Introduction
Intraductal papillary mucinous neoplasms (IPMNs) are
characterized by the papillary proliferation of atypical mucinous
epithelial cells in the pancreatic ductal system, and the affected
pancreatic ducts are often cystically dilated. IPMNs comprise a
spectrum of diseases ranging from adenoma, through in situ
carcinoma, to invasive carcinoma (minimally invasive carcinoma and
invasive carcinoma derived from IPMNs) (1).
There are two recurrence patterns following
resection of IPMNs: Metachronous multifocal occurrence of IPMNs and
distinct pancreatic ductal adenocarcinoma (PDAC) in the remnant
pancreas. Recent reports have demonstrated that distinct PDAC
occurs synchronously or metachronously during the management of
IPMNs, with a frequency of ~10%; thus, IPMNs were recently
recognized as one of the predictors of PDAC (2–5).
We herein report the rare case of a patient who
underwent resection of PDAC that developed in the remnant pancreas
13 years after distal pancreatectomy with splenectomy for
IPMNs.
Case report
A 72-year-old female patient presented to the
Department of Gastroenterological Surgery I, Hokkaido University
Hospital (Sapporo, Japan) for a routine follow-up examination
following distal pancreatectomy with splenectomy ~13 years prior.
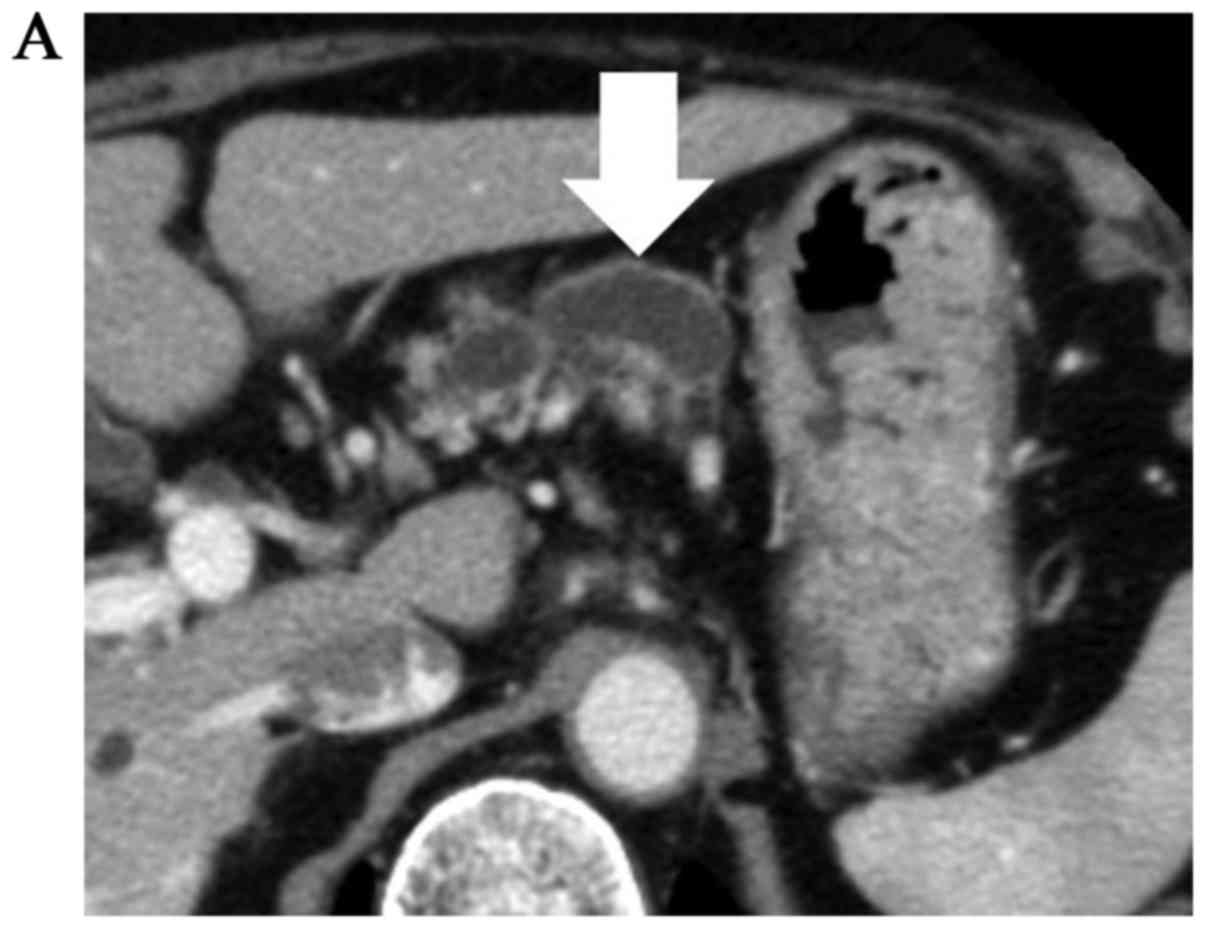
Abdominal computed tomography (CT) revealed a 5.5-cm cystic lesion
in the tail of the pancreas with a mural nodule, and dilation of
the main pancreatic duct (Fig. 1),
with another small cystic lesion in the uncus of the pancreas.
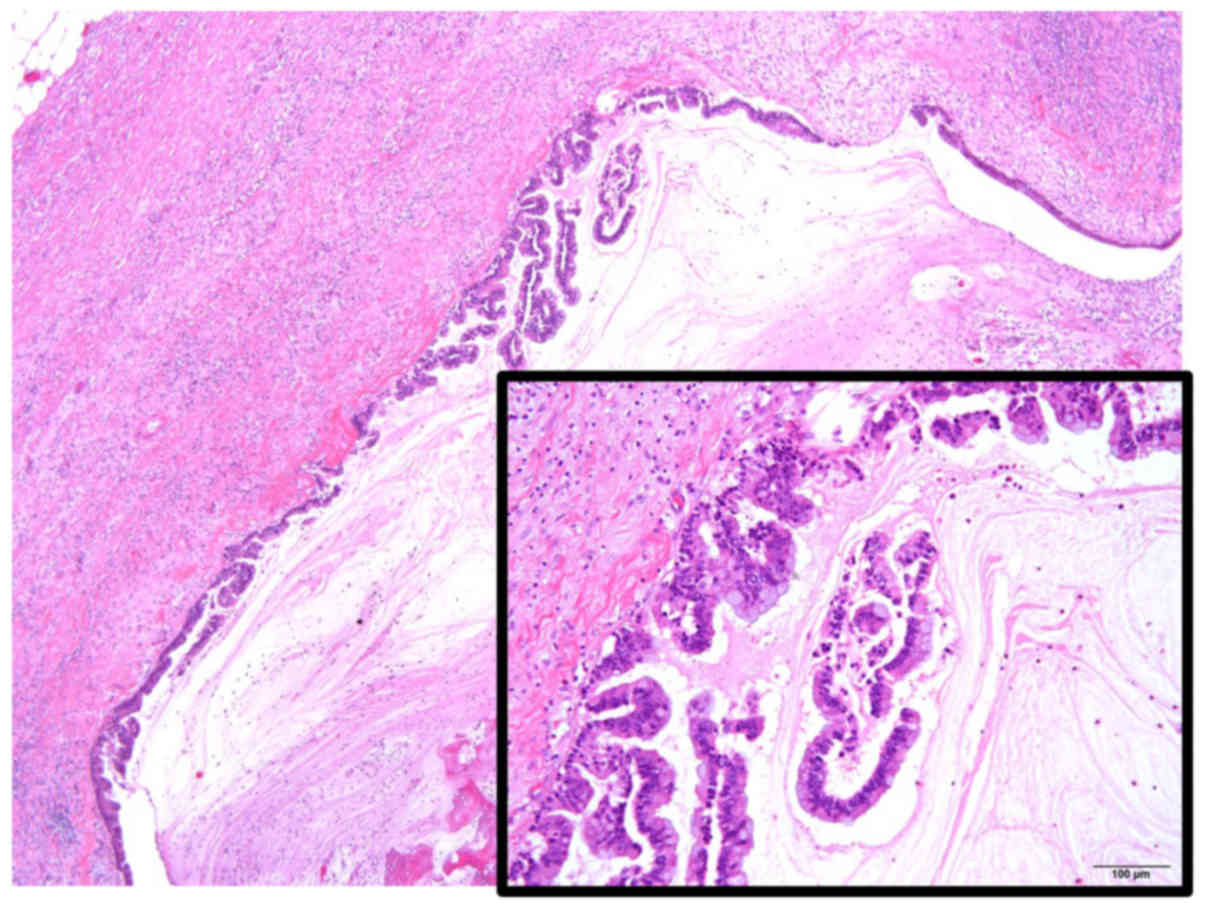
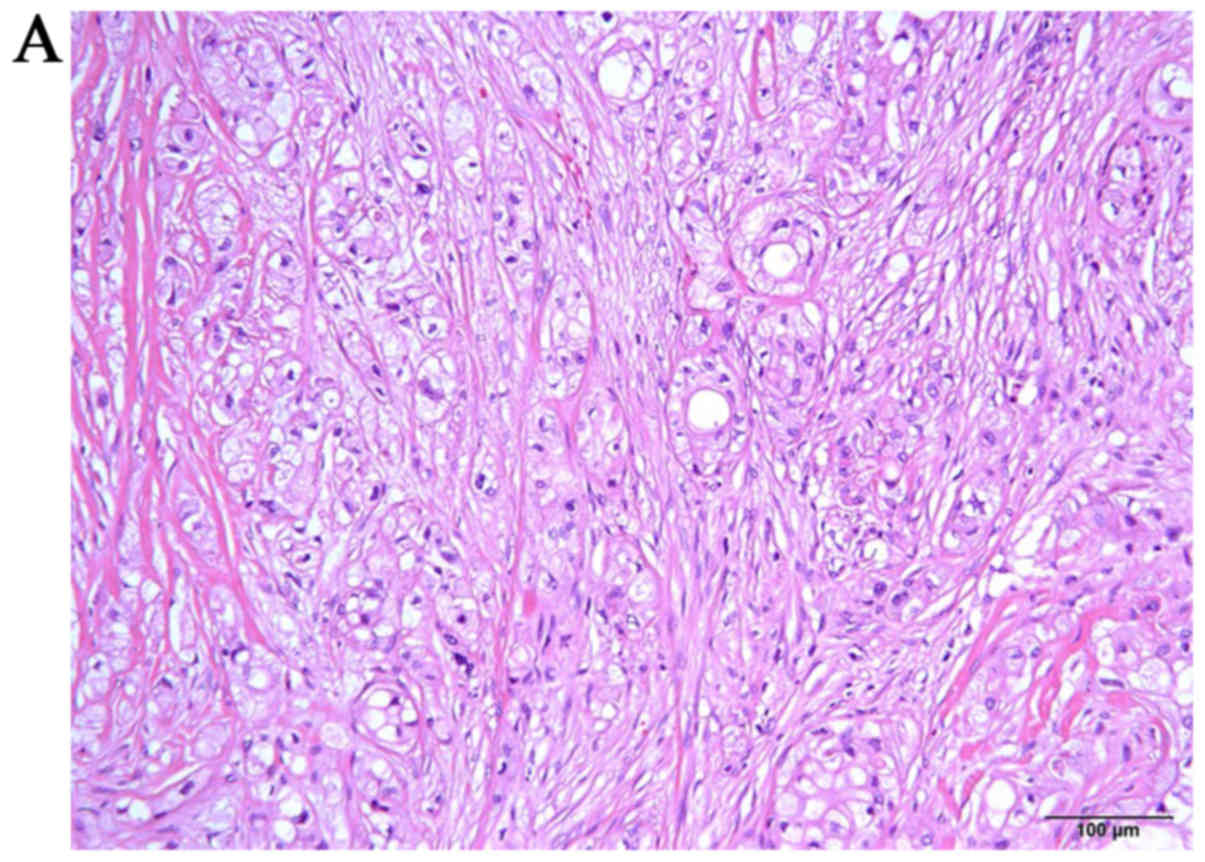
Histopathologically, IPMN with high-grade dysplasia had been
diagnosed after the initial surgery, with negative surgical margins
(Fig. 2). Regular medical follow-up
visits with imaging examinations were performed every 6 months. At
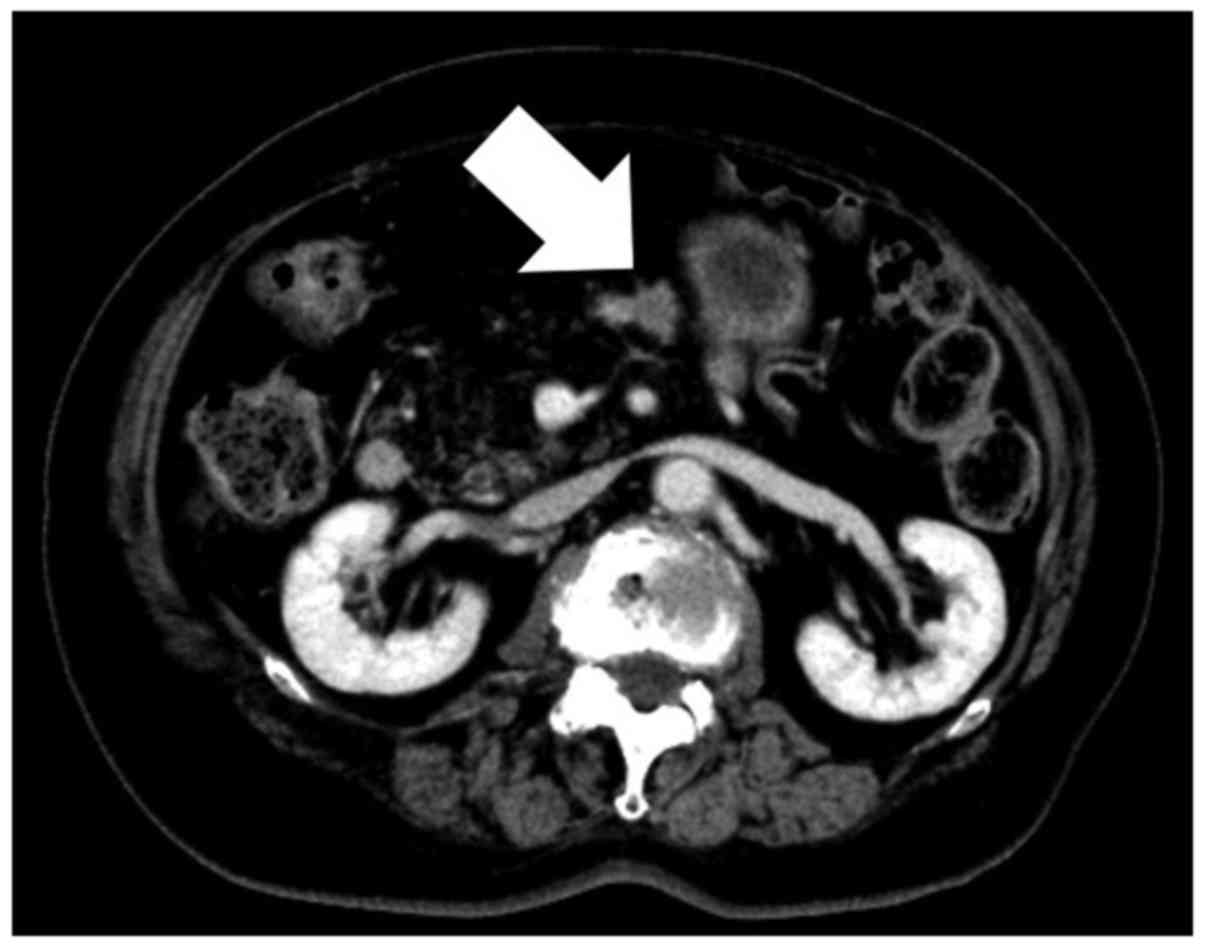
152 months after the initial operation, CT revealed a 2-cm tumor at
the resection margin of the pancreas (Fig. 3). Tumor marker level examination
revealed that the carcinoembryonic antigen and Duke pancreatic
monoclonal antigen type-2 levels were within the reference limits,
whereas carbohydrate antigen 19-9 had increased to 109.7 U/ml
(reference range: 0–36 U/ml) and Span-1 has increased to 60.1 U/ml
(reference range: 0–30 U/ml) (Table
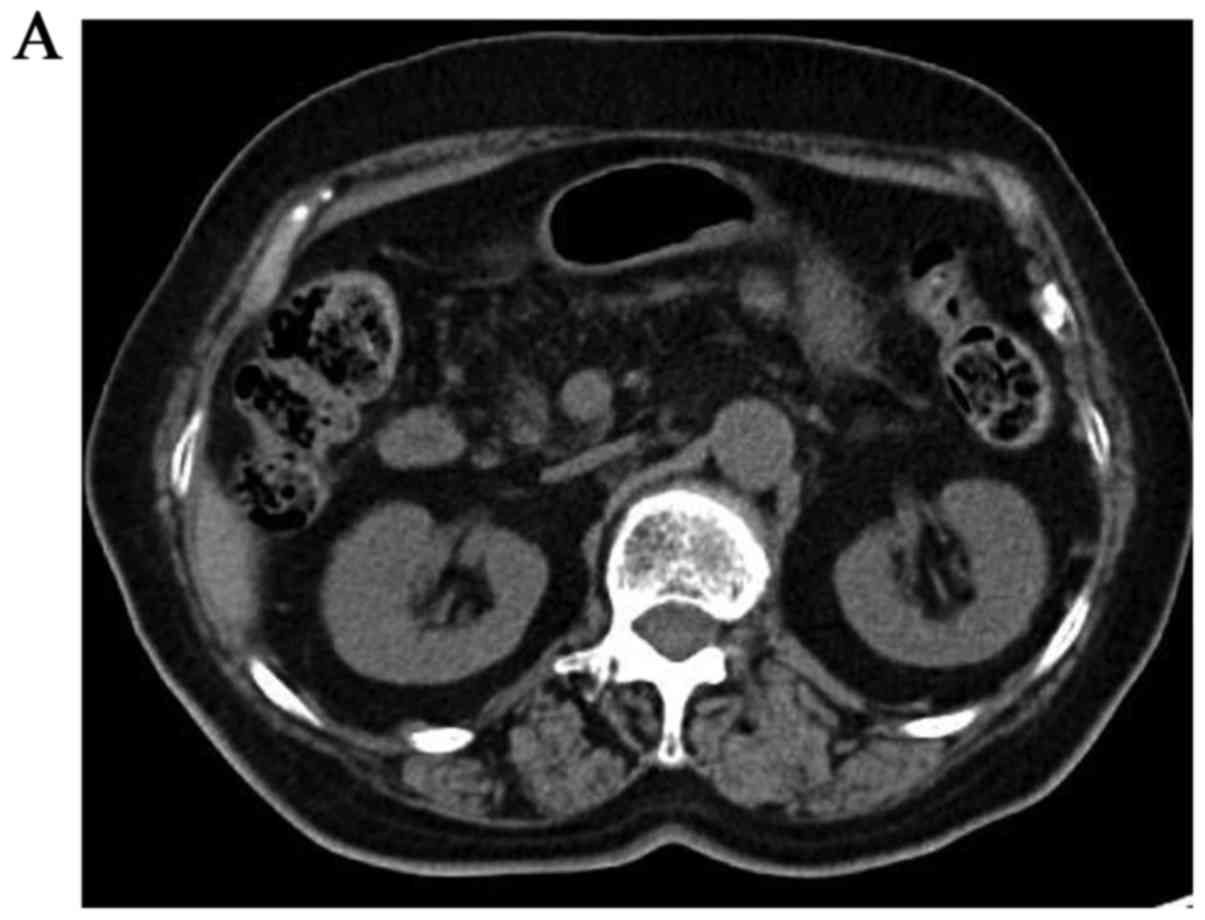
I). The lesion at the resection margin appeared as a hot spot
on fluorodeoxyglucose-positron emission tomography (Fig. 4). Remnant proximal partial
pancreatectomy and partial gastrectomy were performed due to the
patient's age and the presence of comorbidities (diabetes).
Histopathological examination of the lesion at the resection margin
confirmed a poorly to moderately differentiated adenocarcinoma,
which was not derived from the IPMNs (Fig. 5): A metachronous PDAC with vascular
and stomach invasion (T3N0M0; R0; stage IIA). The patient received
S-1 for 6 months after surgery and remained alive without
recurrence at the last follow-up, 17 months after the second
operation.
 | Table I.Laboratory results prior to the second
operation. |
Table I.
Laboratory results prior to the second
operation.
| Laboratory
parameters | Values |
|---|
| White blood
cells | 6,800/µl |
| Red blood cell
count |
408×104/µl |
| Hemoglobin level | 11.3 g/dl |
| Hematocrit | 34.5% |
| Platelet count |
17.0×104/µl |
| HbA1C | 6.1% |
| Total bilirubin | 0.8 mg/dl |
| AST | 34 U/l |
| ALT | 25 U/l |
| BUN | 15 mg/dl |
| Creatinine | 0.58 mg/l |
| Amylase | 54 U/l |
| CEA | 6.1 ng/ml |
| CA19–9 | 109.7 U/ml |
| DUPAN-2 | 143 U/ml |
| Span-1 | 60.1 U/ml |
| Elastase 1 | <80 ng/dl |
The patient consented to the publication of the case
details and associated images.
Discussion
We herein present the case of a patient who
underwent resection of PDAC that developed in the remnant pancreas
13 years after distal pancreatectomy with splenectomy for IPMNs. A
cystic lesion was also detected in the uncus of the remnant
pancreas, which was diagnosed as IPMN. This patient had been
followed up every 6 months after the first operation to detect new
lesions in the remnant pancreas. According to consensus guidelines
and recent reports on IPMNs, branch duct (BD)-IPMNs are less
aggressive compared with main duct (MD)-IPMNs (6,7);
therefore, BD-IPMNs that cause no symptoms and have cysts <30 mm
and no mural nodules, may be followed up with periodic imaging
examinations (1,8). Several recent studies investigated the
development of distinct PDAC during follow-up evaluation of
BD-IPMNs, and the incidence rate ranged from 4.5 to 8% (5,8,9). Thus, BD-IMPNs may be a good predictor
for the early detection of PDACs during observation of BD-IPMNs or
after the resection of IPMNs.
The 2012 international consensus guidelines
recommend that, if patients with multifocal BD-IPMNs have known
IPMNs in the remnant pancreas following IPMN resection, these
patients should be followed up for a short period (3–6 months) by
pancreatic magnetic resonance imaging (MRI)/magnetic resonance
cholangiopancreatography (MRCP) or CT, to establish stability
(1). The patient presented herein
was followed up every 6 months due to the relatively high incidence
of PDAC. By contrast, Ohtsuka et al reported that some
metachronous PDACs were unresectable at diagnosis >10 years
after initial surgery for IPMNs, and performed surveillance every 6
months for the first 2 years after surgery and every 12 months
thereafter. They concluded that, based on their experience, annual
examination was not sufficient to detect all the cases of
resectable PDACs (10). Yogi et
al reported that the main pancreatic duct dilates after
surgery, and that IPMNs in the remnant pancreas after
pancreatectomy for IPMNs were risk factors for recurrence. In that
study, half of the patients (5/10) who had IPMNs in the remnant
pancreas developed recurrence (11).
Hirono et al found that remnant pancreatic recurrence may
occur >5 years after surgical resection, and a second resection
may improve survival for patients with IPMNs or PDAC recurrence in
its early stages; to improve survival, they proposed continual
surveillance of the remnant pancreas every 6 months after the
initial operation to detect remnant pancreatic recurrence in its
early stages (12). Our patient also
presented with an IPMN in the uncus of the pancreas. Based on the
abovementioned reports, it is recommended that patients who have
IPMNs in the remnant pancreas undergo CT, MRI, or MRCP every 6
months. A follow-up examination schedule for IPMNs should be
established, so that PDAC may be detected while it is
resectable.
In summary, PDAC may develop in the remnant pancreas
following pancreatectomy for IPMNs; therefore, careful long-term
follow-up with periodic surveillance, at least every 6 months, is
warranted.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Tanaka M, Fernández-del Castillo C, Adsay
V, Chari S, Falconi M, Jang JY, Kimura W, Levy P, Pitman MB,
Schmidt CM, et al: International consensus guidelines 2012 for the
management of IPMN and MCN of the pancreas. Pancreatology.
12:183–197. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Yamaguchi K, Kanemitsu S, Hatori T,
Maguchi H, Shimizu Y, Tada M, Nakagohri T, Hanada K, Osanai M, Noda
Y, et al: Pancreatic ductal adenocarcinoma derived from IPMN and
pancreatic ductal adenocarcinoma concomitant with IPMN. Pancreas.
40:571–580. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Uehara H, Nakaizumi A, Ishikawa O, Iishi
H, Tatsumi K, Takakura R, Ishida T, Takano Y, Tanaka S and Takenaka
A: Development of ductal carcinoma of the pancreas during follow-up
of branch duct intraductal papillary mucinous neoplasm of the
pancreas. Gut. 57:1561–1565. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Ingkakul T, Sadakari Y, Ienaga J, Satoh N,
Takahata S and Tanaka M: Predictors of the presence of concomitant
invasive ductal carcinoma in intraductal papillary mucinous
neoplasm of the pancreas. Ann Surg. 251:70–75. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Tanno S, Nakano Y, Sugiyama Y, Nakamura K,
Sasajima J, Koizumi K, Yamazaki M, Nishikawa T, Mizukami Y,
Yanagawa N, et al: Incidence of synchronous and metachronous
pancreatic carcinoma in 168 patients with branch duct intraductal
papillary mucinous neoplasm. Pancreatology. 10:173–178. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Sugiyama M, Izumisato Y, Abe N, Masaki T,
Mori T and Atomi Y: Predictive factors for malignancy in
intraductal papillary-mucinous tumours of the pancreas. Br J Surg.
90:1244–1249. 2003. View
Article : Google Scholar : PubMed/NCBI
|
|
7
|
Terris B, Ponsot P, Paye F, Hammel P,
Sauvanet A, Molas G, Bernades P, Belghiti J, Ruszniewski P and
Fléjou JF: Intraductal papillary mucinous tumors of the pancreas
confined to secondary ducts show less aggressive pathologic
features as compared with those involving the main pancreatic duct.
Am J Surg Pathol. 24:1372–1377. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Tanno S, Nakano Y, Nishikawa T, Nakamura
K, Sasajima J, Minoguchi M, Mizukami Y, Yanagawa N, Fujii T, Obara
T, et al: Natural history of branch duct intraductal
papillary-mucinous neoplasms of the pancreas without mural nodules:
Long-term follow-up results. Gut. 57:339–343. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Sawai Y, Yamao K, Bhatia V, Chiba T,
Mizuno N, Sawaki A, Takahashi K, Tajika M, Shimizu Y, Yatabe Y and
Yanagisawa A: Development of pancreatic cancers during long-term
follow-up of side-branch intraductal papillary mucinous neoplasms.
Endoscopy. 42:1077–1084. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Ohtsuka T, Kono H, Tanabe R, Nagayoshi Y,
Mori Y, Sadakari Y, Takahata S, Oda Y, Aishima S, Igarashi H, et
al: Follow-up study after resection of intraductal papillary
mucinous neoplasm of the pancreas; special references to the
multifocal lesions and development of ductal carcinoma in the
remnant pancreas. Am J Surg. 204:44–48. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Yogi T, Hijioka S, Imaoka H, Mizuno N,
Hara K, Tajika M, Tanaka T, Ishihara M, Shimizu Y, Hosoda W, et al:
Risk factors for postoperative recurrence of intraductal papillary
mucinous neoplasms of the pancreas based on a long-term follow-up
study: Proposals for follow-up strategies. J Hepatobiliary Pancreat
Sci. 22:757–765. 2015. View
Article : Google Scholar : PubMed/NCBI
|
|
12
|
Hirono S, Kawai M, Okada K, Miyazawa M,
Shimizu A, Kitahata Y, Ueno M, Yanagisawa A and Yamaue H: Long-term
surveillance is necessary after operative resection for intraductal
papillary mucinous neoplasm of the pancreas. Surgery. 160:306–317.
2016. View Article : Google Scholar : PubMed/NCBI
|