Introduction
Esophageal cancer is the eighth most common type of
cancer worldwide, with >480,000 new cases diagnosed annually
(1). Esophageal cancer has a high
mortality rate (sixth worldwide), causing >400,000 deaths
annually (2). Squamous cell
carcinoma is the most frequently type occurring in Asians,
particularly in China, where it accounts for 70% of global
morbidity (3). However, the
incidence of esophageal adenocarcinoma in Western populations is
rapidly increasing, whereas that of squamous cell carcinoma remains
unchanged (4). Esophagectomy is
considered to be the standard treatment for patients with
resectable esophageal carcinoma, despite a detailed assessment of
preoperative staging showing that 25% of patients treated with
definitive surgery had microscopically positive resection margins
(R1). However, the 5-year survival rate scarcely exceeds 40%
(5); in addition, due to the
morbidity and mortality associated with surgery, this approach is
limited to a minority of medically fit patients.
Since the 1980s, there have been several randomized
clinical trials (RCTs) assessing the efficacy of preoperative
chemoradiotherapy followed by surgery (CRTS) in the treatment of
esophageal cancer. However, the sample-size of these RCTs was
small, with a short-term follow-up and adverse outcomes in the
surgical monotherapy arm of combination treatment trials when
compared with surgery alone (SA) case-series (6). Furthermore, the majority of the trials
did not have sufficient statistical power to produce a definitive
conclusion. Thus, a comprehensive analysis was conducted to compare
the potential objective value of CRTS with SA for resectable
esophageal carcinoma.
As regards the differences between traditional and
cumulative meta-analysis, cumulative meta-analysis refers to a
meta-analysis of the obtained studies in a certain order; those
studies are treated as a continuous whole and multiple
meta-analyzes are performed by accumulating studies sequentially in
a specified sequence (such as publication time). In addition, if a
new test result is published, a new meta-analysis may follow.
Traditional meta-analysis is performed only once, whereas
cumulative meta-analysis is performed several times; the former may
obtain summary results, but cannot distinguish the impact of each
study result on the summary results, whereas the latter does not
only obtain the results of the summary and compare the dynamic
results of summary changes, but also compares the effect of the
newly added studies on overall outcome. The cumulative
meta-analysis is controversial in terms of test level. Some
scholars object to performing multiple meta-analyses due to the
increasing probability of committing class I error, and claim the
test level should be adjusted for each analysis; some scholars
believe that the analysis of the Bayesian theory may be used to
explain, without the need for adjustment.
Based on this theory, the present study aimed to
combine the traditional and cumulative meta-analysis to explore the
pooled results of the relevant studies.
Data collection methods
Search strategy
The relevant articles identified were RCTs retrieved
from Embase, PubMed and The Cochrane Library (issue 4, 2016) and
the deadline for trial publication and/or presentation was October
1st, 2016. The American Society of Clinical Oncology (ASCO) and the
Cochrane Collaboration's Central Register of Controlled Clinical
Trials were searched for updates of the trials. The search terms
were as follows: Esophageal neoplasms, esophageal cancer,
esophageal carcinoma, esophageal tumor, neoadjuvant therapy,
chemoradiotherapy, esophagectomy, resection, surgery and
operation.
Inclusion and exclusion criteria
In our meta-analysis, the study focus was
locoregional resectable esophageal cancer patients who received
either CRTS or SA. The eligible studies were required to meet the
following inclusion criteria: i) Prospective RCTs comparing CRTS
vs. SA in the initial management of resectable esophageal cancer;
ii) outcome indices containing survival data; iii) no significant
differences in baseline characteristics between the CRTS and SA
groups; and iv) definitive follow-up survival number of cases or
survival curve, with a follow-up rate of >95% in the original
RCTs. Studies focusing on patients with esophageal cancer who had
been treated with neoadjuvant chemotherapy alone or radiotherapy
alone, other studies without usable data, letters, editorials, case
reports and reviews were excluded.
Data extraction and quality
assessment
Two investigators independently extracted data to
avoid bias in the course of the extraction. Disagreements were
resolved by consensus or consultation with third parties.
Statistics for each available outcome were extracted from trials in
the light of the key information including patient characteristics,
first author, year of publication, country/region, the regimen of
the CRTS, and tumor histology. The methodological quality
assessment of individual studies followed the Cochrane risk of bias
method.
Statistical analysis
Overall survival rates at 1, 3 and 5 years (OSR1y,
OSR3y and OSR5y, respectively), R0 resection rate, postoperative
mortality, postoperative local recurrence rate and postoperative
distant metastasis rate were extracted and pooled with 95%
confidence intervals (CIs) by adopting the fixed- or random-effects
model where heterogeneity was assessed with the inconsistency
statistic (I2<50%, P>0.05; and I2≥50%,
P≤0.05, respectively). The odds ratio (OR) was estimated with 95%
CI and P-values in both the CRTS and SA groups. All calculations
were performed using Review Manager 5.3 (Nordic Cochrane Centre,
Copenhagen, Denmark), R software version 3.2.2, and STATA version
12.0 (StataCorp LP-College Station, TX, USA). Statistical
significance was set at P<0.05.
Results
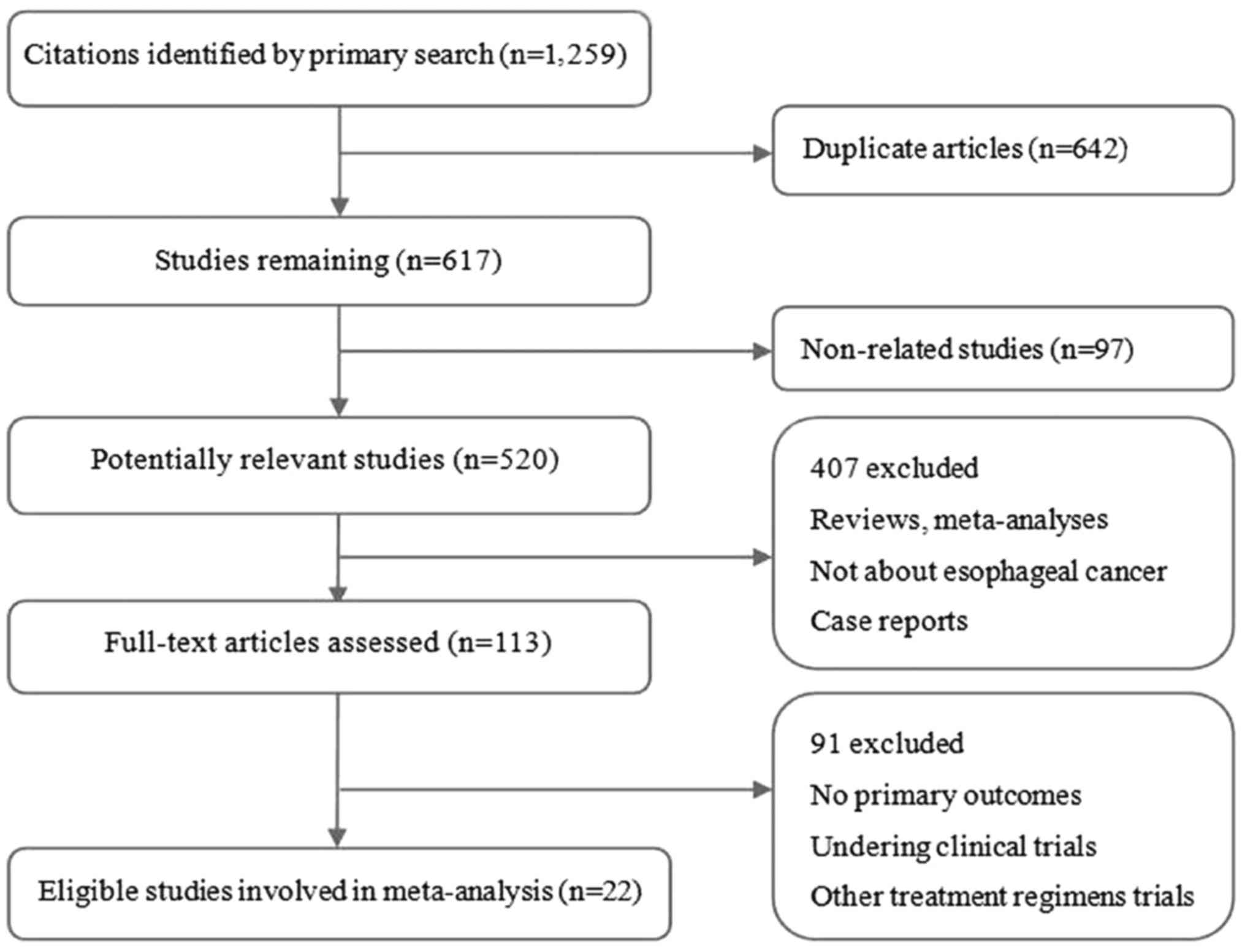
Summary of included studies
A total of 1279 records were identified according to
the search strategy and 22 were finally included in the
meta-analysis after removing duplicated, ineligible and unrelated
studies (Fig. 1). Ten countries,
including China, Australia, Japan, Korea, Thailand, United States
of America, France, The Netherlands, Ireland and Norway, were
included in the RCTs. Of the 22 studies, 20 (7–26)
reported OSR1y, 19 (7–21,24–27)
reported OSR3y and 15 (8,11–13,15–17,19–12,24–27)
reported information on OSR5y after SA or CRTS for resectable
esophageal carcinoma.
As regards pathological type, 5 RCTs (10,15,24,26,27) on
adenocarcinoma and 12 studies (7–9,11,13,14,16,21,23,24,26) on
squamous cell carcinoma investigated OSR1y, OSR3y and OSR5y after
CRTS or SA for resectable esophageal carcinoma.
A total of 9 studies (7,9,11,13–15,23,27,28)
reported R0 resection rate, 10 (9,12–15,16,19–21,26)
included postoperative local recurrence rate and distant metastasis
rate, and 15 (7–12,14–16,20,21,28)
provided postoperative mortality information.
With respect to the treatment efficacy of both
methods in different countries or regions, 8 trials (8,13,14,16–19,23)
collected data from Asian populations, 7 studies (7–11,24–26)
from European populations, and 2 (12,20) from
USA populations. The study characteristics are summarized in
Table I.
 | Table I.Outline of the literature search
findings and the general characteristics of the included
studies. |
Table I.
Outline of the literature search
findings and the general characteristics of the included
studies.
| Study, year | Country | Chemotherapy | Radiotherapy | Pathology | CRTS/SA, n | (Refs.) |
|---|
| Nygaard et
al, 1992 | Norway | 2 cycles: Cisplatin
20 mg/m2 D1-5; bleomycin 5 mg/m2 D1-5 | 35 Gy, 1.75 Gy/fr,
4 weeks | SCC | 47/41 | (7) |
| Apinop et
al, 1994 | Thailand | 2 cycles: Cisplatin
100 mg/m2 D1; 5-FU 1,000 mg/m2 D1-4 | 40 Gy, 2 Gy/fr, 4
weeks | SCC | 35/34 | (8) |
| Le Prise et
al, 1994 | France | 2 cycles: Cisplatin
100 mg/m2 D1,21; 5-FU 600 mg/m2 D2-5, 22–25 | 40 Gy, 20 Gy/10 fr,
2 weeks | SCC | 41/45 | (9) |
| Walsh et al,
1996 | Ireland | 2 cycles: Cisplatin
75 mg/m2 D1; 5-FU 15 mg/kg D1-5 | 40 Gy/15 fr, 3
weeks | AC | 58/55 | (10) |
| Bosset et
al, 1997 | France | 2 cycles: Cisplatin
80 mg/m2 D1-2 | 37 Gy, 3.7 Gy/fr, 2
weeks | SCC | 143/139 | (11) |
| Urba et al,
2001 | USA | 2 cycles: Cisplatin
20 mg/m2 D1-5; 5-FU 300 mg/m2 D1-21; Vin 1
mg/m2 D1-4 | 45 Gy; 1.5 Gy/fr, 3
weeks | SCC 25%, AC
75% | 50/50 | (12) |
| An et al,
2003 | China | 2 cycles: 5-FU 1
mg/m2 D1-5, 21–25; cisplatin 25 mg/m2 D1, 22–25 | 36 Gy, 1.2 Gy/fr, 3
weeks | SCC | 48/49 | (13) |
| Lee et al,
2004 | Korea | 2 cycles: Cisplatin
60 mg/m2 D1; 5-FU 1,000 mg/m2 D3-5 | 45.6 Gy, 1.2 Gy/fr,
4 weeks | SCC 40%, AC
60% | 51/50 | (14) |
| Burmeister et
al, 2005 | Australia | One cycle:
Cisplatin 80 mg/m2 D1; 5-FU 800 mg/m2
D1-4 | 35 Gy/15 fr, 3
weeks | SCC | 128/128 | (15) |
| Law et al,
2006 | China | 5-FU 500
mg/m2 D1-5, D24-28; cisplatin 100 mg/m2
D1,24 | 40 Gy, 2 Gy/fr, 3
weeks | SCC | 170/109 | (16) |
| Natsugoe et
al, 2006 | Japan | Cisplatin 7 mg over
2 h; 5-FU 350 mg over 24 h | 40 Gy, 2 Gy/fr, 4
weeks | SCC | 22/23 | (17) |
| Cao et al,
2007 | China | Cisplatin 20
mg/m2 D1-5, mitomycin 10 mg/m2/day D1, 5-FU
500 mg/m2 D1-5 | 40 Gy, 2 Gy/fr, 4
weeks | SCC | 118/118 | (18) |
| Jin et al,
2008 | China | Cisplatin 20–30
mg/m2, Dl-5, 22–26; paclitaxel 135 mg/m2,
D1,22 | 38–44 Gy, 2
Gy/fraction | SCC 90%, AC
10% | 30/30 | (19) |
| Peng et al,
2008 | China | 2 cycles: 5-FU 500
mg/m2 over 5 days, cisplatin 75 mg/m2 D1 | 40 Gy, 2
Gy/fraction 4 weeks | SCC | 40/40 | (20) |
| Tepper et
al, 2008 | USA | 2 cycles: 5-FU
1,000 mg/m2 D1-4; cisplatin 100 mg/m2 D1 | 50.4 Gy, 1.8 Gy/fr,
5.6 weeks | SCC 25%, AC
75% | 30/26 | (21) |
| Lv et al,
2010 | China | 2 cycles: Cisplatin
20 mg/m2 D1-3, 22–25; paclitaxel 135 mg/m2
D1-3,22–25 | 40 Gy, 2 Gy/fr, 4
weeks | SCC | 119/119 | (22) |
| Jin et al,
2011 | China | 2 cycles: 5-FU 500
mg/m2 D1-5; cisplatin 75 mg/m2 D1 | 50 Gy, 2 Gy/fr, 5
weeks | SCC 90%, AC
10% | 30/30 | (23) |
| van Hagen et
al, 2012 | The
Netherlands | 5-week
chemotherapy: Carboplatin AUC = 2, paclitaxel 50 mg/m2
D1, weekly | 41.4 Gy, 1.8 Gy/fr,
4.6 weeks | SCC 23%, AC 75%LCC
2% | 178/188 | (24) |
| Yang et al,
2012 | China | 2 cycles: Cisplatin
75 mg/m2 D1,22; navelbine 25 mg/m2
D1,8,22,29 | 40 Gy, 2 Gy/fr, 4
weeks | SCC | 54/59 | (25) |
| Bass et al,
2014 | Ireland | 2 cycles: Cisplatin
75 mg/m2 D7; 5-FU 15 mg/kg D 1–5 | 40 Gy/15 fr, 3
weeks | AC | 104/107 | (26) |
| Mariette et
al, 2014 | France | 2 cycles: Cisplatin
75 mg/m2 D1, 5-FU 800 mg/m2 on D1-4 | 45 Gy/25 fr, 5
weeks | SCC 72%, AC
28% | 98/97 | (27) |
| Shapiro et
al, 2015 | The
Netherlands | 5-week
chemotherapy: Carboplatin AUC = 2, paclitaxel 50 mg/m2
D1, weekly | 41.4 Gy, 1.8 Gy/fr,
4.6 weeks | SCC 23%, AC
75% | 178/188 | (28) |
Survival rate
The heterogeneity test at all the time points had a
I2 value of <55%; thus, the fixed-effects model was
used.
OSR1y, OSR3y and OSR5y outcomes of
traditional and cumulative meta-analysis
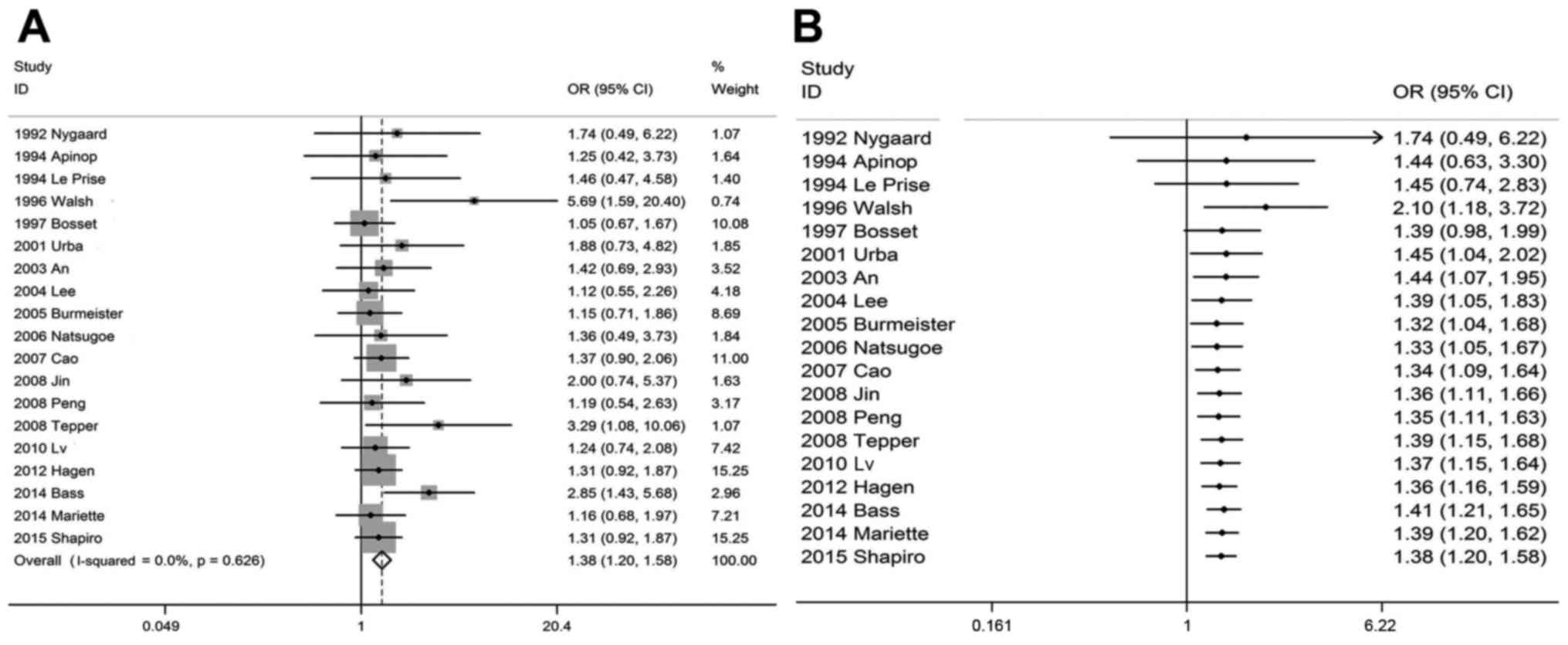
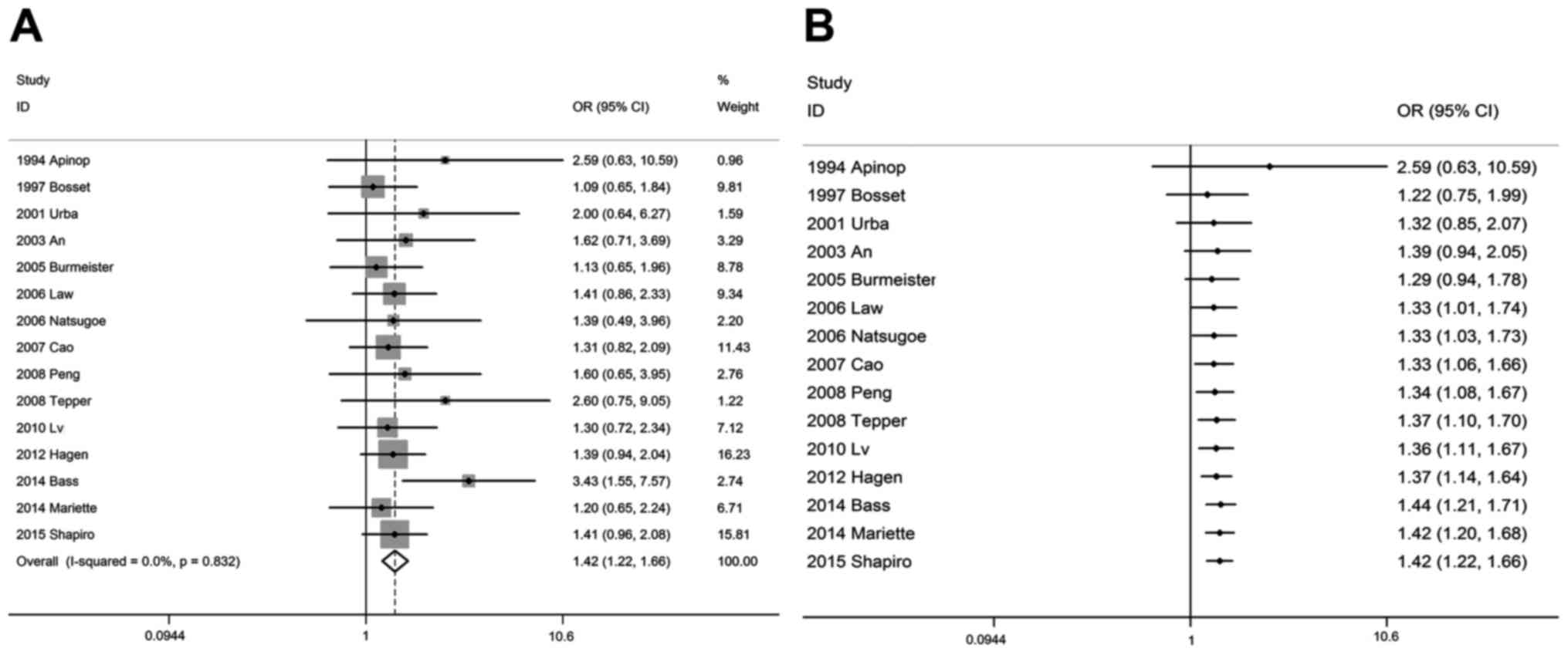
Traditional meta-analysis provided evidence that,
compared with the SA group, the OSR3y and OSR5y were significantly
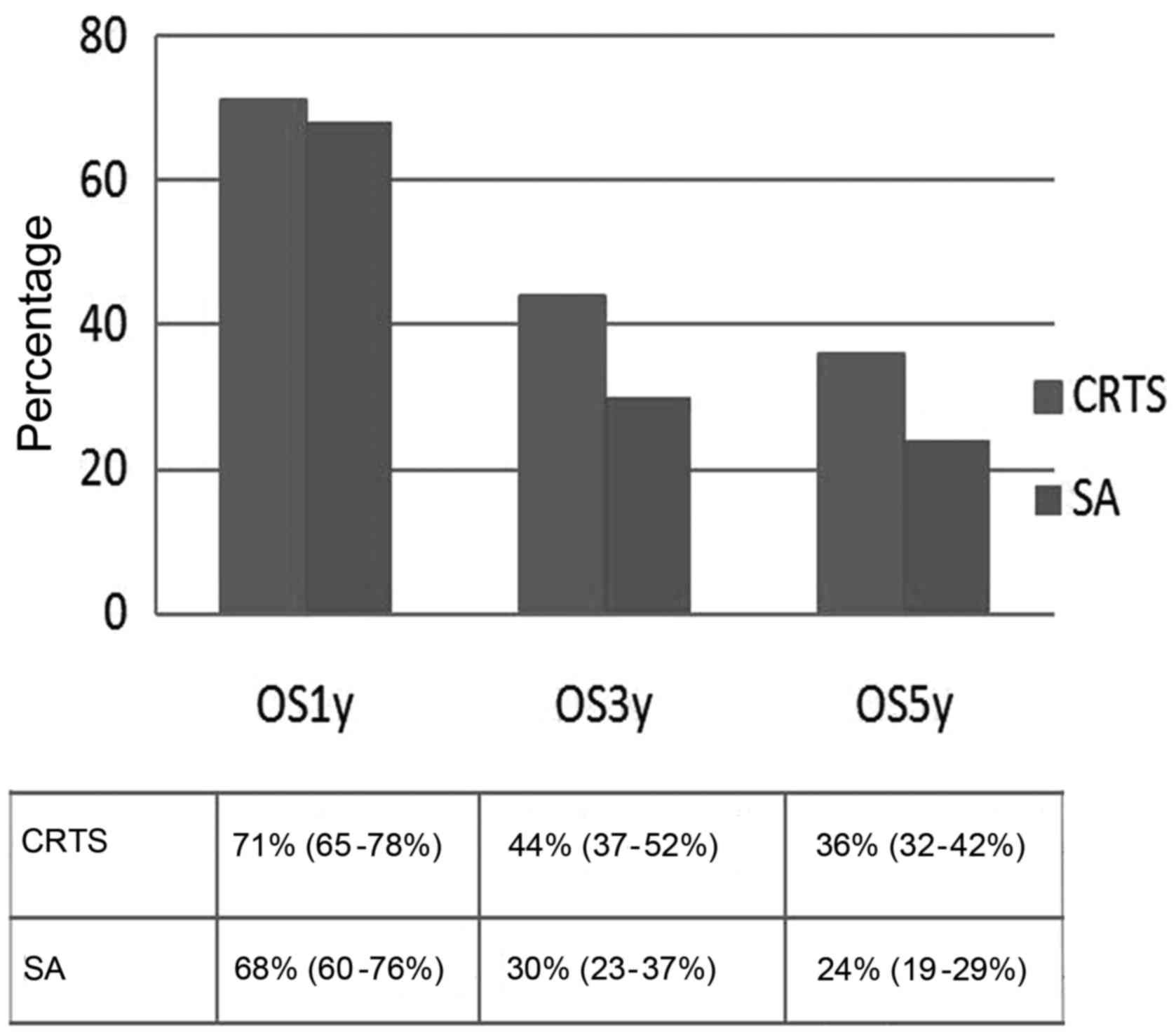
higher in the CRTS group. The pooled OSR3y was 44% (95% CI: 37–52%)
vs. 30% (95% CI: 23–38%), respectively, and the OSR5y was 36% (95%
CI: 32–42%) vs. 24% (95% CI: 19–29%), respectively, with an OR of
1.38 (1.20–1.58, P<0.001) and 1.42 (95% CI: 1.22–1.66,
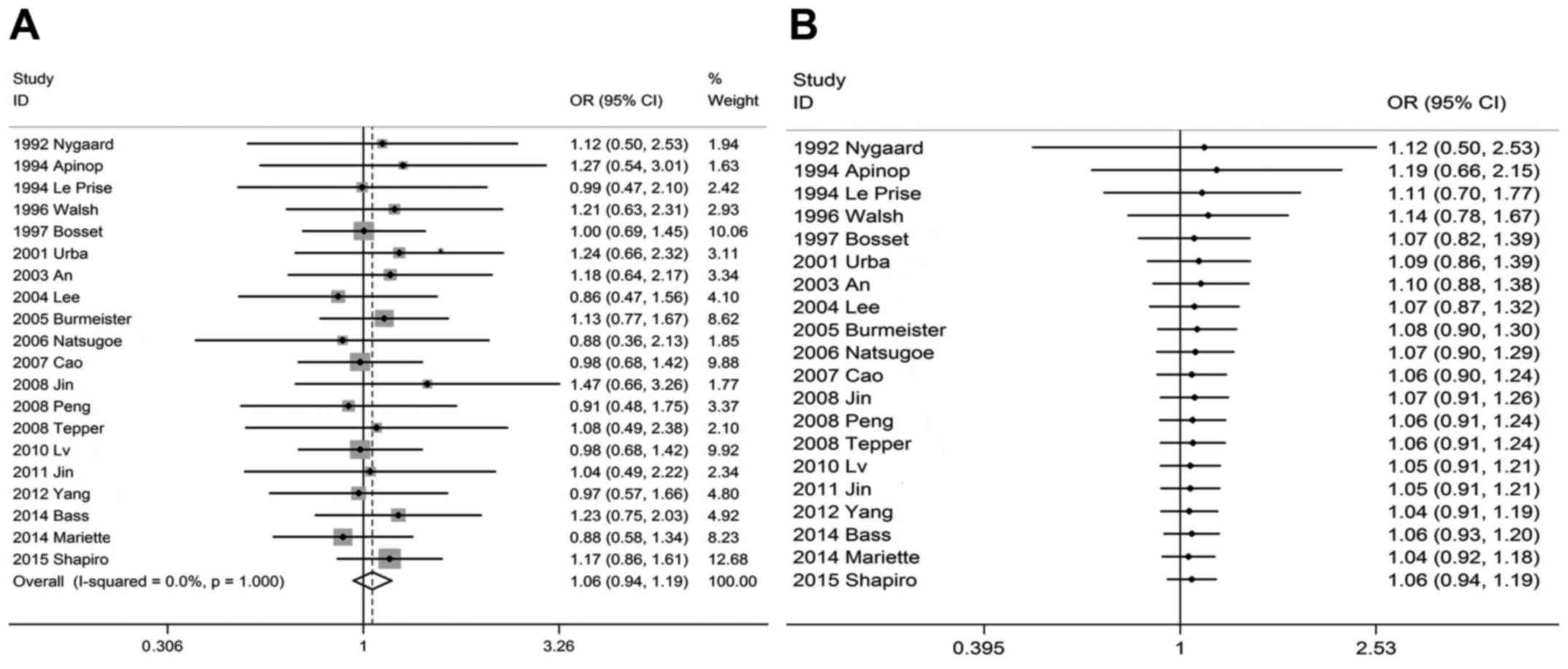
P<0.001), respectively. However, there was no statistically
significant difference in OSR1y between the CRTS and SA groups; the
pooled OSR1y was 71% (95% CI: 65–78%) vs. 68% (95% CI: 60–76%),
respectively, and the OR was 1.06 (95% CI: 0.94–1.19, P=0.348)
(Figs. 2A, 3A, 4A and
5; Table
II).
 | Table II.Survival rate and surgical parameters
of patients with EC by treatment approach. |
Table II.
Survival rate and surgical parameters
of patients with EC by treatment approach.
|
|
| No. of
patients |
|
|
|---|
|
|
|
|
|
|
|---|
| Variables | No. of studies | CRTS | SA | OR (95% CI) | P-value |
|---|
| Survival rate |
|
|
|
|
|
|
OSR1y | 20 | 1,424 | 1,429 | 1.06
(0.94–1.19) | 0.348 |
|
OSR3y | 19 | 1,479 | 1,488 | 1.38
(1.20–1.58) | <0.0001 |
|
OSR5y | 15 | 1,361 | 1,437 | 1.42
(1.22–1.66) | <0.0001 |
| Surgery
conditions |
|
|
|
|
|
| R0
resection rate | 9 | 774 | 874 | 2.76
(2.15–3.53) | <0.0001 |
| Local
recurrence rate | 10 | 668 | 679 | 0.49
(0.36–0.65) | <0.0001 |
| Distant
metastasis rate | 10 | 668 | 679 | 0.76
(0.60–0.97) | 0.02 |
|
Postoperative mortality | 15 | 1,086 | 1,205 | 0.97
(0.72–1.32) | 0.87 |
Cumulative meta-analyses were performed in
chronological order. With the increase in the number of cases, OR
point estimates and 95% CIs of all survival rates tended to be
stable and exhibited an improving trend. When multiple studies with
large sample sizes were added, the effect on the outcome was only a
reduction in the length of the confidence interval, reflecting an
increase in the accuracy of the estimated overall treatment
response. Under the α=0.05 test standard, cumulative meta-analyses
demonstrated there was no statistical difference between CRTS and
SA in terms of OSR1y (Fig. 2B), and
the P-value decreased gradually, stabilizing at P=0.334 (calculated
via Microsoft Excel). As regards OSR3y (Fig. 3B), it was observed that the
difference was initially confirmed to be statistically significant
(OR=2.10, 95% CI: 1.18–3.72, P<0.05) when adding a 113 sample
size study by Walsh et al (10) in 1996 under the selection criteria.
The P-value was >0.05 when subsequent studies were added
successively and the analysis was re-accumulated, and it again
became <0.05 when including a 100 sample size study by Urba
et al (12) in 2001 (OR=1.45,
95% CI: 1.04–2.02, P<0.05). Subsequently, the cumulative
analysis of successively included studies demonstrated that the
difference was statistically significant, with P-values stable at
<0.05. As regards OSR5y (Fig.
4B), cumulative meta-analyses demonstrated that the difference
was initially statistically significant in 2007, when a 102 sample
size study was conducted by Cao et al (18) (OR=1.33, 95% CI: 1.06–1.66,
P<0.05), after which time the P-values were stable at
<0.05.
Surgical factors
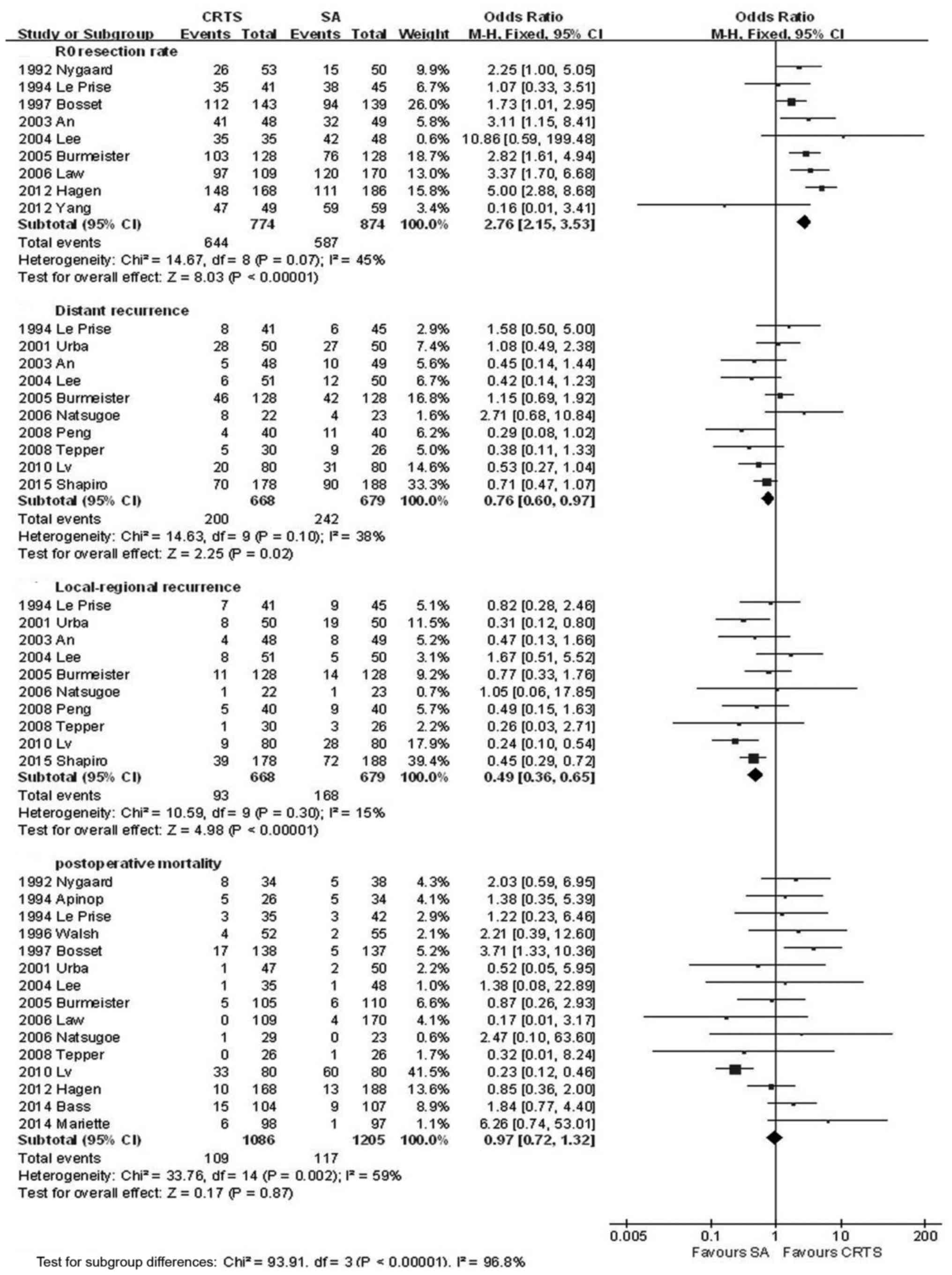
The CRTS group had a significantly higher R0
resection rate and a lower local recurrence and distant metastasis
rate compared with the SA group, with a pooled OR of 2.76 (95% CI:
2.15–3.53, P<0.001), 0.49 (95% CI: 0.36–6.65, P<0.001) and
0.76 (95% CI: 0.60–0.97, P=0.02), respectively; the differences
were statistically significant. However, the incidence of
postoperative mortality in the two groups suggested there was no
significantly statistical difference, with an OR of 0.97 (95% CI:
0.72–1.32, P=0.87) (Fig. 6, Table II).
Subgroup analysis
Survival rate of squamous cell
carcinoma and adenocarcinoma
The pooled OR of squamous cell carcinoma in terms of
OSR3y and OSR5y in the CRTS and SA groups was 1.57 (95% CI:
1.21–2.04, P=0.0006) and 1.69 (95% CI: 1.32–2.16, P<0.0001),
respectively; the differences were statistically significant.
However, there was no statistically significant difference in terms
of OSR1y (OR=1.13, 95% CI: 0.88–1.45, P=0.35). Compared with
adenocarcinoma patients treated with SA, the OSR1y, OSR3y and OSR5y
were significantly higher in CRTS, with an OR of 1.55 (95% CI:
1.09–2.20, P=0.01), 1.77 (95% CI: 1.34–2.36, P<0.0001) and 1.92
(95% CI: 1.34–2.75, P=0.0004), respectively; the differences were
statistically significant (Table
III).
 | Table III.Survival rate by histological type
and continent in EC patients treated with CRTS and SA. |
Table III.
Survival rate by histological type
and continent in EC patients treated with CRTS and SA.
|
|
|
| No. of
patients |
|
|
|---|
|
|
|
|
|
|
|
|---|
| Variables | Overall
survival | No. of studies | CRTS | SA | OR (95% CI) | P-value |
|---|
| Histological
type |
|
|
|
|
|
|
|
SCC | OSR1y | 11 | 647 | 654 | 1.13
(0.88–1.45) | 0.35 |
|
| OSR3y | 10 | 554 | 556 | 1.57
(1.21–2.04) | 0.0006 |
|
| OSR5y | 8 | 622 | 698 | 1.69
(1.32–2.16) | <0.0001 |
| AC | OSR1y | 4 | 295 | 302 | 1.55
(1.09–2.20) | 0.01 |
|
| OSR3y | 5 | 429 | 442 | 1.77
(1.34–2.36) | <0.0001 |
|
| OSR5y | 4 | 371 | 387 | 1.92
(1.34–2.75) | 0.0004 |
| Location |
|
|
|
|
|
|
|
Asia | OSR1y | 8 | 398 | 403 | 1.05
(0.74–1.49) | 0.80 |
|
| OSR3y | 8 | 424 | 424 | 1.81
(1.37–2.40) | <0.0001 |
|
| OSR5y | 7 | 452 | 514 | 1.73
(1.31–2.27) | <0.0001 |
|
Europe | OSR1y | 7 | 669 | 673 | 1.22
(0.96–1.54) | 0.10 |
|
| OSR3y | 8 | 847 | 860 | 1.74
(1.42–2.14) | <0.0001 |
|
| OSR5y | 5 | 701 | 719 | 1.69
(1.35–2.13) | <0.0001 |
|
USA | OSR1y | 2 | 80 | 76 | 1.75
(0.86–3.55) | 0.06 |
|
| OSR3y | 2 | 80 | 76 | 3.55
(1.68–7.49) | 0.0009 |
|
| OSR5y | 2 | 80 | 76 | 2.80
(1.19–6.61) | 0.02 |
Survival rates of different countries
or regions
The subgroup analysis of OSR3y, OSR5y for Asian,
European and American populations were significantly higher in the
CRTS group compared with those in the SA group, and the differences
were all statistically significant (P<0.05). However, when
comparing the OSR1y between the two groups in patients from the
three continents, the difference was not significant (P>0.05;
Table III).
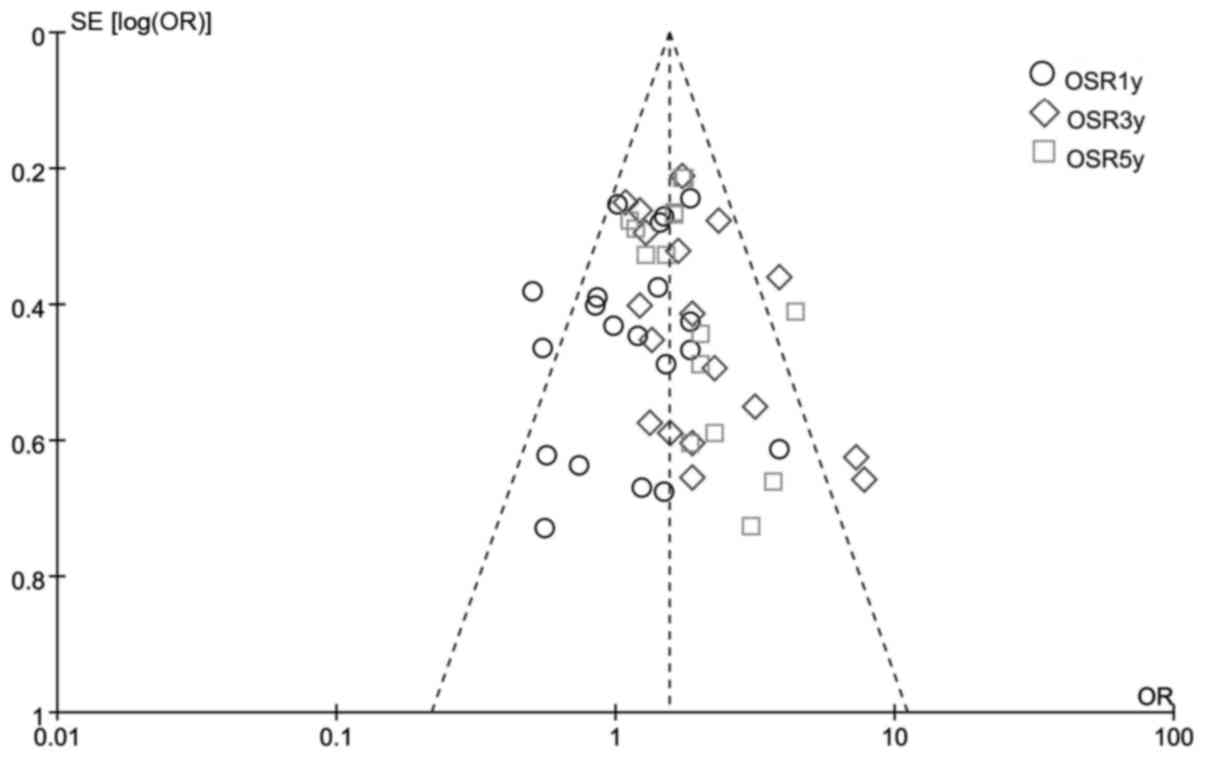
Publication bias
A funnel plot analysis of all the studies was
performed in the meta-analysis of OSR1y, OSR2y and OSR3y between
CRTS and SA. This indicated that the publication bias was low in
the present meta-analysis (Fig.
7).
Discussion
CRT is quickly becoming the neoadjuvant treatment of
choice for patients with resectable esophageal carcinoma prior to
surgery. However, trials and meta-analyses on this subject are
limited and varied, with small sample sizes and heterogeneity of
population distribution characteristics, tumor pathological types,
tumor location, radiation doses, chemotherapy regimens, surgical
approach, postoperative care and adequacy of surgical resections,
despite all the advantages of trimodality therapy.
In the CROSS trial (26), CRTS improved the long-term overall
and progression-free survival in patients with resectable
esophageal carcinoma; this improvement was statistically
significant and clinically relevant for both the adenocarcinoma and
squamous cell carcinoma subtypes. In addition, locoregional control
and distant disease control also improved significantly. However,
Mariette et al (25) reported
that, compared with SA, CRTS with cisplatin plus fluorouracil did
not improve R0 resection rate or survival, but rather enhanced
postoperative mortality in patients with resectable esophageal
carcinoma. Burmeister et al (15) obtained results in a randomised
controlled phase III trial indicating that preoperative CRT with
cisplatin and fluorouracil did not significantly improve
progression-free or overall survival in patients with resectable
esophageal cancer compared with SA.
Meta-analyses on CRTS vs. SA in esophageal cancer,
however, are discordant. In the most recent meta-analysis of 13
studies on CRTS compared with SA in operable patients, the hazard
ratio for all-cause mortality was 0.78 (P<0.001), favoring CRTS.
However, due to the large majority of locally advanced cases
included in the trials and the heterogeneity in staging methods,
there was no definitive conclusion regarding survival benefit for
stage I or II esophageal cancer (29). A meta-analysis of those trials by
Gluud and Krag (30) reported a
short-term survival benefit for neoadjuvant chemoradiotherapy over
surgical monotherapy in adenocarcinoma as well as squamous cell
carcinoma of the esophagus. In addition, a meta-analysis by Huang
et al (31) reported that
CRTS with paclitaxel plus platinum appeared to be a better choice
compared with platinum plus 5-fluorouracil for esophageal cancer,
particularly for squamous cell carcinoma. Wijnhoven et al
(32) performed a secondary
meta-analysis of six published meta-analyses to compare the
differences in the studies included and statistical methods
applied, and found heterogeneity between the RCTs included in the
meta-analyses with regard to the previously mentioned content. Of
note, the majority of RCTs were conducted in the 90s; hence, the
diagnostic methods, staging, treatment delivery and outcome
assessment reflected the clinical practice during tha decade.
Our aim was to conduct a meta-analysis combining the
traditional and cumulative methods. The traditional meta-analysis
revealed that CRTS may improve the long-term survival and surgical
parameters, and reduce locoregional cancer recurrence and distant
metastasis in adenocarcinoma as well as squamous cell carcinoma of
the oesophagus, but there was no significant difference in terms of
short-term survival. We focused more on the integration of various
researches chronologically by using the cumulative meta-analysis.
Clinical trials on a particular research topic constitute an
increasing, open and continuous entity over time. Baum et al
(33) first proposed the concept of
cumulative meta-analysis that was first applied to clinical
practice by Lau et al (34)
on the basis of the traditional meta-analysis, adding studies
sequentially and performing multiple meta-analyses in a sequential
manner based on the time of publication, the size of the sample and
the quality score of the study; whenever a new study is published,
the meta-analysis may be again continued. Unlike traditional
meta-analyses, which are performed only at a certain point in time,
cumulative meta-analysis was performed at each time point in order
to capture the variation tendency of the combined total effect,
which may enable greater use of information, contribute to early
detection of coherent interventions, and facilitate new
research.
From forest plots of cumulative meta-analysis
(performed in chronological order), it was observed that, as the
number of cases increased, the test efficacy increased and the 95%
CI gradually decreased; under the α=0.05 test standard, cumulative
meta-analyses demonstrated there was no statistical difference
between CRTS and SA in terms of OSR1y, and the P-value decreased
gradually and stabilized at 0.334. Therefore, it was concluded that
CRTS did not improve the short-term survival benefit of patients
with esophageal cancer. The difference between the two treatment
approaches in terms of OSR3y was initially confirmed to be
statistically significant (OR=2.10, 95% CI: 1.18–3.72, P<0.05);
when adding a 113 sample size study by Walsh et al (10) under the selected test criteria, it
was observed that the treatment regimen was the same as that of
previous studies, except that the subjects were adenocarcinoma
patients rather than squamous cell carcinoma patients. Thus, it was
hypothesized that CRTS may be more effective in treating esophageal
adenocarcinoma. The same conclusion was reached using the
traditional meta-analysis, as the OR for OSR1y, OSR3y and OSR5y in
adenocarcinoma patients is higher compared with that in squamous
cell carcinoma patients (1.55 vs. 1.13 for OSR1y, 1.77 vs. 1.57 for
OSR3y and 1.92 vs. 1.69 for OSR5y). A meta-analysis conducted by
Hai-Lin et al (35) also
confirmed that CRTS may increase the survival rate of patients with
esophageal adenocarcinoma. However, the P-value was >0.05 when
adding a 282 sample size study by Bosset et al (11) in 1997, possibly due to the cisplatin
monotherapy. The P-value again became <0.05 when a 100 sample
size study by Urba et al (12) in 2001 was included (OR=1.45, 95% CI:
1.04–2.02, P<0.05), in which innovative triple therapy was used,
combining vinblastine with cisplatin and fluorouracil. Liu et
al (36) also reported in 2015
that cisplatin with vinorelbine may achieve a higher pathological
complete response rate and better survival outcomes compared with
cisplatin and fluorouracil in esophageal squamous cell carcinoma.
Subsequently, the cumulative analysis of successively included
studies demonstrated that the difference was statistically
significant, with P-values stable at <0.05. It was demonstrated
that CRTS may improve the 3-year survival benefit of patients with
esophageal cancer. As regards OSR5y, cumulative meta-analyses
demonstrated that the difference was initially found to be
statistically significant in 2007, when a 102 sample size study was
conducted by Cao et al (17)
(OR=1.33, 95% CI: 1.06–1.66, P<0.05), after which the P-values
were stable at <0.05. A study by Wolf et al (37) on long-term outcome of mitomycin C and
5-fluorouracil-based primary CRT for esophageal cancer demonstrated
a significant increase of overall survival (P<0.0001) in the CRT
vs. the radiotherapy alone group, indicating that CRTS may provide
a long-term survival benefit to patients with esophageal cancer.
However, it remains uncertain whether the alteration in the
abovementioned treatment options is the cause of P<0.05, as this
is only a monistic interpretation. From the present analysis, it
was concluded that CRTS was able improve the long-term survival of
patients with esophageal cancer, and may be more effective in
treating esophageal adenocarcinoma. In addition, vinblastine or
mitomycin combined with general chemotherapy were more likely to
improve the long-term survival rate following complete resection,
which may also be a future research focus.
Traditional meta-analysis may be associated with
various types of bias, such as selection, implementation, exit and
measurement bias; the same biases may occur at various time points
in the cumulative meta-analysis and affect the determination of the
overall effect trend. Furthermore, certain information could not be
collected (e.g., the chronological cumulative effect of the
treatment regimen, the difference in efficacy and the quality score
of a single article), which is a major drawback. In addition,
patients included in the present study were in various stages of
the trial, such as adjuvant therapy; patient compliance was also
different, which may affect the results. Furthermore, the 22
included studies differed significantly in sample size; thus, the
contribution to the overall effect was not proportional, which was
another limitation of the cumulative meta-analysis.
In summary, it may be concluded from the cumulative
meta-analysis that CRTS may increase OSR3y and OSR5y by 38%
(P<0.0001) and 42% (P<0.0001), respectively. From the forest
plot, it was observed that the difference in OSR3y and OSR5y was
statistically significant, with P-values stable at <0.05,
indicating that CRTS may improve the patient survival rate.
Therefore, it is recommended that the CRTS regimen is routinely
used for patients with early resectable esophageal cancer. There
are ongoing studies on this subject and, as the results of those
studies are published, it may further elucidate the role of CRTS in
the treatment of early resectable esophageal cancer.
References
|
1
|
Ferlay J, Shin HR, Bray F, Forman D,
Mathers C and Parkin DM: Estimates of worldwide burden of cancer in
2008: GLOBOCAN 2008. Int J Cancer. 127:2893–2917. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Parkin DM, Bray FI and Devesa SS: Cancer
burden in the year 2000. The global picture. Eur J Cancer. 37 Suppl
8:S4–S66. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Siegel RL, Miller KD and Jemal A: Cancer
statistics, 2016. CA Cancer J Clin. 66:7–30. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Devesa SS, Blot WJ and Fraumeni JF Jr:
Changing patterns in the incidence of esophageal and gastric
carcinoma in the United States. Cancer. 83:2049–2053. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Song Y, Li L, Ou Y, Gao Z, Li E, Li X,
Zhang W, Wang J, Xu L, Zhou Y, et al: Identification of genomic
alterations in oesophageal squamous cell cancer. Nature. 509:91–95.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Funk EM and Witte J: Multimodal therapy
for esophageal adenocarcinoma. N Engl J Med. 336:375–376, author
reply 375–376. 1997.PubMed/NCBI
|
|
7
|
Nygaard K, Hagen S, Hansen HS, Hatlevoll
R, Hultborn R, Jakobsen A, Mäntyla M, Modig H, Munck-Wikland E,
Rosengren B, et al: Pre-operative radiotherapy prolongs survival in
operable esophageal carcinoma: A randomized, multicenter study of
pre-operative radiotherapy and chemotherapy. The second
Scandinavian trial in esophageal cancer. World J Surg.
16:1104–1109, discussion 1110. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Apinop C, Puttisak P and Preecha N: A
prospective study of combined therapy in esophageal cancer.
Hepatogastroenterology. 41:391–393. 1994.PubMed/NCBI
|
|
9
|
Le Prise E, Etienne PL, Meunier B, Maddern
G, Ben Hassel M, Gedouin D, Boutin D, Campion JP and Launois B: A
randomized study of chemotherapy, radiation therapy, and surgery
versus surgery for localized squamous cell carcinoma of the
esophagus. Cancer. 73:1779–1784. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Walsh TN, Noonan N, Hollywood D, Kelly A,
Keeling N and Hennessy TP: A comparison of multimodal therapy and
surgery for esophageal adenocarcinoma. N Engl J Med. 335:462–467.
1996. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Bosset JF, Gignoux M, Triboulet JP, Tiret
E, Mantion G, Elias D, Lozach P, Ollier JC, Pavy JJ, Mercier M, et
al: Chemoradiotherapy followed by surgery compared with surgery
alone in squamous-cell cancer of the esophagus. N Engl J Med.
337:161–167. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Urba SG, Orringer MB, Turrisi A,
Iannettoni M, Forastiere A and Strawderman M: Randomized trial of
preoperative chemoradiation versus surgery alone in patients with
locoregional esophageal carcinoma. J Clin Oncol. 19:305–313. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
An FS, Huang JQ, Xie YT, Chen SH and Rong
TH: A prospective study of combined chemoradiotherapy followed by
surgery in the treatment of esophageal carcinoma. Zhonghua Zhong
Liu Za Zhi. 25:376–379. 2003.(In Chinese). PubMed/NCBI
|
|
14
|
Lee JL, Park SI, Kim SB, Jung HY, Lee GH,
Kim JH, Song HY, Cho KJ, Kim WK, Lee JS, et al: A single
institutional phase III trial of preoperative chemotherapy with
hyperfractionation radiotherapy plus surgery versus surgery alone
for resectable esophageal squamous cell carcinoma. Ann Oncol.
15:947–954. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Burmeister BH, Smithers BM, Gebski V,
Fitzgerald L, Simes RJ, Devitt P, Ackland S, Gotley DC, Joseph D,
Millar J, et al Trans-Tasman Radiation Oncology Group, ;
Australasian Gastro-Intestinal Trials Group, : Surgery alone versus
chemoradiotherapy followed by surgery for resectable cancer of the
oesophagus: A randomised controlled phase III trial. Lancet Oncol.
6:659–668. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Natsugoe S, Okumura H, Matsumoto M,
Uchikado Y, Setoyama T, Yokomakura N, Ishigami S, Owaki T and Aikou
T: Randomized controlled study on preoperative chemoradiotherapy
followed by surgery versus surgery alone for esophageal squamous
cell cancer in a single institution. Dis Esophagus. 19:468–472.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Cao XF, He XT, Ji L, Xiao J and Lv J:
Effects of neoadjuvant radiochemotherapy on pathological staging
and prognosis for locally advanced esophageal squamous cell
carcinoma. Dis Esophagus. 22:477–481. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Jin MG, Jiang SC and Chen ZW: Clinical
trial of preoperative concurrent chemoradiation followed by surgery
versus surgery alone for advanced esophageal carcinoma. China J
Cancer Prev Treat. 15:1815–1817. 2008.
|
|
19
|
Peng L, Xie TP, Han YT, et al: Randomized
controlled study on preoperative concurrent chemoradiotherapy
versus surgery alone for esophageal squamous cell carcinoma. Tumor
Chin. 28:620–622. 2008.
|
|
20
|
Tepper J, Krasna MJ, Niedzwiecki D, Hollis
D, Reed CE, Goldberg R, Kiel K, Willett C, Sugarbaker D and Mayer
R: Phase III trial of trimodality therapy with cisplatin,
fluorouracil, radiotherapy, and surgery compared with surgery alone
for esophageal cancer: CALGB 9781. J Clin Oncol. 26:1086–1092.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Lv J, Cao XF, Zhu B, Ji L, Tao L and Wang
DD: Long-term efficacy of perioperative chemoradiotherapy on
esophageal squamous cell carcinoma. World J Gastroenterol.
16:1649–1654. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Jin FL, Zu-Liang HU and Hai-Feng MA:
Treatment effect of neoadjuvant chemoradiotherapy followed by
surgery versus surgery alone in local advanced esophageal
carcinoma. Shiyong Zhongliu Zazhi. 26:523–526. 2011.
|
|
23
|
Yang H, Jh FU, Liu MZ, et al: A
multi-centered randomized controlled study of neo-adjuvant
chemoradiotherapy followed by surgery versus surgery alone for
locally advanced squamous cell carcinoma of esophagus: an interim
analysis). Natl Med J Chin (Chin). 92:1028–1032. 2006.(In
Chinese).
|
|
24
|
Bass GA, Furlong H, O'Sullivan KE,
Hennessy TP and Walsh TN: Chemoradiotherapy, with adjuvant surgery
for local control, confers a durable survival advantage in
adenocarcinoma and squamous cell carcinoma of the oesophagus. Eur J
Cancer. 50:1065–1075. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Mariette C, Dahan L, Mornex F, Maillard E,
Thomas PA, Meunier B, Boige V, Pezet D, Robb WB, Le Brun-Ly V, et
al: Surgery alone versus chemoradiotherapy followed by surgery for
stage I and II esophageal cancer: Final analysis of randomized
controlled phase III trial FFCD 9901. J Clin Oncol. 32:2416–2422.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Shapiro J, van Lanschot JJB, Hulshof MCCM,
van Hagen P, van Berge Henegouwen MI, Wijnhoven BPL, van Laarhoven
HWM, Nieuwenhuijzen GAP, Hospers GAP, Bonenkamp JJ, et al CROSS
study group, : Neoadjuvant chemoradiotherapy plus surgery versus
surgery alone for oesophageal or junctional cancer (CROSS):
Long-term results of a randomised controlled trial. Lancet Oncol.
16:1090–1098. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
van Hagen P, Hulshof MC, van Lanschot JJ,
Steyerberg EW, van Berge Henegouwen MI, Wijnhoven BP, Richel DJ,
Nieuwenhuijzen GA, Hospers GA, Bonenkamp JJ, et al CROSS Group, :
Preoperative chemoradiotherapy for esophageal or junctional cancer.
N Engl J Med. 366:2074–2084. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Law S, Kwong DL, Wong KH, Kwok KF and Wong
J: The effects of neoadjuvant chemoradiation on pTNM staging and
its prognostic significance in esophageal cancer. J Gastrointest
Surg. 10:1301–1311. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Sjoquist KM, Burmeister BH, Smithers BM,
Zalcberg JR, Simes RJ, Barbour A and Gebski V; Australasian
Gastro-Intestinal Trials Group, : Survival after neoadjuvant
chemotherapy or chemoradiotherapy for resectable oesophageal
carcinoma: An updated meta-analysis. Lancet Oncol. 12:681–692.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Gluud LL and Krag A: Banding ligation
versus beta-blockers for primary prevention in oesophageal varices
in adults. Cochrane Database Syst Rev. 8:CD0045442012.
|
|
31
|
Huang TC, Hsu CH, Lin CC and Tu YK:
Systematic review and network meta-analysis: Neoadjuvant
chemoradiotherapy for locoregional esophageal cancer. Jpn J Clin
Oncol. 45:1023–1028. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Wijnhoven BP, van Lanschot JJ, Tilanus HW,
Steyerberg EW and van der Gaast A: Neoadjuvant chemoradiotherapy
for esophageal cancer: A review of meta-analyses. World J Surg.
33:2606–2614. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Baum ML, Anish DS, Chalmers TC, Sacks HS,
Smith H Jr and Fagerstrom RM: A survey of clinical trials of
antibiotic prophylaxis in colon surgery: Evidence against further
use of no-treatment controls. N Engl J Med. 305:795–799. 1981.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Lau J, Antman EM, Jimenez-Silva J,
Kupelnick B, Mosteller F and Chalmers TC: Cumulative meta-analysis
of therapeutic trials for myocardial infarction. N Engl J Med.
327:248–254. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Jin HL, Zhu H, Ling TS, Zhang HJ and Shi
RH: Neoadjuvant chemoradiotherapy for resectable esophageal
carcinoma: A meta-analysis. World J Gastroenterol. 15:5983–5991.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Liu SL, Yang H, Zhang P, Zhang L, Zhao L,
Luo LL, Fu JH, Liu MZ and Xi M: Neoadjuvant chemoradiotherapy with
cisplatin plus vinorelbine versus cisplatin plus fluorouracil for
esophageal squamous cell carcinoma: A matched case-control study.
Radiother Oncol. 116:262–268. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Wolf M, Zehentmayr F, Niyazi M, Ganswindt
U, Haimerl W, Schmidt M, Hölzel D and Belka C: Long-term outcome of
mitomycin C- and 5-FU-based primary radiochemotherapy for
esophageal cancer. Strahlenther Onkol. 186:374–381. 2010.
View Article : Google Scholar : PubMed/NCBI
|