Introduction
Non-small-cell lung cancer (NSCLC) with echinoderm
microtubule associated protein like 4-anaplasitic lymphoma kinase
(EML4-ALK) rearranged has been found to highly respond to
crizotinib, an oral small-molecule ALK tyrosine-kinase inhibitor
(1). Cizotinib is generally well
tolerated, which most common adverse events include visual
dysfunction (60%), nausea (55%), vomiting (47%), diarrhea (60%),
and pitting edema (31%) (1). Severe
skin involvement is comparatively rare reported for cizotinib
(2). Here we firstly report a case
of crizotinib-associated toxic epidermal necrolysis (TEN).
Case report
A 75-year-old Chinese male was admitted to our
hospital for non-productive cough and progressive dyspnea in April
2016. Confirmed by the tumor biopsy, he was diagnosed as
adenocarcinoma lung cancer with pleura and osseous metastasis
(T2NxM1c). As the chromosomal fusion of EML4-ALK was detected by
amplification refractory mutation system, first-line treatment with
crizotinib was started on May 11th 2016. One month later, pulmonary
CT scan suggested that the disease was stable, whereas the patient
was admitted to hospital again for hepatic insufficiency and edema
of lower extremity, which both were due to crizotinib. Reduced
glutathione and magnesium isoglycyrrhizinate were administered for
liver protection, and furosemide was administered to promote
diuresis. On July 6th, 56 days after initiation of crizotinib
treatment, the patient suddenly developed skin rash presenting as
itchy papules on the trunk without symptom before or simultaneously
skin eruption. The rash quickly became diffuse papules with
vesicles, which progressed to flaccid bullae. Moreover, the
patients developed oral ulcers and conjunctivitis. Skin biopsy was
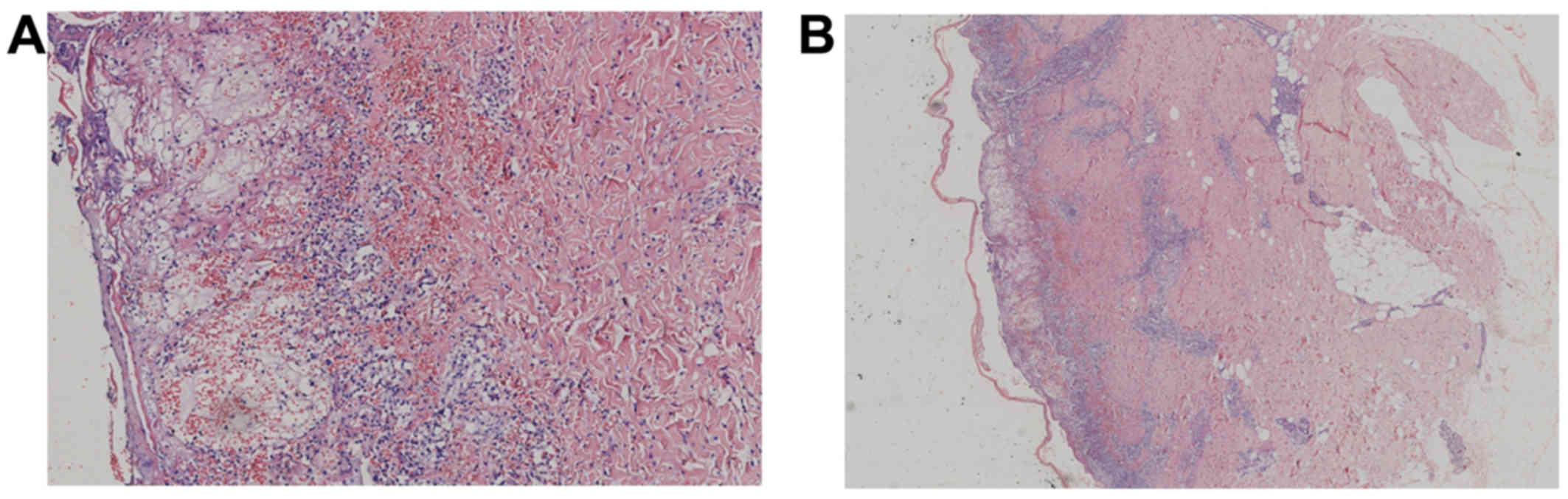
performed after consulting the dermatologist. Histopathological
analysis suggested various cutaneous changes (Fig. 1A and B). In the epidermis, a large
number of cells showed eosinophilic degeneration, swelling and
necrosis, with unclear cell boundary, karyopyknosis or karyolysis.
Multiple vacuoles and fissures were also observed inside the
epidermis. Besides, some cells of the basal layer showed
liquefactive degeneration. The epidermis-dermal junction was not
clear and present interface dermatitis changes. Moreover, in the
upper and middle dermis, perivascular inflammatory cells
infiltration and erythrocyte overflew were detected. A diagnosis of
Stevens-Johnson syndrome (SJS) was made. Written informed consent
was obtained from the patient and the present study was approved by
the Institutional Ethics Committee of Hangzhou First People's
Hospital.
Crizotinib was immediately discontinued and the
patient received dexamethasone (5 mg, i.v., daily), cetirizine and
topical steroids. Five days later, the patient's condition
deteriorated with a fever of above 38.5°C, as well as diffuse rash
expanded to the limbs and face. At last, the affected area reached
>50% of his body surface, and the patient was diagnosed as TEN,
according to the quantitative evaluation of the surface of affected
skin. The patient was then treated with methylprednisolone (40 mg
i.v. bid), intravenous Human Normal Immunoglobulin (20 g i.v. gtt
qd) and anti-infective therapy. However, his symptoms was not
relieved after 10 days of treatment, and the skin lesions still
progressed quickly (Fig. 2),
Nikolski sign was present (Fig. 2C).
The patient died 22 days after the discontinuation of
crizotinib.
Discussion
Crizotinib is a clinically available ALK inhibitor,
with previously reported severe adverse events majorly including
esophageal ulcer (3), diffuse
alveolar damage (4), and erythema
multiforme (2). Up to date, only two
cases of SJS were reported which were developed after single
molecule targeted therapy acting against the EGFR (epidermal growth
factor receptor) and ALK, both were related with afatinib (5,6).
To our knowledge, this case is the first report of
crizotinib-associated TEN. SJS and TEN are two overlapping
syndromes of burning-like severe skin lesions and characterized by
skin detachment. Both syndromes are life-threatening skin
conditions, with mortality rates of 1–10% for SJS and 20–40% for
TEN (7). The only differentiate
criterion for the two syndromes is the quantitative evaluation of
the range of affected skin. For SJS, the affected skin area is less
than 10% of Total Body Surface Area (TBSA), whereas the proportion
is >30% for TEN. If the skin involvement ranges between 10 to
30%, it is defined as overlapping syndrome (7). The most well-known causes for the above
syndromes are certain medications, but in very rare cases, the
coexistence of cancer can also have an impact on the incidence of
SJS and TEN (7). Several cutaneous
adverse cases have also recently been reported while more and more
immune checkpoint inhibition were used in cancer treatment
(8,9).
The diagnosis of SJS or TEN is mainly based on
clinical parameters during the acute phase. In our case,
keratinocyte apoptosis followed by necrosis is the pathogenic cause
of the widespread epidermal detachment observed in this patient.
Since the patient have not taken any other suspicious drugs beside
crizotinib, both before and during the development of skin lesions,
we consider TEN was most likely induced by crizotinib. It was
reported that Blacks and Asian patients were at a higher risk than
Caucasian (10). Some researchers
found a strong association between HLA genotype and SJS/TEN in Han
Chinese (11,12). In this case, it happened more than 1
month after the causative drug was initiated, and because of its
potential increased mortality we should try to find TEN predict
factors before crizotinib treatment.
In conclusion, clinicians should be aware of SJS and
TEN during the usage of crizotinib in NCSLC patients, when patients
develop symptoms including generalized skin rash followed by a few
flaccid bullae, separation of large sheets of epidermis from the
dermis, and mucositis of the mouth and genital area. Immediate
crizotinib discontinuation and adequate treatment of skin diseases
are required in these cases.
Acknowledgements
The present study is supported by funding from the
Health Foundation of Hangzhou City Zhejiang Province (grand no.
2015 Z003).
Competing interests
The authors declare that they have no competing
interests.'
References
|
1
|
Shaw AT, Kim DW, Nakagawa K, Seto T, Crinó
L, Ahn MJ, De Pas T, Besse B, Solomon BJ, Blackhall F, et al:
Crizotinib versus chemotherapy in advanced ALK-positive lung
cancer. N Engl J Med. 368:2385–2394. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Sawamura S, Kajihara I, Ichihara A,
Fukushima S, Jinnin M, Yamaguchi E, Kohrogi H and Ihn H:
Crizotinib-associated erythema multiforme in a lung cancer patient.
Drug Discov Ther. 9:142–143. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Park J, Yoshida K, Kondo C, Shimizu J,
Horio Y, Hijioka S and Hida T: Crizotinib-induced esophageal
ulceration: A novel adverse event of crizotinib. Lung Cancer.
81:495–496. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Tamiya A, Okamoto I, Miyazaki M, Shimizu
S, Kitaichi M and Nakagawa K: Severe acute interstitial lung
disease after crizotinib therapy in a patient with
EML4-ALK-positive non-small-cell lung cancer. J Clin Oncol.
31:e15–e17. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Doesch J, Debus D, Meyer C, Papadopoulos
T, Schultz ES, Ficker JH and Brueckl WM: Afatinib-associated
Stevens-Johnson syndrome in an EGFR-mutated lung cancer patient.
Lung Cancer. 95:35–38. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Honda Y, Hattori Y, Katsura S, Terashima
T, Manabe T, Otsuka A and Miyachi Y: Stevens-Johnson syndrome-like
erosive dermatitis possibly related to afatinib. Eur J Dermatol.
26:413–414. 2016.PubMed/NCBI
|
|
7
|
Rosen AC, Balagula Y, Raisch DW, Garg V,
Nardone B, Larsen N, Sorrell J, West DP, Anadkat MJ and Lacouture
ME: Life-threatening dermatologic adverse events in oncology.
Anticancer Drugs. 25:225–234. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Vivar KL, Deschaine M, Messina J, Divine
JM, Rabionet A, Patel N, Harrington MA and Seminario-Vidal L:
Epidermal programmed cell death-ligand 1 expression in TEN
associated with nivolumab therapy. J Cutan Pathol. 44:381–384.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Collins LK, Chapman MS, Carter JB and
Samie FH: Cutaneous adverse effects of the immune checkpoint
inhibitors. Curr Probl Cancer. 41:125–128. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Frey N, Jossi J, Bodmer M, Bircher A, Jick
SS, Meier CR and Spoendlin J: The epidemiology of Stevens-Johnson
syndrome and toxic epidermal necrolysis in the UK. J Invest
Dermatol. 137:1240–1247. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Man CB, Kwan P, Baum L, Yu E, Lau KM,
Cheng AS and Ng MH: Association between HLA-B*1502 allele and
antiepileptic drug-induced cutaneous reactions in Han Chinese.
Epilepsia. 48:1015–1018. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Chung WH, Hung SI, Hong HS, Hsih MS, Yang
LC, Ho HC, Wu JY and Chen YT: Medical genetics: A marker for
Stevens-Johnson syndrome. Nature. 428:4862004. View Article : Google Scholar : PubMed/NCBI
|