Introduction
Peritoneal malignant mesothelioma (PMM) is a rare
and aggressive neoplasm that arises from the lining mesothelial
cells of the peritoneum and spreads extensively within the confines
of the abdominal cavity. Even though asbestos is the most important
factor for mesothelioma, recent studies have focused on other
causal factors, including radiation (1). The incidence of secondary malignancies
after radiation therapy is increasing because of the improved
prognosis of cancer survivors owing to the development of
anticancer therapies. Radiation plays a pivotal role in treating
several cancers in organs such as the uterine cervix, testis,
breast, and prostate (2). Therefore,
secondary malignancies after radiation are recently recognized as a
major problem (3). Secondary
cancers, such as bladder, kidney, rectal, uterine corpus, and
ovarian cancer, after radiation therapy for uterine cervical
cancer, are well-known events (4,5). We
herein report our experience with a case of PMM after radiation
therapy for cervical cancer, along with a review of the pertinent
literature.
Case report
A 34-year-old Japanese woman, gravida 2, para 2,
without any history of cancer or asbestos exposure, had visited
another clinic because of atypical genital bleeding for a month;
she was referred to our hospital for evaluation of a uterine
cervical mass. T2-weighted magnetic resonance imaging (MRI)
revealed the presence of a 29×21 mm mass at the uterine cervix. The
tumor was diagnosed as squamous cell carcinoma (SCC),
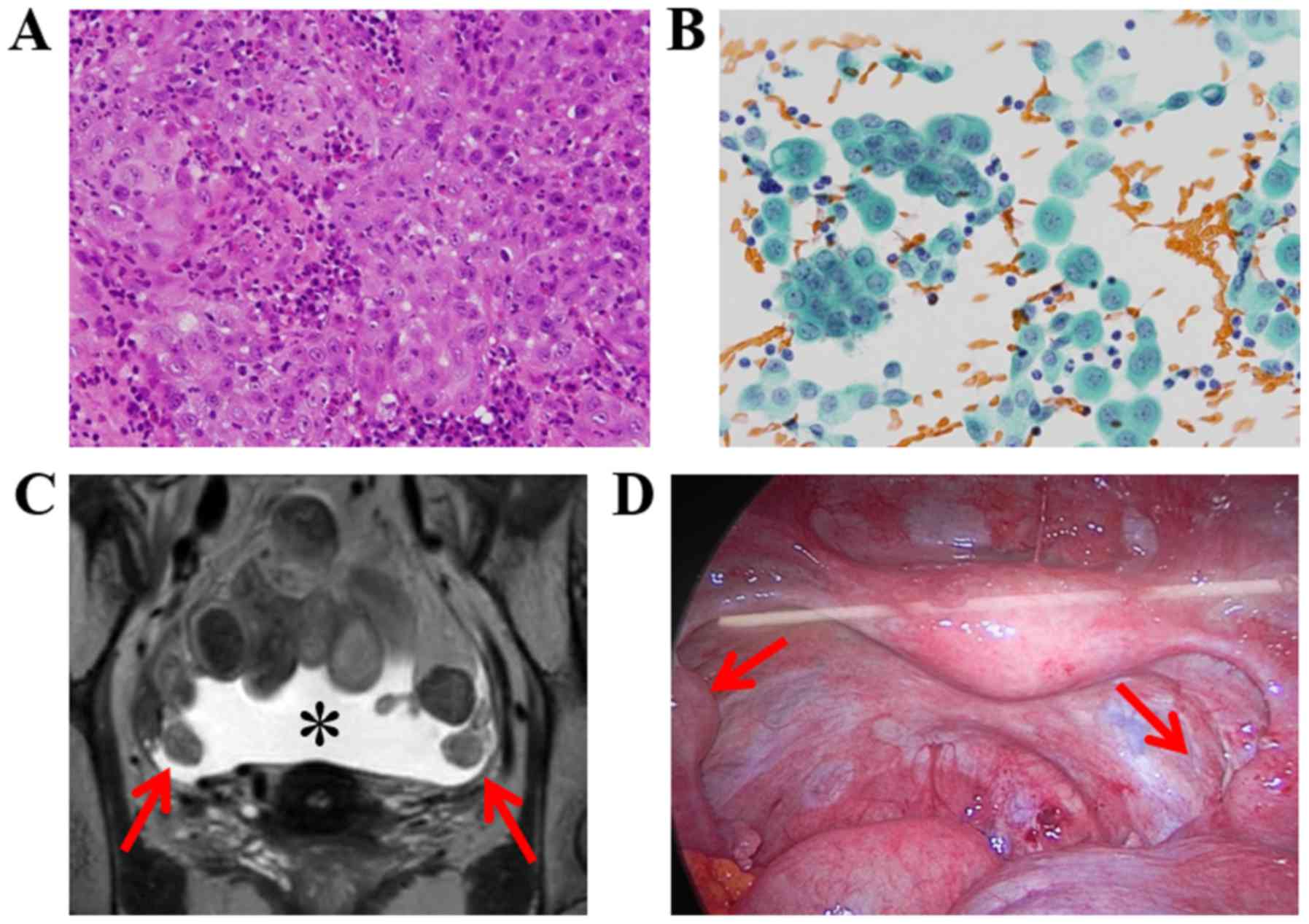
non-keratinizing type, by target biopsy under colposcopy (Fig. 1A). The serum levels of tumor markers
of SCC antigen and cancer antigen 125 (CA125) were 229.0 ng/ml and
54.4 U/ml, respectively. The patient was diagnosed with uterine
cervical cancer, at International Federation of Gynecology and
Obstetrics (FIGO) stage IIB (T2bN0M0). The patient underwent
concurrent chemoradiotherapy (CCRT), with 54 Gray of whole pelvis
radiation and 20 Gray/4 fractions of high-dose-rate intracavitary
brachytherapy combined with weekly cisplatin administration. After
the primary therapy, complete response was achieved and the patient
had been followed up constantly with routine medical examinations.
After 54 months of CCRT, transvaginal ultrasonography revealed the
existence of ascites at the rectouterine pouch. Two months later,
peritoneal cytology by abdominal paracentesis showed mesothelial
cells with mild atypia, suggestive of reactive mesothelial cells
(Fig. 1B); the patient was diagnosed
with a reactive mesothelium and was closely followed up. After 62
months of CCRT, MRI indicated 20-mm masses in both of the
salpinges, with low intensity on T2 weighted images, and pelvic
ascites (Fig. 1C). The serum levels
of SCC and CA125 were 0.9 ng/ml and 506.1 U/ml, respectively. As
the elevation pattern of the serum tumor markers was different from
primary cervical SCC and the presence of ascites is rarely seen in
recurrence of cervical SCC, we considered the possibility of not
recurrence but secondary malignancy. A laparoscopic examination was
performed to determine the pathological diagnosis, and it revealed
white muddy ascites, bilateral intra-tubal masses (Fig. 1D), and multiple peritoneal
dissemination areas of a maximum size of 10 mm. Left salpingectomy
and peritoneal biopsy were performed. Intraoperative peritoneal
cytology showed atypical mesothelial cells with binuclear and
enlarged irregular nuclei. Macroscopically, there was a 20-mm solid
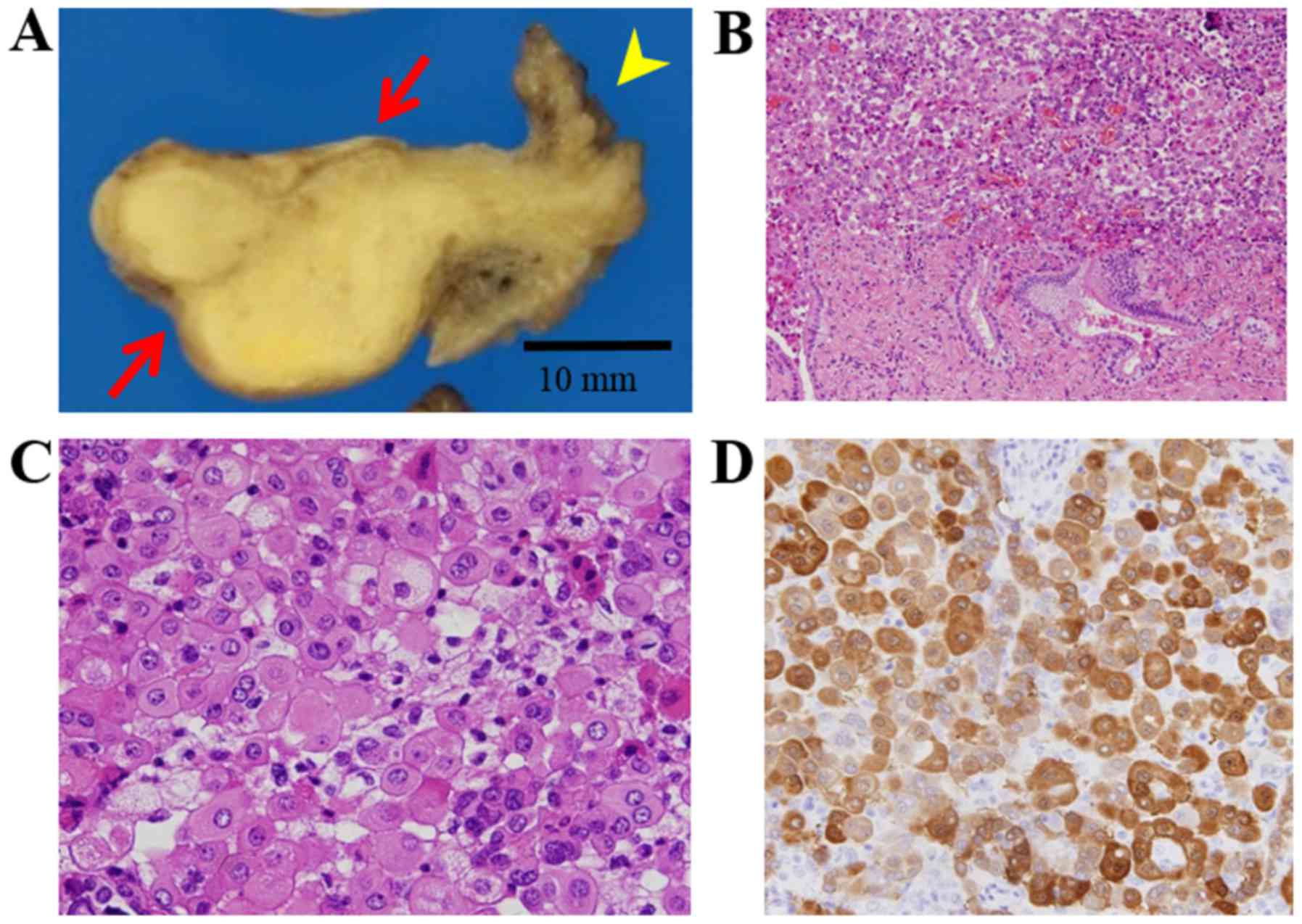
mass in the ampulla of the uterine tube (Fig. 2A). Histological examination showed
the presence of mesothelial, round tumor cells with mild to
moderate pleomorphism and prominent nucleoli, strongly suggesting
the possibility of malignant mesothelioma. In the main tumor
located intra-tubally, however, there was no continuity between the
background tubal epithelium and the malignant mesothelioma
(Fig. 2B, C). Immunohistochemistry
(IHC) showed positive staining for calretinin (Fig. 2D), thrombomodulin, mesothelin, D2-40,
CK20, and glucose transporter 1, and negative staining for CK7,
estrogen receptor, progesterone receptor, pax-8, CD146, CEA,
epithelial membrane antigen (EMA), epithelial specific antigen
(MOC31), claudin 4, and BRCA1-associated protein-1 (BAP1).
Additional examination of fluorescence in situ hybridization
(FISH) revealed no findings of homozygous p16 deletion. Based on
these findings, the patient was diagnosed with PMM, epithelioid
type. She underwent systemic chemotherapy with cisplatin plus
pemetrexed, and stable disease status has been obtained for 3
months.
Discussion
PMM accounts for 17–32% of mesotheliomas in women,
who are usually middle-aged or elderly. The association between the
exposure to asbestos and PMM is less strong than it is for pleural
mesothelioma, particularly among women (6). Ascites is present in most cases. The
present case was observed in a young adult woman who received
radiotherapy for cervical cancer and who had no asbestos exposure.
Because of the unlikely recurrence pattern from cervical SCC
considering both tumor marker levels and the presence of ascites,
we performed surgical resection of the tumors. The main location of
the malignant mesothelioma is intra-tubal; however, to the best of
our knowledge, there are no reports of tubal-origin malignant
mesothelioma. We made the diagnosis of PMM because of an absence of
continuity between the background tubal epithelium and the tumors,
and a presence of multiple peritoneal disseminations.
Retrospectively, as the findings of initial peritoneal cytology
showed a potential of PMM, we might have been able to suggest the
histological examination for the patient earlier. Our experience
suggests the importance of histopathological and
immunohistochemical examination in cases of an atypical
tumorigenesis pattern after primary treatment.
Radiotherapy for cervical cancer seems to increase
the risk for developing high-grade endometrial cancer and
carcinosarcoma (7). Although
radiation therapy for several cancers is known to increase the risk
for PMM (8), PMM after radiation
therapy for cervical cancer is extremely rare; to the best of our
knowledge, only 2 reports have been published so far (9,10). In
the previous 2 cases of PMM after radiation therapy for cervical
cancer, limited information about pathological findings has been
described (9,10). In this report, we present detailed
information about the tumor markers, histological subtype, IHC, and
p16 homologous deletion status. In particular, tumor markers and
IHC data are helpful in the differential diagnosis. The
histological subtype, BAP-1 expression on IHC, and p16 homologous
deletions on FISH are some of the prognostic factors for PMM
(11,12). We believe that our report will
contribute to the diagnosis of PMM after radiotherapy for cervical
cancer.
In general, the period between the occurrence of
primary and secondary malignancies is 10 or more years (3). The mean interval for the development of
high-grade endometrial cancer and carcinosarcoma after radiotherapy
for cervical cancer was 14 years (7). However, in all the 3 cases, including
our current case, the tumor had been diagnosed as PMM within 10
years after radiation therapy for cervical cancer, and our case had
the shortest interval, being within 5 years (9,10). We
suggest PMM tends to develop within 10 years after radiotherapy in
patients with cervical cancer. Physicians need to pay adequate
attention for secondary PMM, especially within 10 years after
radiotherapy.
In conclusion, we herein report the third case of
PMM after radiation therapy for cervical cancer. Our case
demonstrates the possibility of PMM occurrence within 10 years
after radiotherapy, and indicates the importance of histological
and immunohistochemical examination, especially in cases of an
atypical tumorigenesis pattern from the primary cancer.
Acknowledgements
We appreciate the advice and expertise of Dr Kenzo
Hiroshoma, Department of Pathology, Tokyo Women's Medical
University Yachiyo Medical Center, Dr Toshiaki Kawai, Department of
Pathology and Laboratory Medicine, National Defense Medical
College, and Dr Teruaki Oka, Division of Pathology, Kanto Central
Hospital. We appreciate the advice of Professor Keiichi Fujiwara,
Professor Kosei Hasegawa, and Dr Hiroyuki Yoshida, Department of
Gynecologic Oncology, Saitama Medical University International
Medical Center.
Glossary
Abbreviations
Abbreviations:
|
PMM
|
peritoneal malignant mesothelioma
|
|
SCC
|
squamous cell carcinoma
|
|
CA125
|
cancer antigen 125
|
|
CCRT
|
concurrent chemoradiotherapy
|
|
MRI
|
magnetic resonance imaging
|
|
FIGO
|
International Federation of Gynecology
and Obstetrics
|
|
IHC
|
immunohistochemistry
|
|
BAP1
|
BRCA1-associated protein-1
|
|
FISH
|
in situ hybridization
|
References
|
1
|
Jasani B and Gibbs A: Mesothelioma not
associated with asbestos exposure. Arch Pathol Lab Med.
136:262–267. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
National Council on Radiation Protection
and Measurements (NCRP) Scientific Committee 1–17, . Second Primary
Cancers and Cardiovascular Disease After RadiotherapyNCRP Report
No. 170. Bethesda, MD: National Council on Radiation Protection and
Measurements; 2011
|
|
3
|
Travis LB, Ng AK, Allan JM, Pui CH,
Kennedy AR, Xu XG, Purdy JA, Applegate K, Yahalom J, Constine LS,
et al: Second malignant neoplasms and cardiovascular disease
following radiotherapy. J Natl Cancer Inst. 104:357–370. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Chaturvedi AK, Engels EA, Gilbert ES, Chen
BE, Storm H, Lynch CF, Hall P, Langmark F, Pukkala E, Kaijser M, et
al: Second cancers among 104,760 survivors of cervical cancer:
Evaluation of long-term risk. J Natl Cancer Inst. 99:1634–1643.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Kleinerman RA, Boice JD Jr, Storm HH,
Sparen P, Andersen A, Pukkala E, Lynch CF, Hankey BF and Flannery
JT: Second primary cancer after treatment for cervical cancer. An
international cancer registries study. Cancer. 76:442–452. 1995.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Spirtas R, Heineman EF, Bernstein L, Beebe
GW, Keehn RJ, Stark A, Harlow BL and Benichou J: Malignant
mesothelioma: Attributable risk of asbestos exposure. Occup Environ
Med. 51:804–811. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Pothuri B, Ramondetta L, Eifel P, Deavers
MT, Wilton A, Alektiar K, Barakat R and Soslow RA:
Radiation-associated endometrial cancers are prognostically
unfavorable tumors: A clinicopathologic comparison with 527
sporadic endometrial cancers. Gynecol Oncol. 103:948–951. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Farioli A, Ottone M, Morganti AG,
Compagnone G, Romani F, Cammelli S, Mattioli S and Violante FS:
Radiation-induced mesothelioma among long-term solid cancer
survivors: A longitudinal analysis of SEER database. Cancer Med.
5:950–959. 2016. View
Article : Google Scholar : PubMed/NCBI
|
|
9
|
Babcock TL, Powell DH and Bothwell RS:
Radiation-induced peritoneal mesothelioma. J Surg Oncol. 8:369–372.
1976. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Beier KM, Gallup DG, Burgess R and Stock
RJ: Occurrence of malignant peritoneal mesothelioma after surgery
and irradiation for cervical cancer. Gynecol Oncol. 17:375–380.
1984. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Cerruto CA, Brun EA, Chang D and
Sugarbaker PH: Prognostic significance of histomorphologic
parameters in diffuse malignant peritoneal mesothelioma. Arch
Pathol Lab Med. 130:1654–1661. 2006.PubMed/NCBI
|
|
12
|
Singhi AD, Krasinskas AM, Choudry HA,
Bartlett DL, Pingpank JF, Zeh HJ, Luvison A, Fuhrer K, Bahary N,
Seethala RR and Dacic S: The prognostic significance of BAP1, NF2,
and CDKN2A in malignant peritoneal mesothelioma. Mod Pathol.
29:14–24. 2016. View Article : Google Scholar : PubMed/NCBI
|