Introduction
Endoscopic biliary stenting is an established
technique used to treat obstructive jaundice and cholangitis.
Self-expandable metallic stents (SEMS) are now used more often than
plastic stents (PS) for palliative drainage for unresectable distal
malignant biliary strictures because of their longer duration of
stent patency (1–5). Isayama et al compared covered
SEMS (CSEMS) and uncovered SEMS (USEMS) in a randomized study and
reported that CSEMS had a longer duration of patency because they
prevented tumor ingrowth (6). The
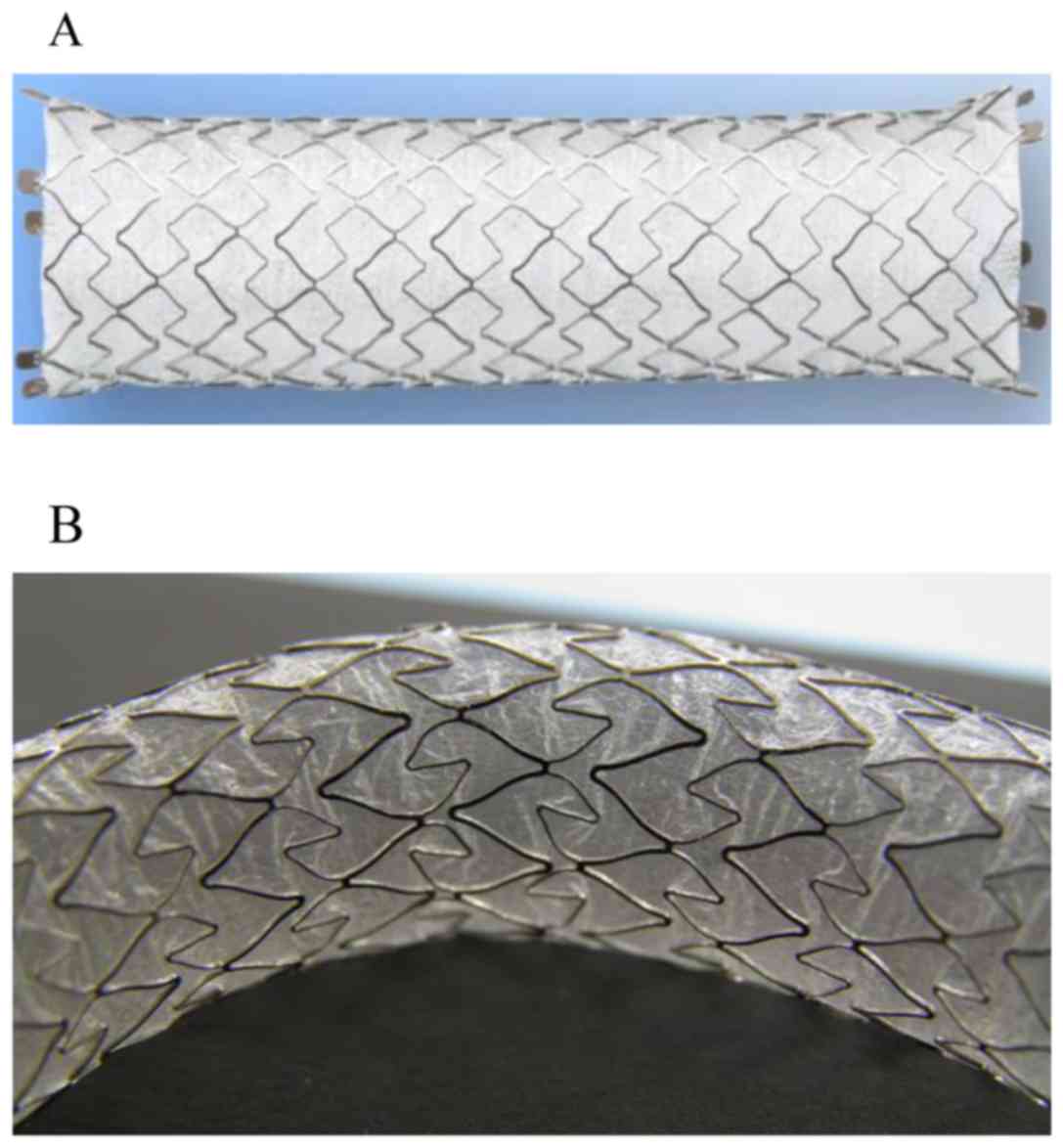
X-Suit NIR® Covered Biliary Metallic Stent (Olympus
Medical Systems, Tokyo, Japan) is a laser-cut CSEMS used as the
first-choice device for treating unresectable distal malignant
biliary strictures at the Department of Gastroenterology, Saitama
Medical University International Medical Center because it allows
easy positioning due to its characteristic of having minimal stent
shortening (Fig. 1).
After SEMS placement, the presence of recurrent
biliary obstruction (RBO) can affect the duration of stent patency.
Considering recent advancements in chemotherapy, re-intervention
for RBO will be crucial. Different treatment approaches are used
depending on the cause of RBO. Replacing the old SEMS with a new
SEMS is the most common re-intervention approach for RBO used in
recent studies (7–10). However, the CSEMS removal reported to
date involved only braided stents. Endoscopic removal of laser-cut
CSEMS has not been reported. Thus, we herein report the efficacy
and safety of endoscopically removing laser-cut CSEMS.
Case report
Patients
The X-Suit NIR® Covered Biliary Metallic
Stents, which are a type of laser-cut CSEMSs, were placed in 64
consecutive patients with unresectable distal malignant biliary
strictures at Saitama Medical University International Medical
Center between October 2014 and December 2016. We performed
endoscopic sphincterotomy (EST) before stent placement and placed
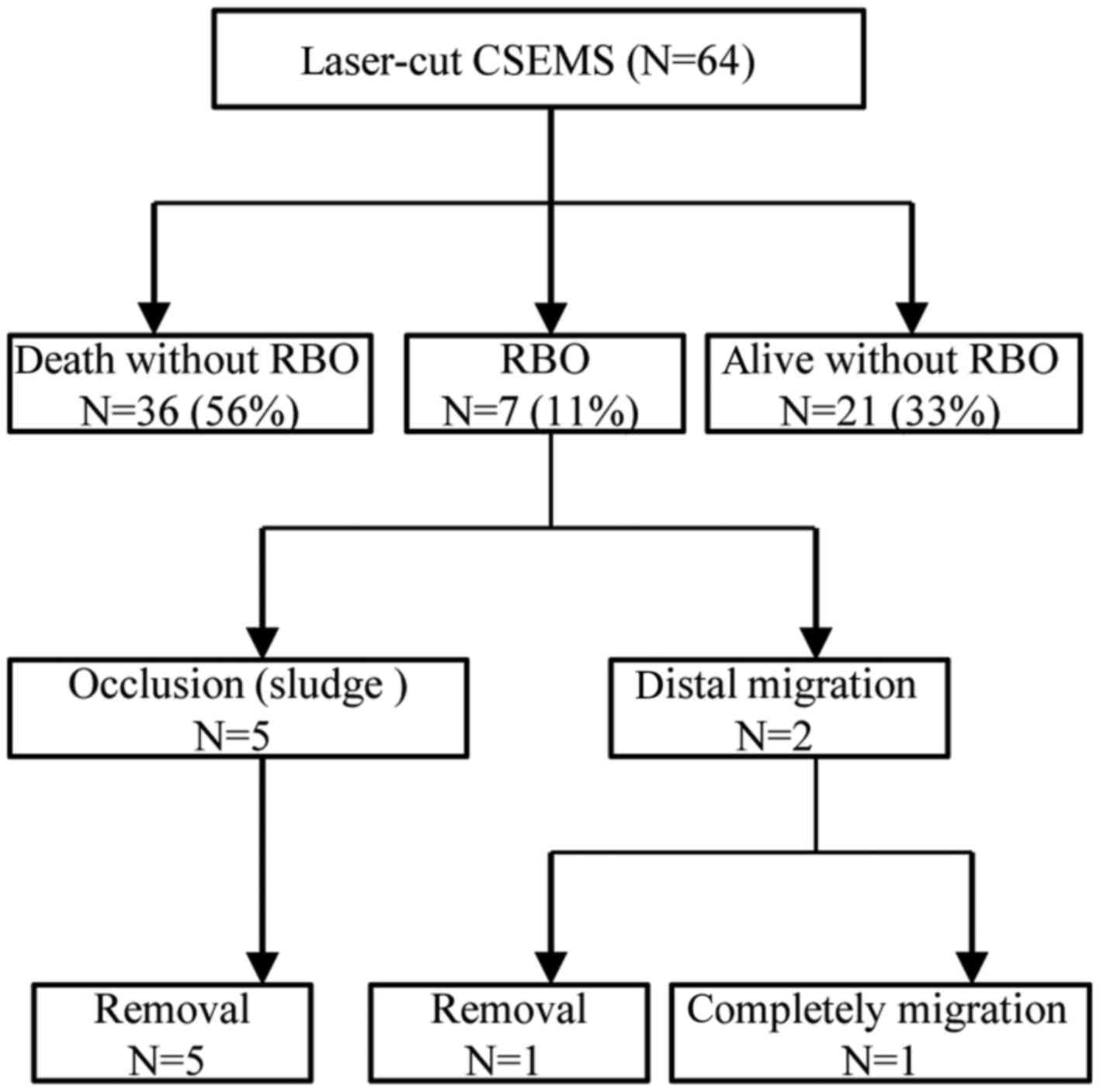
laser-cut CSEMSs across the papilla. RBO was reported in 7 patients
(10.9%) after stenting. With the exception of the 1 patient with
complete stent migration. Complete migration refers to CSEMS in the
gastrointestinal tract or outside the body.
Stent removal
Our indications for CSEMS removal are as follow: i)
Stent occlusion because of overgrowth or sludge within the stent;
ii) distal migration with impaction against the duodenal wall
opposite the papilla. Distal migration has the risk of not only
associated occlusion but also formation of erosions and ulcers from
mechanical irritation, and serious gastrointestinal tract
perforation.
Patients with poor general health conditions or
terminal malignancy who are expected to survive for >2 months
are not indicated to undergo stent removal. They are appropriate
for stent cleaning or plastic stent insertion.
A therapeutic duodenoscope (TJF-260 V and JF-260V,
Olympus Medical Systems, Tokyo, Japan) was used for endoscopic
stent removal. Snare forceps (SD-5L-1) or V-shaped grasping forceps
(FG-44NR-1) (Olympus Medical Systems) were used to hold the stent.
The stent held with the forceps was gradually pulled towards the
papilla by repeated pushing and clockwise torsion of the endoscope.
The proximal end of the visible part of stent was held again, and
the entire stent was eventually pulled out into the duodenum.
Subsequently, the stent and the endoscope were carefully removed
together from the patient's body while under endoscopy and
fluoroscopy guidance to avoid damage to the surrounding organs. All
patients received information on the procedure and provided consent
in advance to receive the treatment. This study was conducted in
compliance with the Declaration of Helsinki and the Ethical
Guidelines for Medical and Health Research Involving Human
Subjects. This study was approved by the Institutional Review Board
of Saitama Medical University International Medical Center
(approval no. 16–293).
The duration of stent placement (from placement to
endoscopic removal), procedural success rate, procedural duration,
and the occurrence of accidental complications were evaluated.
Results
Endoscopic stent removal was performed in 6 patients
with RBO. The male-to-female ratio was 2:4. The median age was 73.5
years (range, 39–83 years). The underlying disorder was pancreatic
cancer in 3 patients and distal biliary cancer in 3 patients. The
cause of RBO was stent occlusion with sludge in 5 patients and
partial distal stent migration in 1 patient. Two patients received
chemotherapy after the initial stenting (Table I).
 | Table I.Characteristics of patients who needed
stent removal. |
Table I.
Characteristics of patients who needed
stent removal.
| Characteristic | Laser-cut CSEMS |
|---|
| Number of
patients | 6 |
| Age, years |
|
| Median
(range) | 73.5 (39–83) |
| Sex |
|
|
Male/female | 2 (33.3%)/4
(66.7%) |
| Primary disease |
|
|
Pancreatic cancer | 3 (50%) |
| Bile duct
cancer | 3 (50%) |
| Clinical stage
(UICC) |
|
| Stage
III | 2 (33.3%) |
| Stage
IV | 4 (66.6%) |
| Chemotherapy | 2 (33.3%) |
| Recurrent biliary
obstruction |
|
| Occlusion
(Sludge) | 5 (83.3%) |
| Distal
migration | 1 (16.7%) |
The mean duration of stent placement (from placement
to endoscopic removal) was 156±37.9 days (range, 117–205 days) in
the patients who underwent stent removal. The procedural success
rate was 100% (6/6). The mean procedural duration was 11.8 min
(range, 5–24 min). Snare forceps were used in 5 patients while both
snare forceps and grasping forceps were used in 1 patient for stent
removal. The procedure was lengthy because the stent was carefully
removed to avoid stent fracture. No complications, such as
gastrointestinal tract damage, occurred during stent removal. A
SEMS was placed in all patients after the removal of the old stent
(Table II). The outcomes of the 64
patients who underwent X-Suit NIR® Covered Biliary
Metallic Stent placement are shown (Fig.
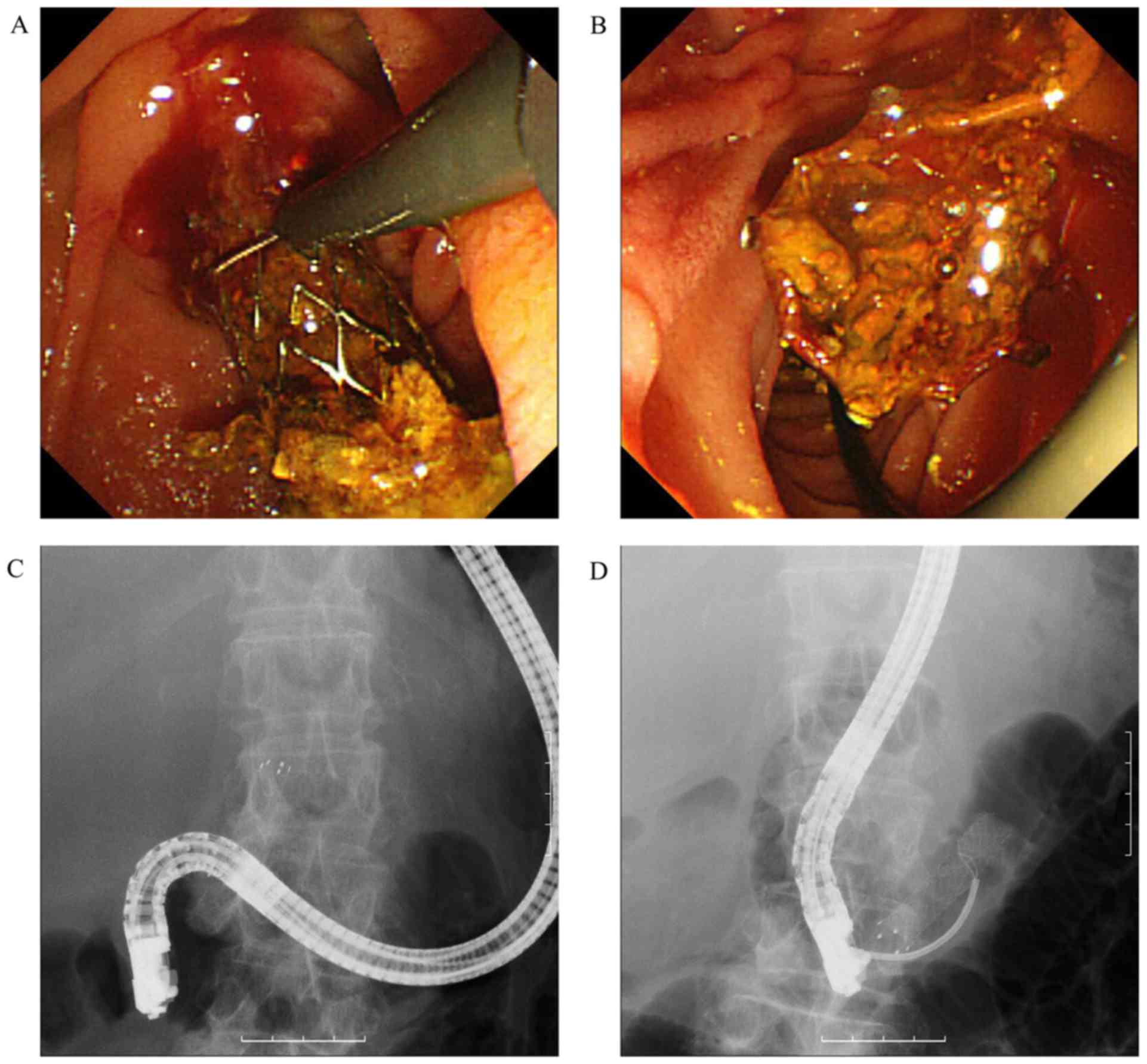
2). We show a representative case (Case 5) of a patient who
underwent safe occluded stent removal with careful endoscopic
procedures using snare forceps. This patient had a new CSEMS placed
following stent occlusion with sludge that occurred at 201 days
after the initial stenting as shown in (Fig. 3).
 | Table II.Outcome of stent removal. |
Table II.
Outcome of stent removal.
| Case | Disease | Patency (d) | Procedure time
(min) | Outcome | Additional
procedure |
|---|
| Occlusion
(Sludge) |
|
|
|
|
|
| 1 | Bile duct ca. | 117 | 18 | Success | New SEMS |
| 2 | Bile duct ca. | 150 | 24 | Success | New SEMS |
| 3 | Pancreatic ca. | 129 | 5 | Success | New SEMS |
| 4 | Bile duct ca. | 205 | 6 | Success | New SEMS |
| 5 | Pancreatic ca. | 201 | 10 | Success | New SEMS |
| Distal migration |
|
|
|
|
|
| 6 | Pancreatic ca. | 134 | 8 | Success | New SEMS |
Discussion
With the recent advances in chemotherapy, patients
may survive for a long time after SEMS placement. Therefore, the
importance of re-intervention for RBO is increasing. Tumor
ingrowth/mucosal hyperplasia, tumor overgrowth, sludge with/without
stones, hemobilia, food impaction, and bile duct kinking as well as
partial migration of the SEMS are listed as possible causes of RBO
in the 2014 Tokyo criteria (11).
The usefulness of CSEMS for preventing ingrowth in distal malignant
biliary strictures has also been reported (6,12).
Braided CSEMSs like the Niti-S stents (TaeWoong Medical Co., Seoul,
Korea) are feasible and effective for maliganct biliary
obstruction. Other investigators have reported that stent removal
is relatively sinple (8,10). However, braided CSEMSs are sometimes
difficult to place in the appropriate position because they have
much shortening function. Thus, the positioning of braided CSEMS
must be decided considering shortening. On the other hand,
laser-cut CSEMSs have limited shortening function. Thus, the
appropriate position can be decided relatively easy.
Re-intervention approaches for RBO include stent
cleaning with a balloon catheter and stent-in-stent placement.
Togawa et al compared the re-intervention approaches used in
74 patients with CSEMS failure and reported short-term stent
patency (<2 months) for changing the old stent to a PS,
stent-in-stent placement of a PS, and stent cleaning. The
researchers recommend changing the old stent to a new CSEMS or
stent-in-stent placement of a CSEMS (13). Bleeding of the orifice of the papilla
can occur at stent removal. Thus, we need to remove the CSEMS
gradually and carefully. When CSEMSs are placed for a long period,
the covering membrane can break, which can causes tumor ingrowth.
As pulling the CSEMS forcibly can damage the bile duct mucosa, the
mobility of the CSEMS should be checked before removal.
According to a recent report, the success rate of
SEMS removal ranges between 0–100% for USEMSs and 77.8–92.9% for
CSEMSs. A new SEMS was placed after old stent removal in 58.8–96.6%
of patients from whom a SEMS was removed (Table III) (7–10).
USEMSs are more difficult to remove because the metallic stent
becomes implanted in the tissue due to ingrowth. To our knowledge,
removal of laser-cut USEMS has failed in all patients reported to
date. Familiari et al explained this finding by the fact
that braided-mesh SEMSs are resistant to longitudinal traction
because of the crisscross mesh structure and are thus easy to
remove, while laser-cut SEMSs which have a zigzag design with no
crossing struts in the mesh are not resistant to longitudinal
traction and easily tear on removal (8).
 | Table III.Review of stent removal. |
Table III.
Review of stent removal.
| Authors | Type of SEMS | Success rate
success/total number (%) | Rate of new SEMS
success/total number (%) | (Refs.) |
|---|
| Kahaleh et
al |
|
|
|
|
|
|
| USEMS | Braided | 4/4 (100) |
| (7) |
|
| CSEMS | Braided | 13/14 (92.9) |
|
|
|
| Total |
| 17/18 (94.4) | 10/17 (58.8) |
|
| Familiari et
al |
|
|
|
|
|
|
| USEMS | Braided | 5/10 (50) |
|
|
|
|
| Laser-cut | 0/3 (0) |
| (8) |
|
| CSEMS | Braided | 24/26 (92.3) |
|
|
|
| Total |
| 29/39 (74.4) | 28/29 (96.6) |
|
| Shin et
al |
|
|
|
|
|
|
| USEMS | Braided | 0/5 (0) |
|
|
|
|
| Laser-cut | 0/3 (0) |
| (9) |
|
| CSEMS | Braided | 19/22 (86.4) |
|
|
|
| Total |
| 19/30 (63.3) | 17/19 (89.5) |
|
| Ishii et
al |
|
|
|
|
|
|
| USEMS | Braided | 0/1 (0) |
| (10) |
|
| CSEMS | Braided | 14/18 (77.8) |
|
|
|
| Total |
| 14/19 (73.7) | 12/14 (85.7) |
|
| Current study | CSEMS | Laser-cut | 6/6 (100) | 6/6 (100) |
|
Removal of laser-cut CSEMS has not been previously
reported. We successfully removed X-Suit NIR® Covered
Biliary Metallic Stents, which are a type of laser-cut CSEMS, under
endoscopic guidance in 6/6 patients. The reason for the successful
removal of laser-cut SEMSs may be that the stents were covered with
a double-layer of silicone and polyurethane, which makes the stent
more resistant to longitudinal traction than laser-cut USEMSs are
and which results in less ingrowth. Braided CSEMSs have a
crisscross mesh structure. Since the entire stent shrinks and
straightens once held by snare forceps, it is easy to pull into the
forceps channel of the endoscope and remove. In contrast, laser-cut
CSEMSs have no crisscross mesh structure and may be torn when
pulled longitudinally. Thus, we must pull laser-cut CSEMSs
gradually and carefully. Furthermore, laser-cut CSEMSs do not
straighten when held by snare forceps. Thus, they can not be pulled
into the working channel of the endoscope. Once the laser-cut CSEMS
is pulled into the duodenum, the stent and the endoscope can
carefully removed together from the patient's body under endoscopy
and fluoroscopy guidance to avoid damage to the surrounding
organs.
In conclusion, the X-Suit NIR® Covered
Biliary Metallic Stents were safely removed from all patients.
Further accumulation of patient data will be necessary since the
follow-up duration after CSEMS placement was relatively short and a
small number of patients were evaluated.
References
|
1
|
Davids PH, Groen AK, Rauws EA, Tytgat GN
and Huibregtse K: Randomised trial of self-expanding metal stents
versus polyethylene stents for distal malignant biliary
obstruction. Lancet. 340:1488–1492. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Prat F, Chapat O, Ducot B, Ponchon T,
Pelletier G, Fritsch J, Choury AD and Buffet C: A randomized trial
of endoscopic drainage methods for inoperable malignant strictures
of the common bile duct. Gastrointest Endosc. 47:1–7. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Kaassis M, Boyer J, Dumas R, Ponchon T,
Coumaros D, Delcenserie R, Canard JM, Fritsch J, Rey JF and Burtin
P: Plastic or metal stents for malignant stricture of the common
bile duct? Results of a randomized prospective study. Gastrointest
Endosc. 57:178–182. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Moss AC, Morris E, Leyden J and MacMathuna
P: Do the benefits of metal stents justify the costs? A systematic
review and meta-analysis of trials comparing endoscopic stents for
malignant biliary obstruction. Eur J Gastroenterol Hepatol.
19:1119–1124. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Isayama H, Yasuda I, Ryozawa S, Maguchi H,
Igarashi Y, Matsuyama Y, Katanuma A, Hasebe O, Irisawa A, Itoi T,
et al: Results of a Japanese multicenter, randomized trial of
endoscopic stenting for non-resectable pancreatic head cancer
(JM-test): Covered wallstent versus doublelayer stent. Dig Endosc.
23:310–315. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Isayama H, Komatsu Y, Tsujino T, Sasahira
N, Hirano K, Toda N, Nakai Y, Yamamoto N, Tada M, Yoshida H, et al:
A prospective randomised study of ‘covered’ versus ‘uncovered’
diamond stents for the management of distal malignant biliary
obstruction. Gut. 53:729–734. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Kahaleh M, Tokar J, Le T and Yeaton P:
Removal of self-expandable metallic Wallstents. Gastrointest
Endosc. 60:640–644. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Familiari P, Bulajic M, Mutignani M, Lee
LS, Spera G, Spada C, Tringali A and Costamagna G: Endoscopic
removal of malfunctioning biliary self-expandable metallic stents.
Gastrointest Endosc. 62:903–910. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Shin HP, Kim MH, Jung SW, Kim JC, Choi EK,
Han J, Lee SS, Seo DW and Lee SK: Endoscopic removal of biliary
self-expandable metallic stents: A prospective study. Endoscopy.
38:1250–1255. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Ishii K, Itoi T, Sofuni A, Itokawa F,
Tsuchiya T, Kurihara T, Tsuji S, Ikeuchi N, Umeda J, Moriyasu F and
Tsuchida A: Endoscopic removal and trimming of distal
self-expandable metallic biliary stents. World J Gastroenterol.
17:2652–2657. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Isayama H, Hamada T, Yasuda I, Itoi T,
Ryozawa S, Nakai Y, Kogure H and Koike K: TOKYO criteria 2014 for
transpapillary biliary stenting. Dig Endosc. 27:259–264. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Kitano M, Yamashita Y, Tanaka K, Konishi
H, Yazumi S, Nakai Y, Nishiyama O, Uehara H, Mitoro A, Sanuki T, et
al: Covered self-expandable metal stents with an anti-migration
system improve patency duration without increased complications
compared with uncovered stents for distal biliary obstruction
caused by pancreatic carcinoma: A randomized multicenter trial. Am
J Gastroenterol. 108:1713–1722. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Togawa O, Isayama H, Tsujino T, Nakai Y,
Kogure H, Hamada T, Sasaki T, Yashima Y, Yagioka H, Arizumi T, et
al: Management of dysfunctional covered self-expandable metallic
stents in patients with malignant distal biliary obstruction. J
Gastroenterol. 48:1300–1307. 2013. View Article : Google Scholar : PubMed/NCBI
|