Introduction
Three-dimensional (3D) cell culture models are
considered an important tool in cancer research (1–3) and
anticancer drug development (2).
Owing to intercellular communication and cell-matrix interactions
(3–5), multicellular spheroids (MCS) reproduce
the in vivo situation more effectively than two-dimensional
(2D) monolayer cell culture systems. MCS can be used to evaluate
cell proliferation, apoptosis, differentiation, gene expression and
metabolism, and the tumour response to radiochemotherapy (4). Moreover, MCS can imitate the biological
properties of tumour invasion and micrometastasis (4,6). There
are several known techniques for spheroid generation, e.g. hanging
drop (7,8). The liquid overlay technique has been
described as the most suitable technique for reproducible spheroid
preparation (3). On a non-adhesive
surface, cells adhere to each other and start forming MCS (5,9,10). The generation of MCS has been
described in few moderately- and poorly-differentiated oral
squamous cell carcinoma (OSCC) cell lines (11). Although studying the complex tumour
characteristics from all the different degrees of malignancy is
vital, no previous study demonstrating the MCS formation in a
highly differentiated OSCC cell line has been conducted. In the
present investigation, using the liquid overlay technique, we
generate MCS from highly differentiated OSCC cell line BHY for the
first time.
Materials and methods
Cell culture
Highly differentiated OSCC cell line BHY was
obtained from the German Collection of Cell Cultures and
Microorganisms (DSZM, Braunschweig, Germany). Cell line was
cultivated in 90% Dulbecco's MEM (4.5 g/l glucose) with 10% h.i.
FBS and 1% Penicillin/Streptomycin in a humidified incubator at
37°C with 5% CO2.
Tumour doubling time
For calculation of the tumour doubling time, 1,1
million cells were seeded in a 25 cm2 cell culture flask
and cell density was counted after 48 and 72 h, respectively.
Spheroid preparation
A total of 50 µl of the agarose solution (0.15 g of
agarose mixed with 10 ml of DMEM) were added to each well of a
96-well microtiter plate, and was let to cool down for 30 min. BHY
cell suspension was prepared using a trypsin/EDTA solution. Cells
were counted in a Neubauer counting chamber.
In independent experiments 5,000, 10,000 and 15,000
cells per well were used. Since the use of 10,000 and 15,000 cells
did not result in the formation of single compact spheroids, only
the spheroids with 5,000 initial cells were used for the further
studies.
A total of 480,000 cells in 19.2 ml DMEM were used
for spheroid initiation. 200 µl of cell suspension was transferred
to each well of the agarose-coated 96-well microtiter plate (5,000
cells per well). Since no further increase in sizes could be
observed, BHY MCS were harvested after 3 days of incubation, using
a pasteur pipette. MCS and its supernatant were transferred from
each well into a 50-ml falcon tube, where MCS sedimented. DMEM
supernatant was removed, and 20 ml of formalin was added for 5 h of
fixation. Sediment was transferred into a 0.5 ml e-cup, and
embedded with 3% agarose solution. The resulting agarose block was
used for paraffin embedding, using the automated embedding
workstation Excelsior ES (Thermo Fisher Scientific, Waltham,
Massachusetts, USA). All measurements were performed in three
independent experiments. Over all, a total of 32 BHY spheroids were
used for further evaluation. Two independent investigators
evaluated all tissue sections by using a Zeiss Axioskop 2 plus
light microscope (Zeiss, Goettingen, Germany).
Evaluation of spheroid size
MCS size was analysed after 3 days on 2 µm tissue
sections. The diameter of each spheroid was measured by using the
AxioVision software (version 4.8; Zeiss, Goettingen, Germany). By
assuming a spherical shape, the volume of BHY MCS was calculated
using the following equation: V=4/3 x π x
(d/2)3.
Results
The analysis of the tumour doubling time of
monolayer cell culture revealed a doubling time of ~72 h.
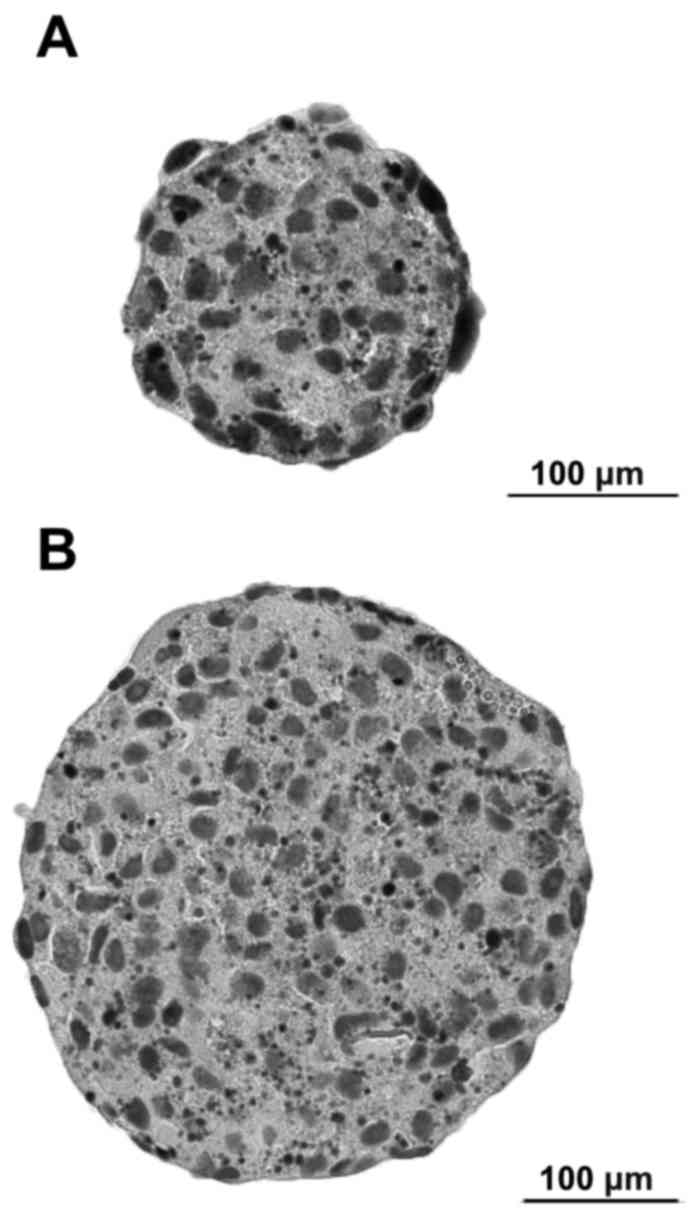
Highly differentiated OSCC cell line BHY formed
different sized compact MCS within 24 h (Fig. 1). After 48 h, spheroids showed a
compact body. After 72 h, no further increase in size could be
observed. Spheroid compactness decreased with increasing incubation
time over three days.
Diameter of the analysed spheroids ranged from 46,76
µm to 233,26 µm [mean 123.99 µm ±59.44 standard deviation (SD)].
Volume ranged from 5,35x104 µm3 to
6,65x106 µm3 [mean
1.74x106 µm3 ± 2.20 SD] (Table I).
 | Table I.Size of each spheroids. |
Table I.
Size of each spheroids.
| Spheroid (N) | Diameter [µm] | Volume
(µm3) |
|---|
| 1 | 115,96 |
8,16×105 |
| 2 |
66,76 |
1,56×105 |
| 3 | 122,53 |
9,63×105 |
| 4 | 233,26 |
6,65×106 |
| 5 | 112,72 |
7,50×105 |
| 6 | 101,81 |
5,53×105 |
| 7 | 208,53 |
4,75×106 |
| 8 |
72,74 |
2,02×105 |
| 9 |
46,76 |
5,35×104 |
| 10 |
97,69 |
4,88×105 |
| 11 | 117,79 |
8,56×105 |
| 12 |
69,10 |
1,73×105 |
| 13 | 129,48 |
1,14×106 |
| 14 |
78,87 |
2,57×105 |
| 15 | 128,61 |
1,11×106 |
| 16 | 227,22 |
6,14×106 |
| 17 | 105,55 |
6,16×105 |
| 18 |
96,42 |
4,69×105 |
| 19 | 217,79 |
5,41×106 |
| 20 |
73,10 |
2,05×105 |
| 21 |
94,40 |
4,40×105 |
| 22 |
55,79 |
9,09×104 |
| 23 | 134,75 |
1,28×106 |
| 24 |
57,11 |
9,75×104 |
| 25 |
66,28 |
1,52×105 |
| 26 |
73,17 |
2,05×105 |
| 27 | 210,22 |
4,86×106 |
| 28 | 211,22 |
4,93×106 |
| 29 |
95,29 |
4,53×105 |
| 30 | 221,85 |
5,72×106 |
| 31 | 209,72 |
4,83×106 |
| 32 | 115,26 |
8,02×105 |
Discussion
In the present investigation, for the first time, we
generated reproducible MCS from highly differentiated OSCC cell
line BHY by using the liquid overlay technique.
The advantage of the used method lies in its easy
practicability compared to other protocols described in the
literature that use the Matrigel, Hanging Drops, or doing Ultra-Low
Attachment Assays (2,11,12).
Lee and colleagues compared the spheroid formation
of four oral cancer cell lines (SCC9, SCC9β6, SCC9β6KDFyn, and
SCC9β6D1) using agarose-coated tissue, and let a 0.6% agarose
solution dry overnight at room temperature (11). The SCC9β6D1 cell line formed
diminutive clusters containing 2–4 cells. In contrast, the
SCC9β6KDFyn cell line generated large oversized spheres. The
aforementioned method to generate spheroids seems to be very
sensitive. In our study, the agarose solution was left to cool down
at room temperature for only 30 min. An over-dried agarose solution
might have a significant influence on spheroid stability.
The present results are contrary to those described
by Adcock and colleagues, who described an increased average
spheroid diameter with an increasing initial cell number per well
plate, using the CAL-27 cell line from the tongue squamous cell
carcinoma (2). While 5,000 BHY
cells, resulted in one compact spheroid, adding 10,000 or 15,000
cells to each well plate led to the formation of small aggregates
of cells, instead of one compact spheroid in our investigation.
This study shows that it is possible to rapidly
generate spheroids with homogeneous size distribution, which is a
prerequisite for drug screening (1,10).
Within 3 days, BHY MCS grew to 46,76–233,26 µm in diameter. This is
comparable to the results presented by Adcock et al, where
CAL-27 MCS grew to 50–100 µm in diameter within 3 to 4 days
(2). Hagemann et al also
showed similar results by generating CAL-27 MCS that reached a
diameter of 1–3 mm within 5 days (12).
The harvesting procedure of MCS was a critical step
in the present protocol. Contrary to the method used by Gebhard
et al, who harvested MCS using a plastic pipette of 2 mm tip
diameter and centrifuged them to cell pellets (13), in the present investigation MCS were
carefully harvested using a pasteur pipette and were not
centrifuged. The use of a plastic pipette and centrifugation
disrupted BHY MCS structure in the present investigation.
BHY is a slow-growing (doubling time of ~72 h) cell
line, established from the tumour of the lower alveolus of a
Japanese male, which was highly invasive towards the mandible and
the oral floor (14). It has a
stable growth on agarose, which means a reliable measure of the
metastatic capability of the cancer cells (15). In the present study, BHY spheroids
did not follow the typical growth patterns, with proliferating
cells next to the oxygen supply and nutrients, and quiescent and
necrotic cells farthest away from the capillaries, as described
before (9).
However, a better understanding of the molecular
mechanisms underlying tumour resistance to apoptotic cell death is
expected to provide the basis for a rational approach to develop
molecular targeted therapies (16).
Although BHY is a slow growing, difficult to
cultivate highly differentiated OSCC cell line, it forms
reproducible compact spheroids on an agarose surface. The
generation of MCS using the liquid overlay technique is a simple
method that can be used for further cancer research and anticancer
drug development.
References
|
1
|
Friedrich J, Seidel C, Ebner R and
Kunz-Schughart LA: Spheroid-based drug screen: Considerations and
practical approach. Nat Protoc. 4:309–324. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Adcock AF, Trivedi G, Edmondson R,
Spearman C and Yang L: Three-dimensional (3D) cell cultures in
cell-based assays for in vitro evaluation of anticancer drugs. J
Anal Bioanal Tech. 6:2472015. View Article : Google Scholar
|
|
3
|
Metzger W, Sossong D, Bächle A, Pütz N,
Wennemuth G, Pohlemann T and Oberringer M: The liquid overlay
technique is the key to formation of co-culture spheroids
consisting of primary osteoblasts, fibroblasts and endothelial
cells. Cytotherapy. 13:1000–1012. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Ivascu A and Kubbies M: Rapid generation
of single-tumor spheroids for high-throughput cell function and
toxicity analysis. J Biomol Screen. 11:922–932. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Santini MT and Rainaldi G:
Three-dimensional spheroid model in tumor biology. Pathobiology.
67:148–157. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Mueller-Klieser W: Tumor biology and
experimental therapeutics. Crit Rev Oncol Hematol. 36:123–139.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Timmins NE, Dietmair S and Nielsen LK:
Hanging-drop multicellular spheroids as a model of tumour
angiogenesis. Angiogenesis. 7:97–103. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Timmins NE and Nielsen LK: Generation of
multicellular tumor spheroids by the hanging-drop method. Methods
Mol Med. 140:141–151. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Yuhas JM, Li AP, Martinez AO and Ladman
AJ: A simplified method for production and growth of multicellular
tumor spheroids. Cancer Res. 37:3639–3643. 1977.PubMed/NCBI
|
|
10
|
Kelm JM, Timmins NE, Brown CJ, Fussenegger
M and Nielsen LK: Method for generation of homogeneous
multicellular tumor spheroids applicable to a wide variety of cell
types. Biotechnol Bioeng. 83:173–180. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Lee C, Lee C, Atakilit A, Siu A and Ramos
DM: Differential spheroid formation by oral cancer cells.
Anticancer Res. 34:6945–6949. 2014.PubMed/NCBI
|
|
12
|
Hagemann J, Jacobi C, Hahn M, Schmid V,
Welz C, Schwenk-Zieger S, Stauber R, Baumeister P and Becker S:
Spheroid-based 3D cell cultures enable personalized therapy testing
and drug discovery in head and neck cancer. Anticancer Res.
37:2201–2210. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Gebhard C, Gabriel C and Walter I:
Morphological and immunohistochemical characterization of canine
osteosarcoma spheroid cell cultures. Anat Histol Embryol.
45:219–230. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Kawamata H, Nakashiro K, Uchida D, Harada
K, Yoshida H and Sato M: Possible contribution of active MMP2 to
lymph-node metastasis and secreted cathepsin L to bone invasion of
newly established human oral-squamous-cancer cell lines. Int J
Cancer. 70:120–127. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Lin CJ, Grandis JR, Carey TE, Gollin SM,
Whiteside TL, Koch WM, Ferris RL and Lai SY: Head and neck squamous
cell carcinoma cell lines: Established models and rationale for
selection. Head Neck. 29:163–188. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Fulda S: Tumor resistance to apoptosis.
Int J Cancer. 124:511–515. 2009. View Article : Google Scholar : PubMed/NCBI
|