Compr Canc
Netw. 13:448–475. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Brown LM, Chen BE, Pfeiffer RM, Schairer
C, Hall P, Storm H, Pukkala E, Langmark F, Kaijser M, Andersson M,
et al: Risk of second non-hematological malignancies among 376,825
breast cancer survivors. Breast Cancer Res Treat. 106:439–451.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Kirova YM, De Rycke Y, Gambotti L, Pierga
JY, Asselain B and Fourquet A; Institut Curie Breast Cancer Study
Group, : Second malignancies after breast cancer: The impact of
different treatment modalities. Br J Cancer. 98:870–874. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Shilkrut M, Belkacemi Y and Kuten A;
Association of Radiotherapy and Oncology of the Mediterranean arEa
(AROME), : Secondary malignancies in survivors of breast cancer:
How to overcome the risk. Crit Rev Oncol Hematol. 84 Suppl
1:e86–e89. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Vidal-Millan S, Zeichner-Gancz I,
Flores-Estrada D, Vela-Rodríguez BE, Vazquez-López MI, Robles-Vidal
CD, Ramirez-Ugalde MT and Chávez-MacGregor M: A descriptive study
of second primary malignancies associated to breast cancer in a
mexican Hispanic population. Med Oncol. 22:17–22. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Jensen JD, Knoop A, Ewertz M and Laenkholm
AV: ER, HER2, and TOP2A expression in primary tumor, synchronous
axillary nodes and asynchronous metastases in breast cancer. Breast
Cancer Res Treat. 132:511–521. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Tanaka F, Li M, Hanaoka N, Bando T, Fukuse
T, Hasegawa S and Wada H: Surgery for pulmonary nodules in breast
cancer patients. Ann Thorac Surg. 79:1711–1715. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Rena O, Papalia E, Ruffini E, Filosso PL,
Oliaro A, Maggi G and Casadio C: The role of surgery in the
management of solitary pulmonary nodule in breast cancer patients.
Eur J Surg Oncol. 33:546–550. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Fan J, Chen D, Du H, Shen C and Che G:
Prognostic factors for resection of isolated pulmonary metastases
in breast cancer patients: A systematic review and meta-analysis. J
Thorac Dis. 7:1441–1451. 2015.PubMed/NCBI
|
|
20
|
Dieci MV, Barbieri E, Piacentini F,
Ficarra G, Bettelli S, Dominici M, Conte PF and Guarneri V:
Discordance in receptor status between primary and recurrent breast
cancer has a prognostic impact: A single-institution analysis. Ann
Oncol. 24:101–108. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Amir E, Miller N, Geddie W, Freedman O,
Kassam F, Simmons C, Oldfield M, Dranitsaris G, Tomlinson G,
Laupacis A, et al: Prospective study evaluating the impact of
tissue confirmation of metastatic disease in patients with breast
cancer. J Clin Oncol. 30:587–592. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Thompson AM, Jordan LB, Quinlan P,
Anderson E, Skene A, Dewar JA and Purdie CA; Breast Recurrence in
Tissues Study Group, : Prospective comparison of switches in
biomarker status between primary and recurrent breast cancer: The
breast recurrence in tissues study (BRITS). Breast Cancer Res.
12:R922010. View
Article : Google Scholar : PubMed/NCBI
|
|
23
|
Welter S, Jacobs J, Krbek T, Tötsch M and
Stamatis G: Pulmonary metastases of breast cancer. When is
resection indicated? Eur J Cardiothorac Surg. 34:1228–1234. 2008.
View Article : Google Scholar : PubMed/NCBI
|