Introduction
Approximately 690,000 new cases of head and neck
cancer (including oral cavity, lip, nasopharynx, other pharynx and
larynx) were reported worldwide in 2012. Head and neck cancers
represent approximately 4.8% of all cancer cases (1). The cumulative overall 5-year survival
remains poor at approximately 50% (2). The improvement observed in the last
decade in head and neck cancer survival may be due to a higher
incidence of human papilloma virus associated head and neck cancer
rather than to more effective therapy (3,4).
Radiation therapy is an important part of head and
neck cancer therapy. Radiotherapy is used in addition to surgical
treatment (neoadjuvant or adjuvant) or as the primary treatment,
often in combination with chemotherapy (5,6).
Melanoma-associated antigens-A (MAGE-A) are a part
of the cancer/testis antigen (CTA) family (7). Recognized by T-cells, the MAGE-A
proteins are a promising target for cancer immunotherapy and cancer
vaccination (8–10). Expression of MAGE-A has been found in
stem cells, placentae, ovaries, testes (7,11) and
malignancies, such as bronchial carcinoma, malignant melanoma,
breast cancer, urothelial carcinoma, prostate cancer and head and
neck cancer (12). Notably, MAGE-A
expression is found in leukoplakia with dysplasia, or carcinoma
in situ but not in ulcers, lichen planus or leukoplakia
without dysplasia (13). MAGE-A
tumor antigens are frequently expressed in head and neck squamous
cell carcinoma (HNSCC) primary tumors, recurrent tumors as well as
in lymph node metastasis (14). High
expression levels of MAGE-A in HNSCC tumors are predictive for a
poor overall survival (15,16). The correlation between the expression
of a particular MAGE-A subgroup and the treatment efficiency of
chemotherapeutic drugs was reported by our group earlier (17–19). Han
et al showed that there is a significant correlation between
high MAGE-A9 expression levels and a low overall survival in
laryngeal squamous cell carcinoma patients (20). Similar findings were reported for
hepatocellular and renal cell carcinoma as well as breast, ovarian,
lung and colorectal cancer (21–26).
Because radiotherapy is the backbone of non-surgical
treatment and is often used in addition to surgical treatment for
head and neck cancer, an investigation of MAGE-A9 expression in
this context is crucial.
Thus, the present study was designed to address the
following two important aspects: First, to investigate the
prognostic value of MAGE-A9 expression in HNSCC patients, and,
second, analyse the influence of irradiation on MAGE-A9 expression
to obtain a more detailed understanding of the interactions between
irradiation and MAGE-A9 as a potential marker of
radioresistance.
Materials and methods
Data collection
Paraffin-embedded head and neck squamous cell
carcinoma (HNSCC) tissue samples were collected from the archives
of the Department of Pathology at the University of Würzburg. All
samples with follow-up data of at least a year were included in our
study. The tumour sites were located on the tongue, lip, tonsil,
cheek, palate, and oropharynx.
This study was approved by the Ethics Committee of
the University of Würzburg; the reference number is 20160508
01.
Immunohistochemical staining of
MAGE-A9
MAGE-A9 expression levels in all tissue samples were
analysed by immunohistochemical staining with the MAGE-A9 antibody
(sc-130811; Santa Cruz Biotechnology, Inc., Dallas, TX, USA)
diluted to a concentration of 1:10. The detection was performed
using the DAKO Advance+ detection system (DakoCytomation, Pathology
Products Dako Deutschland GmbH, Hamburg, Germany) with DAB as the
chromogen. Hematoxylin was used for counterstaining. The staining
intensity was analysed by using the immunoreactive score (IRS) of
Remmele and Steger (27). The
immunoreactive score (IRS) was calculated as the product of the
amount of staining (SI) (0: No reaction; 1: Weak reaction; 2:
Moderately high reaction; 3: Strong reaction) and the number of
positive cells (PP) (0: Negative; 1: <10% positive cells; 2:
10–50% positive cells; 3: 21–80% positive cells; 4: >80%
positive cells) (27). The mean IRS
was calculated, and patients with an IRS below the mean were
considered ‘MAGE-A9 low’, and the others were considered ‘MAGE-A9
high’.
Cell lines
The cell lines were cultured in an atmosphere of 5%
CO2/95% air at 37°C. We split the cells two to three
times per week. All cell lines were isolated from male patients at
the Cancer Institute of the University of Pittsburgh (28) (for details see Table I). The cells were cultured in a
low-glucose DMEM medium (Gibco, Carlsbad, USA) supplemented with
10% foetal bovine serum (Gibco, Carlsbad, USA), 2 mM L-Glutamine
(Biochrom, Berlin, Germany) and 1% Pen/Strep (Promocell,
Heidelberg, Germany).
 | Table I.Names, origin, TNM-classification and
grading of the cell lines. |
Table I.
Names, origin, TNM-classification and
grading of the cell lines.
| Cell line | Origin |
TNM-classification | Grading |
|---|
| PCI 1–1 | Larynx
(glottis) | pT2 N0 M0 | G2 |
| PCI 9–1 | Base of tongue | pT4 N3 M0 | G2 |
| PCI 13-1 | Retromolar
triangle | pT4 pN1 M0 | G3 |
| PCI 52 | Aryepiglottic
fold | pT2 N0 M0 | G2 |
| PCI 68-1 | Tongue | pT4 N0 M0 | G1 |
Irradiation
For the X-irradiation, a 6 MV Siemens linear
accelerator (Siemens, Concord, CA, USA) was used at a dose rate of
2 Gy/min.
RNA isolation and reverse
transcription-quantitative polymerase chain reaction (RT-qPCR)
After separating the cells from the culture plate,
RNA isolation was performed using the TRIzol reagent (Ambion,
Carlsbad, CA, USA). The RNA concentration was measured using a Nano
Drop 2000 (Thermo Fisher Scientific, Waltham, MA, USA). The RNA
concentration was determined at a wavelength of 260 nm, using fully
desalinated water as the blank. All samples were adjusted to a
concentration of 0.2 µg RNA/ml.
Reverse transcription was performed in two steps. In
the first step, the samples were cleaned of DNA contamination using
a gDNA Wipeout Buffer (Qiagen, Venlo, the Netherlands), by heating
to 42°C for 2 min and then cooling on ice. In the second step, the
reverse transcription was performed using the QuantiTect Reverse
Transcription kit (Qiagen). The incubation temperature was 42°C for
15 min, and the denaturation temperature was 95°C for 3 min. The
detailed compositions of the reverse transcription reactions, step
1 and 2 are shown in the Tables II
and III.
 | Table II.Composition reverse transcription
step 1. |
Table II.
Composition reverse transcription
step 1.
| gDNA Wipeout
buffer, 7X | 2 µl |
|---|
| Template RNA | 5 µl |
| RNase-free
water | 7 µl |
 | Table III.Composition reverse transcription
step 2. |
Table III.
Composition reverse transcription
step 2.
| Quantiscript
reverse transcriptase | 1 µl |
|---|
| Quantiscript RT
buffer, 5X | 4 µl |
| RT primer mix | 1 µl |
| Purified RNA
sample | 14 µl |
PCR was performed using a QuantiTect SYBR-Green PCR
kit 200, QuantiTect Primer Assays (MAGE-A9: Hs_MAGEA9_1_SG,
QT00230874 and β-actin: Hs_ACTB _2_SG, QT0168047) (all from Qiagen)
and a C1000 Thermal Cycler (Bio-Rad, Hercules, CA, USA). The
composition of the PCR and the PCR cycling conditions are provided
in the Tables IV and V. The quantitative expression levels of the
tested genes were calculated as a ratio to the expression level of
β-actin.
 | Table IV.Composition of PCR. |
Table IV.
Composition of PCR.
| SYBR-Green Master
mix | 12.5 µl |
|---|
| Quantitect primer
assay | 2.75 µl |
| cDNA | 2.5 µl |
| H2O | 7.25 µl |
 | Table V.PCR protocol. |
Table V.
PCR protocol.
| Repeats | PCR-step | Duration | Temperature |
|---|
| ×1 | Polymerase
activation | 15 min | 95°C |
| ×40 | Denaturation | 15 sec | 94°C |
| ×40 | Annealing | 30 sec | 50–60°C |
| ×40 | Elongation | 30 sec | 72°C |
| ×40 | Read data |
|
|
Cells were plated in Petri dishes 24 h before
irradiation with a dose of 2 Gy. Cells were harvested, and RNA was
isolated 24 and 48 h after irradiation. Non-irradiated cells were
used as controls.
Clonogenic survival assay
Cell survival was analysed using a clonogenic
survival assay. The number of irradiated cells plated in Petri
dishes depended on the radiation dose and the cell line. The
irradiated cells were cultured in a humidified atmosphere (5%
CO2 at 37°C) for two weeks. At the end of the two weeks,
the cells were fixed using methanol and acetic acid in a ratio of
3:1 and stained using 0.1% crystal violet. All colonies consisting
of >50 cells were included for analysis.
The mean survival data were fitted to the following
linear quadratic (LQ) model: SF=exp(−αX-βX2), where SF is the
survival fraction, X is the irradiation dose, and α and β are the
fitted parameters.
Statistics
All statistical calculations were carried out using
GraphPad PRISM 6.04 (GraphPad Software, Inc., La Jolla, CA, USA).
Comparisons of clinical parameters between the MAGE-A9 high and low
group were carried out by an unpaired t test.
The tumour samples were allocated to a group with
either a lower or a higher level of MAGE-A9 expression compared to
the median level. The overall survival of the patients in the two
groups was compared using a Kaplan-Meier plot with a log-rank
test.
We analysed the impact of the irradiation dose (2
Gy) on the MAGE-A9-fold change with the use of a Wilcoxon
matched-pairs signed rank test. The correlation between the
clonogenic cell survival and the expression of MAGE-A9 subgroups
was determined using linear regression. A P-value <0.05 was
considered significant.
Results
Patient data
We included a total of 37 patients in this
retrospective analysis. Based on their MAGE-A9 IRS, 19 patients
were assigned to the ‘MAGE-A9 high’ group, and 18 patients were
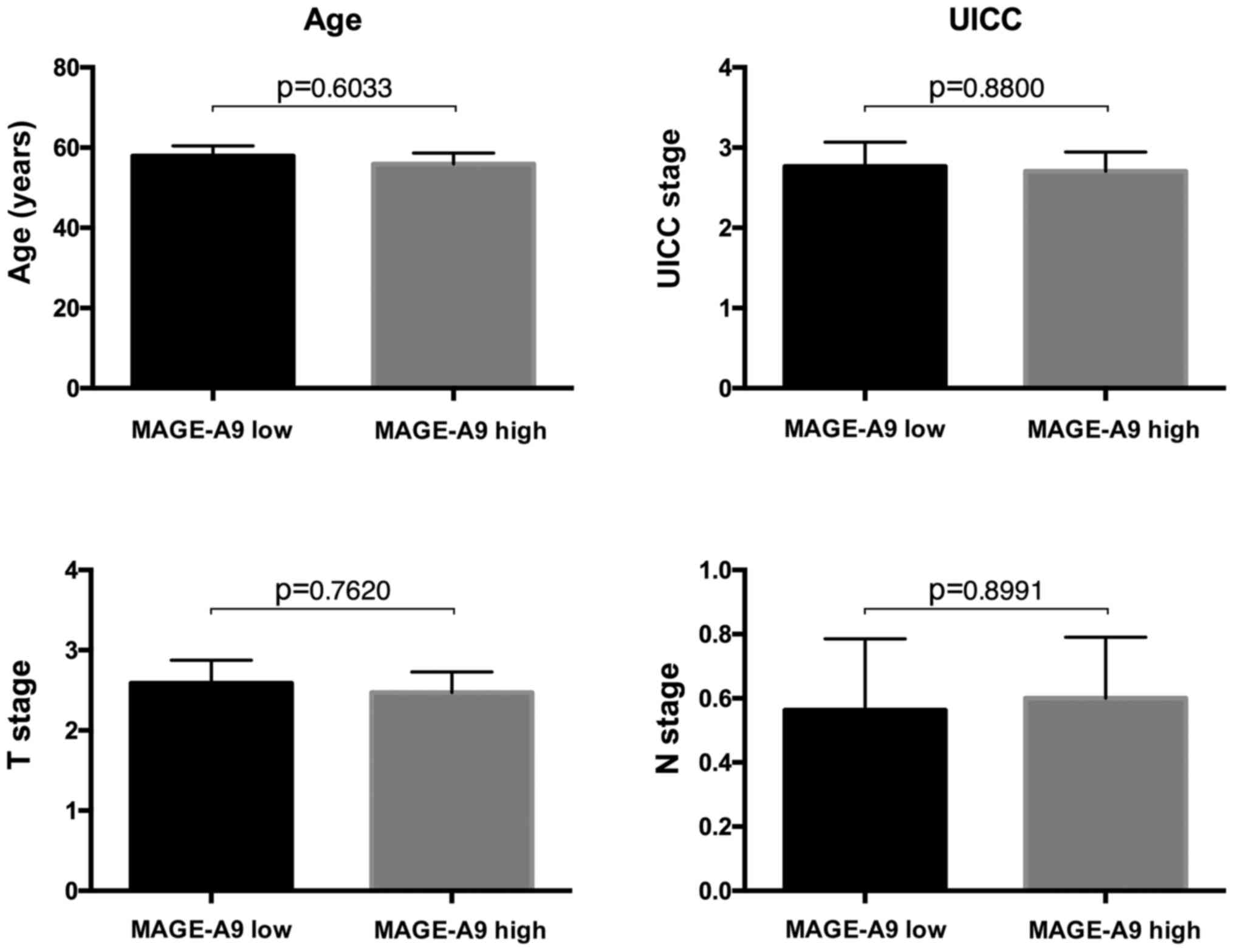
assigned to the ‘MAGE-A9 low’ group. The mean age was 57.88 years
(SD ± 11.20) in the MAGE-A9 high group and 55.92 years (SD ± 11.51)
in the MAGE-A9 low group. The mean Union for International Cancer
Control (UICC) stage was 2.765 (SD ± 1.251) and 2.706 (SD ± 0.9852)
for MAGE-A9 high and MAGE-A9 low, respectively. The mean T stage
was 2.588 (SD ± 1.176) in the high expression group and 2.471 (SD ±
1.068) in the low expression group. The mean N stage was 0.5625 (SD
± 0.8921) for the high expression group and 0.6000 (SD ± 0.7368)
for the low expression group. None of these parameters showed
significant differences between the two groups (age P=0.6033; UICC
P=0.8800; T stage P=0.7620; N stage P=0.8991) (Fig. 1). For three patients, data on the
UICC stage, T stage and N stage were not available (two in the
MAGE-A9 low group, one in the MAGE-A9 high group).
MAGE-A9 expression is correlated with
a shorter overall survival
Patient survival was significantly (P=0.0468) longer
in the group with low MAGE-A9 expression compared to the group with
high MAGE-A9 expression (Fig. 2).
The surviving fraction after 5 years was 47.4% for the high MAGE-A9
expression group and 73.7% for the low expression group.
Radiosensitivity is different in the
cell lines
Cell line-specific survival after treatment with
radiotherapy was measured with a clonogenic survival assay. As
shown in Table VI, there were
distinct differences in the cell line-specific survival among the
investigated cell lines. Measuring the survival fraction after
radiation with 2 Gy (SF2), the cell line PCI 9–1 had the lowest SF2
(SF2=0.184), and the cell line PCI 68-1 had the highest SF2
(SF2=0.479) (Table VI).
Intermediate SF2s were determined for the cell lines PCI 13-1
(SF2=0.421), PCI 52 (SF2=0.349) and PCI 1-1 (SF2=0.239). In
addition, a calculation of the radiation dose, which was necessary
to achieve a surviving fraction of 0.1 (D10), suggests
that the PCI 9-1 cell line was the most radiosensitive cell line
(D10=2.693 Gy), and the PCI 68-1 cell line was the most
radioresistant cell line (D10=5.745 Gy) (Table VI). Similar to the SF2 measurements,
the second highest D10 was observed in the cell line PCI
13-1 (D10=4.533 Gy), the third highest in the cell line
PCI 52 (D10=3.998 Gy) and the second lowest in the cell
line PCI 1–1 (D10=3.228 Gy), as shown in Table VI.
 | Table VI.Mean value of the surviving fraction
(SF) after irradiation with 2 Gy, D10 (Gy), α (Gy-1) and β
(Gy-2). |
Table VI.
Mean value of the surviving fraction
(SF) after irradiation with 2 Gy, D10 (Gy), α (Gy-1) and β
(Gy-2).
| Cell line | SF2 | D10 (Gy) | α (Gy-1) | β (Gy-2) |
|---|
| PCI 1–1 | 0.239±0.010 | 3.228 | 0.311±0.011 | 1.4E-15±0.003 |
| PCI 9–1 | 0.184±0.017 | 2.693 | 0.357±0.030 | 0.005±0.006 |
| PCI 13-1 | 0.421±0.029 | 4.533 | 0.162±0.021 | 0.013±0.004 |
| PCI 52 | 0.349±0.058 | 3.998 | 0.207±0.059 | 0.011±0.014 |
| PCI 68-1 | 0.479±0.034 | 5.745 | 0.152±0.021 | 0.004±0.003 |
Irradiation induces MAGE-A9
expression
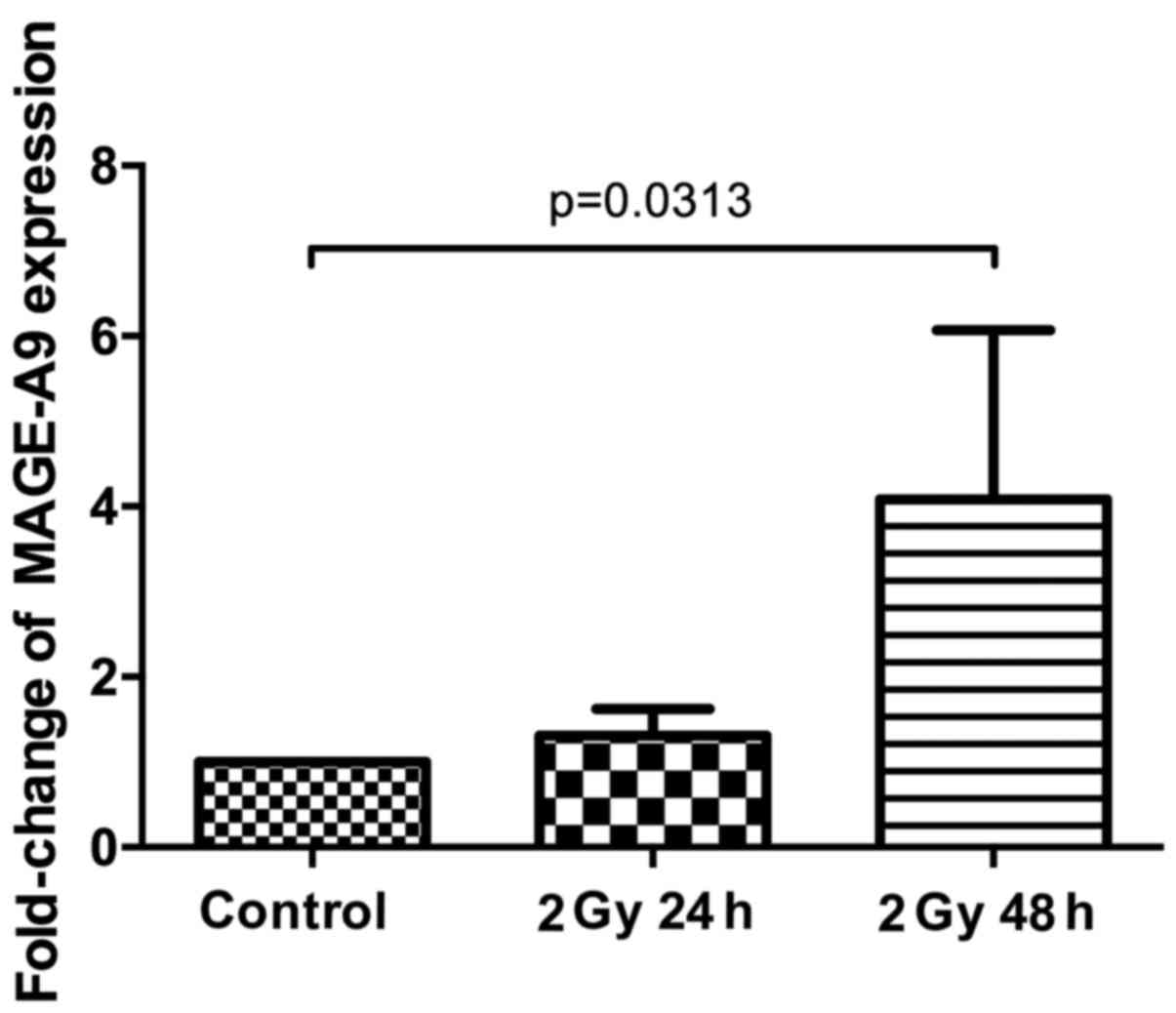
We next analysed the fold-change of MAGE-A9
expression by RT-PCR in relation to the non-irradiated control
cells, adjusted to the housekeeping gene β-actin. After 24 h, the
average MAGE-A9 expression was increased by 1.31 (PCI 1–1: 1.74;
PCI 9-1: 1.12; PCI 13-1: 0.43; PCI 52: 1.00; PCI 68-1: 2.24);
however, this fold-change was not significantly different from that
of the non-irradiated controls (P=0.3125). After 48 h, the average
MAGE-A9 expression was increased by 4.08-fold compared to the
non-irradiated controls (PCI 1-1: 3.24; PCI 9-1: 1.95; PCI 13-1:
1.63; PCI 52: 1.62; PCI 68-1: 11.95). Compared to the
non-irradiated controls, the enhancement of MAGE-A9 expression was
statistically significant (P=0.0313) (Fig. 3). Additionally, the fold-change of
MAGE-A9 expression was time-dependent as we observed an increase in
the expression level of all of the cell lines from the 24 h
measurements to the 48 h measurements. Based on a Wilcoxon
matched-pairs signed rank test, this finding is also statistically
significant (P=0.0313).
Importantly, the most prominent fold-change of
MAGE-A9 expression was observed in the PCI 68-1 cell line (24 h
after irradiation: 2.24, 48 h after irradiation: 11.95). Based on
the clonogenic assay, the PCI 68-1 cell line was also the most
radioresistant cell line (D10=5.75 Gy). However, the
correlation between MAGE-A9-fold change and radiosensitivity of the
five investigated cell lines was not statistically significant
(R2=0.5648, P=0.1430) but showed a trend.
Discussion
To the best of our knowledge, we are the first to
report on irradiation and MAGE-A9 expression in head and neck
cancer cells. We show a correlation between poor overall survival
and MAGE-A9 expression in a cohort of head and neck cancer
patients. Furthermore, we provide evidence that MAGE-A9 mRNA
expression is induced by irradiation. This effect is time-dependent
and significant. The most radioresistant cell line showed the most
prominent increase in MAGE-A9 expression; however, linear
regression analysis did not show a statistically significant
correlation between MAGE-A9 induction and radiosensitivity for any
tested cell line.
Adjuvant radiotherapy in addition to surgery is an
essential element of therapy for closed or positive margin and/or
nodal positive HNSCC to improve locoregional tumour control
(5,29,30).
Primary radiotherapy is a potentially curative option in surgical
unresectable HNSCC, often in combination with radiosensitizing
chemotherapy (6). Both adjuvant and
primary radiotherapies have a positive influence on disease
eradication and overall patient survival (5,6,29,30).
The nearly exclusive expression of the MAGE-A family
members in malignancies makes them an interesting subject for
cancer research (16,13,31). As
shown by Figueiredo et al, MAGE-A tumour antigens are
frequently expressed in HNSCC (31).
Simultaneous cytoplasmic and nuclear protein expression of MAGE-A
has been identified as an independent marker for poor survival in
HNSCC patients (15). Compared to
cancer/testis antigens, e.g., New York oesophageal squamous cell
carcinoma-1 (NY-ESO-1), MAGE-A antigens are significantly more
often expressed in epithelial cancers (123 (3.4%) vs. 815 (22.2%)
of 3668 epithelial cancer cases), particular in HNSCC (6 (3.8%) vs.
71 (41.7%) of 158 HNSCC cases) (32). MAGE-A1-6 expression in patients
sputum correlated with higher incidences of a second primary cancer
and poor oncologic outcome in larynx and hypopharynx squamous cell
carcinoma patients (33), and high
MAGE-A expression levels are correlated with malignant
transformation of oral leukoplakia (34).
Recently, it has been shown that MAGE-A9 expression
in laryngeal, hepatocellular and renal cell carcinoma, as well as
in breast, ovarian, lung and colorectal cancer, is a negative
prognostic marker (20–26). Based on our patient cohort, we can
confirm a significant correlation (P=0.0468) between high MAGE-A9
expression levels and lower overall patient survival. Importantly,
both groups were well balanced in terms of age, UICC stage, T stage
and N stage. These parameters are well-known confounds of overall
survival in head and neck cancer patient cohorts. One limitation of
our study is that patients HPV status was unknown. However, the age
of approximately 60 years, high T stage and low N stage indicate a
more classical, HPV-negative cohort. Additionally, the tissue
collection was performed approximately 15 years prior to this
study, when the rate of HPV-related HNSCC was lower.
There are only a few studies concerning the role of
MAGE-A expression in cancer cells. Various groups have reported on
the direct and indirect inhibitory effects of MAGE-A proteins on
the function of the tumour suppressor p53 (35–37),
suggesting a role of MAGE-A proteins in DNA damage response.
Recently the effect of MAGE-A11 expression on cisplatin resistance
in HNSCC cells could be shown (38).
The results of the qPCR showed a clear and
time-dependent increase of MAGE-A9 mRNA expression after
irradiation with 2 Gy. Interestingly, Picard et al observed
an induction of MAGE-A9 expression in bladder cancer cells after
treatment with chemotherapeutic drugs as the methylation inhibitor
5-AZA-DC was induced (39). A
further upregulation of MAGE-A9 expression was observed by
co-incubation of the deacetylase inhibitors MS-275 and
4-phenylbutyrate together with 5-AZA-DC but not with either agent
alone (39). The gene silencing by
promoter hypermethylation and histone deacetylation were shown for
the subgroups MAGE-A1, MAGE-A2, MAGE-A3 and MAGE-A12 in different
human cancer cells (40).
Cacan et al showed that irradiation with 5 Gy
increases the histone acetylation in colorectal cancer cells
(41). It could be hypothesized that
the irradiation-induced histone acetylation causes MAGE-A9
upregulation after irradiation. Based on The Cancer Genome Atlas
data, MAGE-A9 copy number gain or amplification is highly
significantly correlated with Rac1 gain or amplification
(P<0.001). Rac1 is one of the key proteins responsible for
cancer cell motility and tissue invasion while interacting for
example with the cytoskeletal protein lamellipodium. Additionally,
the co-occurrence of alterations in MAGE-A9 and Rac1 is highly
significantly associated with poorer overall survival (median
survival in the altered group: 35.45 months; in the unaltered
group: 67.81 months; P=0.00299) in HNSCC (42,43).
Rac1 is a known factor contributing to chemoresistance and
radioresistance, particularly in HNSCC (44–46).
Our findings provide evidence for a link between
irradiation and MAGE-A9 expression. Because irradiation is a major
treatment in head and neck cancer, and given that MAGE-A9
expression has been shown to be a marker of a worse prognosis in
several tumor entities, including head and neck cancer, this link
warrants further investigation.
MAGE-A9 protein could be an interesting candidate to
become part of a biomarker panel for personalized therapy, because
of the prognostic characteristics and the potential role as a
target protein.
High MAGE-A9 expression levels correlated
significantly with shorter patient survival in head and neck
squamous cell carcinoma patients. MAGE-A9 mRNA levels were
significantly elevated by irradiation with 2 Gy in five head and
neck squamous cell carcinoma cell lines. The most prominent
fold-change in MAGE-A9 expression was observed in the most
radioresistant cell line. Thus, MAGE-A9 may play a role in
radioresistance in head and neck cancer.
Acknowledgements
Language editing support was provided by the
American Journal Experts (AJE). The present study was supported by
the Interdisciplinary Center for Clinical Research Würzburg (IZKF
Würzburg, to S. Hartmann).
Competing interests
The authors declare that they have no competing
interests.’
Glossary
Abbreviations
Abbreviations:
|
MAGE-A
|
melanoma associated antigen-A
|
|
UICC
|
union for international cancer
control
|
|
IRS
|
immunoreactive score
|
|
SI
|
amount of staining
|
|
PP
|
number of positive cells
|
|
SF2
|
surviving fraction at 2 Gy
|
|
D10
|
dose in Gy for gaining SF10
|
|
SC
|
standardized coefficient
|
|
NY-ESO-1
|
New York oesophageal squamous cell
carcinoma-1
|
References
|
1
|
Ferlay J, Soerjomataram I, Dikshit R, Eser
S, Mathers C, Rebelo M, Parkin DM, Forman D and Bray F: Cancer
incidence and mortality worldwide: Sources, methods and major
patterns in GLOBOCAN 2012. Int J Cancer. 136:E359–E386. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Gupta S, Kong W, Peng Y, Miao Q and
Mackillop WJ: Temporal trends in the incidence and survival of
cancers of the upper aerodigestive tract in Ontario and the United
States. Int J Cancer. 125:2159–2165. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Pulte D and Brenner H: Changes in survival
in head and neck cancers in the late 20th and early 21st century: A
period analysis. Oncologist. 15:994–1001. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Fakhry C, Westra WH, Li S, Cmelak A, Ridge
JA, Pinto H, Forastiere A and Gillison ML: Improved survival of
patients with human papillomavirus-positive head and neck squamous
cell carcinoma in a prospective clinical trial. J Natl Cancer Inst.
100:261–269. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Zelefsky MJ, Harrison LB, Fass DE,
Armstrong JG, Shah JP and Strong EW: Postoperative radiation
therapy for squamous cell carcinomas of the oral cavity and
oropharynx: Impact of therapy on patients with positive surgical
margins. Int J Radiat Oncol Biol Phys. 25:17–21. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Adelstein DJ, Li Y, Adams GL, Wagner H Jr,
Kish JA, Ensley JF, Schuller DE and Forastiere AA: An intergroup
phase III comparison of standard radiation therapy and two
schedules of concurrent chemoradiotherapy in patients with
unresectable squamous cell head and neck cancer. J Clin Oncol.
21:92–98. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Scanlan MJ, Simpson AJ and Old LJ: The
cancer/testis genes: Review, standardization, and commentary.
Cancer Immun. 4:12004.PubMed/NCBI
|
|
8
|
Sang M, Lian Y, Zhou X and Shan B: MAGE-A
family: Attractive targets for cancer immunotherapy. Vaccine.
29:8496–8500. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Scanlan MJ, Gure AO, Jungbluth AA, Old LJ
and Chen YT: Cancer/testis antigens: An expanding family of targets
for cancer immunotherapy. Immunol Rev. 188:22–32. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Atanackovic D, Altorki NK, Stockert E,
Williamson B, Jungbluth AA, Ritter E, Santiago D, Ferrara CA,
Matsuo M, Selvakumar A, et al: Vaccine-induced CD4+ T cell
responses to MAGE-3 protein in lung cancer patients. J Immunol.
172:3289–3296. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Müller-Richter UD, Dowejko A, Zhou W,
Reichert TE and Driemel O: Different expression of MAGE-A-antigens
in foetal and adult keratinocyte cell lines. Oral Oncol.
44:628–633. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Sang M, Wang L, Ding C, Zhou X, Wang B,
Wang L, Lian Y and Shan B: Melanoma-associated antigen genes-an
update. Cancer Lett. 302:85–90. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Krauss E, Rauthe S, Gattenlöhner S,
Reuther T, Kochel M, Kriegebaum U, Kübler AC and Müller-Richter UD:
MAGE-A antigens in lesions of the oral mucosa. Clin Oral Investig.
15:315–320. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Laban S, Giebel G, Klümper N, Schröck A,
Doescher J, Spagnoli G, Thierauf J, Theodoraki MN, Remark R,
Gnjatic S, et al: MAGE expression in head and neck squamous cell
carcinoma primary tumors, lymph node metastases and respective
recurrences-implications for immunotherapy. Oncotarget.
8:14719–14735. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Laban S, Atanackovic D, Luetkens T, Knecht
R, Busch CJ, Freytag M, Spagnoli G, Ritter G, Hoffmann TK, Knuth A,
et al: Simultaneous cytoplasmic and nuclear protein expression of
melanoma antigen-A family and NY-ESO-1 cancer-testis antigens
represents an independent marker for poor survival in head and neck
cancer. Int J Cancer. 135:1142–1152. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Cuffel C, Rivals JP, Zaugg Y, Salvi S,
Seelentag W, Speiser DE, Liénard D, Monnier P, Romero P, Bron L and
Rimoldi D: Pattern and clinical significance of cancer-testis gene
expression in head and neck squamous cell carcinoma. Int J Cancer.
128:2625–2634. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Hartmann S, Meyer TJ, Brands RC, Haubitz
IR, Linz C, Seher A, Kübler AC and Müller-Richter UD: MAGE-A
expression clusters and antineoplastic treatment in head and neck
cancer. Int J Mol Med. 35:1675–1682. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Hartmann S, Kriegebaum U, Küchler N,
Brands RC, Linz C, Kübler AC and Müller-Richter UD: Correlation of
MAGE-A tumor antigens and the efficacy of various chemotherapeutic
agents in head and neck carcinoma cells. Clin Oral Investig.
18:189–197. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Hartmann S, Kriegebaum U, Küchler N,
Lessner G, Brands RC, Linz C, Schneider T, Kübler AC and
Müller-Richter UD: Efficacy of cetuximab and panitumumab in oral
squamous cell carcinoma cell lines: Prognostic value of MAGE-A
subgroups for treatment success. J Craniomaxillofac Surg.
41:623–629. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Han L, Jiang B, Wu H, Zhang S and Lu X:
Expression and prognostic value of MAGE-A9 in laryngeal squamous
cell carcinoma. Int J Clin Exp Pathol. 7:6734–6742. 2014.PubMed/NCBI
|
|
21
|
Gu X, Fu M, Ge Z, Zhan F, Ding Y, Ni H,
Zhang W, Zhu Y, Tang X, Xiong L, et al: High expression of MAGE-A9
correlates with unfavorable survival in hepatocellular carcinoma.
Sci Rep. 4:66252014. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Hatiboglu G, Pritsch M, Macher-Goeppinger
S, Zöller M, Huber J, Haferkamp A, Pahernik S, Wagener N and
Hohenfellner M: Prognostic value of melanoma-associated antigen A9
in renal cell carcinoma. Scand J Urol. 47:311–322. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Xu X, Tang X, Lu M, Tang Q, Zhang H, Zhu
H, Xu N, Zhang D, Xiong L, Mao Y and Zhu J: Overexpression of
MAGE-A9 predicts unfavorable outcome in breast cancer. Exp Mol
Pathol. 97:579–584. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Xu Y, Wang C, Zhang Y, Jia L and Huang J:
Overexpression of MAGE-A9 is predictive of poor prognosis in
epithelial ovarian cancer. Sci Rep. 5:121042015. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Zhai X, Xu L, Zhang S, Zhu H, Mao G and
Huang J: High expression levels of MAGE-A9 are correlated with
unfavorable survival in lung adenocarcinoma. Oncotarget.
7:4871–4881. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Zhan W, Zhang Z, Zhang Y, Ma J, Wu T, Gu
Y, Li Y and Yang J: Prognostic value of MAGE-A9 expression in
patients with colorectal cancer. Clin Res Hepatol Gastroenterol.
40:239–245. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Remmele W and Stegner HE: Recommendation
for uniform definition of an immunoreactive score (IRS) for
immunohistochemical estrogen receptor detection (ER-ICA) in breast
cancer tissue. Pathologe. 8:138–140. 1987.(In German). PubMed/NCBI
|
|
28
|
Heo DS, Snyderman C, Gollin SM, Pan S,
Walker E, Deka R, Barnes EL, Johnson JT, Herberman RB and Whiteside
TL: Biology, cytogenetics, and sensitivity to immunological
effector cells of new head and neck squamous cell carcinoma lines.
Cancer Res. 49:5167–5175. 1989.PubMed/NCBI
|
|
29
|
Cooper JS, Pajak TF, Forastiere AA, Jacobs
J, Campbell BH, Saxman SB, Kish JA, Kim HE, Cmelak AJ, Rotman M, et
al: Postoperative concurrent radiotherapy and chemotherapy for
high-risk squamous-cell carcinoma of the head and neck. N Engl J
Med. 350:1937–1944. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Bernier J, Domenge C, Ozsahin M,
Matuszewska K, Lefèbvre JL, Greiner RH, Giralt J, Maingon P,
Rolland F, Bolla M, et al: Postoperative irradiation with or
without concomitant chemotherapy for locally advanced head and neck
cancer. N Engl J Med. 350:1945–1952. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Figueiredo DL, Mamede RC, Proto-Siqueira
R, Neder L, Silva WA Jr and Zago MA: Expression of cancer testis
antigens in head and neck squamous cell carcinomas. Head Neck.
28:614–619. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Kerkar SP, Wang ZF, Lasota J, Park T,
Patel K, Groh E, Rosenberg SA and Miettinen MM: MAGE-A is more
highly expressed than NY-ESO-1 in a systematic immunohistochemical
analysis of 3668 cases. J Immunother. 39:181–187. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Lee KD, Lee HS, Kim SW, Park T, Hong JC,
Chang HK, Jung SB, Jeon CH and Park JW: Clinical significance of
melanoma-associated antigen A1-6 expression in sputum of patients
with squamous cell carcinoma of the larynx and hypopharynx. Head
Neck. 38 Suppl 1:E736–E740. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Ries J, Agaimy A, Vairaktaris E, Kwon Y,
Neukam FW, Strassburg LH and Nkenke E: Evaluation of MAGE-A
expression and grade of dysplasia for predicting malignant
progression of oral leukoplakia. Int J Oncol. 41:1085–1093. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Marcar L, Maclaine NJ, Hupp TR and Meek
DW: Mage-A cancer/testis antigens inhibit p53 function by blocking
its interaction with chromatin. Cancer Res. 70:10362–10370. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Monte M, Simonatto M, Peche LY, Bublik DR,
Gobessi S, Pierotti MA, Rodolfo M and Schneider C: MAGE-A tumor
antigens target p53 transactivation function through histone
deacetylase recruitment and confer resistance to chemotherapeutic
agents. Proc Natl Acad Sci USA. 103:pp. 11160–11165. 2006;
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Yang B, Oherrin SM, Wu J, Reagan-Shaw S,
Ma Y, Bhat KM, Gravekamp C, Setaluri V, Peters N, Hoffmann FM, et
al: MAGE-A, mMage-b, and MAGE-C proteins form complexes with KAP1
and suppress p53-dependent apoptosis in MAGE-positive cell lines.
Cancer Res. 67:9954–9962. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Hartmann S, Zwick L, Scheurer MJJ, Fuchs
AR, Brands RC, Seher A, Böhm H, Kübler AC and Müller-Richter UDA:
MAGE-A11 expression contributes to cisplatin resistance in head and
neck cancer. Clin Oral Investig. Oct 15–2017.(Epub ahead of print).
View Article : Google Scholar
|
|
39
|
Picard V, Bergeron A, Larue H and Fradet
Y: MAGE-A9 mRNA and protein expression in bladder cancer. Int J
Cancer. 120:2170–2177. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Wischnewski F, Pantel K and Schwarzenbach
H: Promoter demethylation and histone acetylation mediate gene
expression of MAGE-A1, -A2, -A3, and -A12 in human cancer cells.
Mol Cancer Res. 4:339–349. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Cacan E, Greer SF and Garnett-Benson C:
Radiation-induced modulation of immunogenic genes in tumor cells is
regulated by both histone deacetylases and DNA methyltransferases.
Int J Oncol. 47:2264–2275. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Gao J, Aksoy BA, Dogrusoz U, Dresdner G,
Gross B, Sumer SO, Sun Y, Jacobsen A, Sinha R, Larsson E, et al:
Integrative analysis of complex cancer genomics and clinical
profiles using the cBioPortal. Sci Signal. 6:pl12013. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Cerami E, Gao J, Dogrusoz U, Gross BE,
Sumer SO, Aksoy BA, Jacobsen A, Byrne CJ, Heuer ML, Larsson E, et
al: The cBio cancer genomics portal: An open platform for exploring
multidimensional cancer genomics data. Cancer Discov. 2:401–404.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Yan Y, Hein AL, Etekpo A, Burchett KM, Lin
C, Enke CA, Batra SK, Cowan KH and Ouellette MM: Inhibition of RAC1
GTPase sensitizes pancreatic cancer cells to γ-irradiation.
Oncotarget. 5:10251–10270. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Skvortsov S, Jimenez CR, Knol JC,
Eichberger P, Schiestl B, Debbage P, Skvortsova I and Lukas P:
Radioresistant head and neck squamous cell carcinoma cells:
Intracellular signaling, putative biomarkers for tumor recurrences
and possible therapeutic targets. Radiother Oncol. 101:177–182.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Skvortsov S, Dudás J, Eichberger P,
Witsch-Baumgartner M, Loeffler-Ragg J, Pritz C, Schartinger VH,
Maier H, Hall J, Debbage P, et al: Rac1 as a potential therapeutic
target for chemo-radioresistant head and neck squamous cell
carcinomas (HNSCC). Br J Cancer. 110:2677–2687. 2014. View Article : Google Scholar : PubMed/NCBI
|