Introduction
Uterine sarcomas are relatively rare types of
malignancies, comprising 1–3% of uterine malignancies (1). There are numerous histological
subtypes, and it is general consensus that the most common
histological subtype is leiomyosarcoma (LMS), accounting for 60–70%
of all cases, followed by endometrial stromal sarcoma (ESS),
~15–20% cases, worldwide (2).
However, in Japan, carcinosarcoma (CS) represents 46% of uterine
sarcoma diagnoses, followed by LMS (36%) and then ESS (13%)
(3). Uterine sarcomas are some of
the most malignant and aggressive tumors among the gynecologic
malignancies, and often present with hematogenous metastasis in the
early stages. Furthermore, they are basically refractory to
treatments for relapsed tumors, such as chemotherapy and
radiotherapy, resulting in a poor prognosis and a high recurrence
rate. The recurrence rate has been reported to be 45–73% in LMS
(4,5), 37% in ESS (6) and >50% in CS (7). The median survival times of patients
with ESS, LMS and CS have been reported to be 76, 31 and 28 months,
respectively (3). Hysterectomy with
bilateral oophorectomy is often performed as the primary standard
treatment for early-stage uterine sarcomas. Conversely, the
management strategy for patients with recurrent uterine sarcomas
has not been well established. The efficacy of resection for lung
metastasis of uterine malignancies has been demonstrated in prior
studies (8–11); nevertheless, no consensus has been
reached regarding the optimal treatment strategy at the time of
relapse due to their rarity. The objective of the present study was
to evaluate the efficacy of secondary cytoreductive surgery (SCS)
for the treatment of recurrent uterine sarcomas.
Patients and methods
Patients
A retrospective study was conducted at the
Department of Obstetrics and Gynecology, Nagoya University (Nagoya,
Japan) using a computerized database, and 35 females with
pathologically verified sarcoma who underwent primary or recurrent
treatment between January 2002 and December 2015 were identified.
In the 35 patients with uterine sarcomas, 18 patients who
experienced recurrent disease in the study period were included in
the present study. Clinicopathological data from patients' medical
records were collected, including the age, pathological diagnosis,
stage, initial treatment, details of treatment, relapse
information, follow-up and survival data. The tumor stage was based
on the criteria of the International Federation of Gynecology and
Obstetrics (2008). Written informed consent to collect and use
their data for clinical research was acquired from all patients.
The study protocol was approved by the Ethics Committee of Nagoya
University Graduate School of Medicine (approval no.
2013-0078).
Follow-up
At the conclusion of the initial treatment, all
patients underwent a strict follow-up regime, consisting of the
following clinical checkups: Pelvic examination, ultrasonographic
scans, magnetic resonance imaging (MRI), periodic computed
tomography (CT) and/or positron emission tomography (PET).
Recurrence was defined as the presence of a relapsed tumor based on
the results of CT and/or MRI and/or PET-CT and/or ultrasound.
Statistical analysis
Patient data were censored at the time of mortality
or final contact due to causes other than uterine sarcoma or
disease-associated complications. The distributions of
clinicopathological events were evaluated using Fisher's exact
test. Kaplan-Meier curves were generated from the survival data.
All statistical analyses were performed with EZR version 1.32
(Saitama Medical Center, Jichi Medical University, Saitama, Japan),
which is a graphical user interface for R (The R Foundation for
Statistical Computing, Vienna, Austria). Specifically, EZR is a
modified version of the R commander designed to add statistical
functions frequently used in biostatistics. A value of P<0.05
was considered to indicate a statistically significant
difference.
Results
Patients' characteristics
Details of the patients' characteristics are listed
in Table I. During the study period,
a total of 18 patients with recurrent uterine sarcomas were
identified. Of these, 8 (44.4%), 6 (33.3%) and 3 (16.7%) patients
had a diagnosis of LMS, CS and ESS, respectively. The one remaining
(5.6%) patient had another type of uterine sarcoma. The median age
at the time of SCS was 61.5 years (range, 33–71 years) and the
median follow-up time, including deceased patients, was 29.7 months
[range, 2.7–358.3 months (due to initial treatment in 1986 for one
case)]. A total of 9 (50.0%), 6 (33.3%) and 3 (16.7%) patients were
diagnosed with FIGO stage I, III and IV, respectively. All patients
underwent primary debulking surgery as the initial treatment. A
total of 17 patients (88.9%) underwent at least
hysterectomy-including surgery, and 4 patients received pelvic
lymphadenectomy as well as hysterectomy (22.2%). The pathological
diagnosis of the 4 patients who underwent pelvic lymphadenectomy
was CS in all cases. The tumor was completely removed by primary
surgery in 16 patients (88.9%). Among the 18 patients with
recurrent uterine sarcomas, SCS was performed in 9 patients. The
SCS group contained 3 patients with ESS, while there were no
patients with this histology in the non-SCS group. Additionally,
the rate of having a progression-free interval (PFI) >6 months
was higher in the SCS patient group than in the non-SCS patient
group [SCS group, 88.9% (8/9) vs. non-SCS group, 44.4% (4/9);
P=0.0231 (Fisher's exact test)].
 | Table I.Patients' characteristics. |
Table I.
Patients' characteristics.
|
|
| SCS |
|---|
|
|
|
|
|---|
|
|
| Yes | No |
|---|
|
|
|
|
|
|---|
| Characteristics | Total n | n (%) | n (%) |
|---|
| Total | 18 | 9 | 9 |
| Median age at
recurrence | 61.5 | 62.0 | 54 |
| Range
(years) | 33–71 | 33–71 | 37–68 |
| Median follow-up
(Mo) | 29.7 | 90.5 | 22.9 |
| Range
(months) | (2.7–358.3) | (11.1–358.3) | (2.7–58.9) |
| Histological
type |
|
|
|
| LMS | 8 | 3 (33.3) | 5 (55.6) |
| CS | 6 | 2 (22.2) | 4 (44.4) |
| ESS | 3 | 3 (33.3) | 0 (0) |
|
Others | 1 | 1 (11.1) | 0 (0) |
| FIGO stage |
|
|
|
| I | 9 | 7 (77.8) | 2 (22.2) |
| II | 0 | 0 (0) | 0 (0) |
| III | 6 | 2 (22.2) | 4 (44.4) |
| IV | 3 | 0 (0) | 3 (33.3) |
| Initial surgery |
|
|
|
|
ATH+BSO | 13 | 7 (77.8) | 6 (66.7) |
|
ATH+BSO+PLN | 4 | 2 (22.2) | 2 (22.2) |
| Tumor
resection | 1 | 0 (0) | 1 (11.1) |
| Residual tumor at
primary surgery |
|
|
|
|
Complete | 16 | 8 (88.9) | 8 (88.9) |
|
Incomplete | 2 | 1 (11.1) | 1 (11.1) |
| No. of total
chemotherapy regimens |
|
|
|
| 0 | 2 | 2 (22.2) | 0 (0) |
| 1 | 5 | 1 (11.1) | 4 (44.4) |
| 2 | 11 | 6 (66.7) | 5 (55.6) |
|
Progression/recurrence-free interval |
|
|
|
| ≥6
months | 12 | 8 (88.9) | 4 (44.4) |
| <6
months | 6 | 1 (11.1) | 5 (55.6) |
| Outcomes |
|
|
|
| NED | 3 | 3 (33.3) | 0 |
| AWD | 2 | 2 (22.2) | 0 |
| DOD | 13 | 4 (44.4) | 9 (100) |
Analysis of treatment outcomes and
recurrence
Table II lists the
recurrence sites for 18 patients who experienced recurrent uterine
sarcomas. In the SCS group, 5/9 patients had confined pelvic
recurrences, 3 patients had extra-pelvic diseases, including
pulmonary metastasis, and one patient had intra- and extra-pelvic
recurrence. Conversely, of nine patients in the non-SCS group, 4
had confined pelvic recurrences, 2 had extra-pelvic diseases,
including pulmonary metastasis, and 3 had intra- and extra-pelvic
recurrence. The details of the SCS are presented in Table III. It is notable that 4 patients
had a number of surgeries following SCS, the highest number being 8
further surgical procedures. Furthermore, no serious complications
of the SCS were observed in the current study. As salvage therapy
for 9 patients treated without SCS, 8 underwent subsequent
chemotherapy and 1 patient was treated with radiotherapy.
 | Table II.The site of recurrence in patients
with or without SCS. |
Table II.
The site of recurrence in patients
with or without SCS.
|
|
| SCS |
|---|
|
|
|
|
|---|
|
|
| Yes | No |
|---|
|
|
|
|
|
|---|
| Recurrence
site | Total n | n (%) | n (%) |
|---|
| Intrapelvic
recurrence | 9 | 5 (55.6) | 4 (44.4) |
| Extrapelvic
recurrence | 6 | 3 (33.3) | 3 (33.3) |
| Both | 3 | 1 (11.1) | 2 (22.2) |
 | Table III.Details of secondary cytoreductive
surgery. |
Table III.
Details of secondary cytoreductive
surgery.
|
|
|
|
| SCS |
|
|
|
|
|---|
|
|
|
|
|
|
|
|
|
|
|---|
| Case | Histology | Rec. site | No. metastasis | Surgery | Time (min) | Blood loss
(ml) | RT | No. of surgery | Outcomes | PFI (months) |
|---|
| 1 | Others | Pelvis | 14 | Met | 315 | 2,007 |
Incompletea | 1 | DOD | 3.1 |
| 2 | CS | Pelvis | 8 | PM, Col | 389 | 1,940 |
Completeb | 2 | DOD | 6.9 |
| 3 | LS | Omentum | 1 | BSO, OM | N/A | N/A | Complete | 9 | DOD | 8.6 |
| 4 | LS | Pelvis | 1 | Met | 103 | 147 | Complete | 1 | NED | 63.7 |
| 5 | ESS | Lung | 6 | Met | 127 | 20 | Complete | 3 | NED | 48.0 |
| 6 | ESS | Lung, pelvis | 3 | Met | 1,073 | 6,362 | Complete | 3 | AWD | 31.7 |
| 7 | ESS | Inguinal LN | 1 | Met | 41 | 28 |
Residualc | 1 | DOD | 14.4 |
| 8 | CS | Pelvis | 1 | Met, Col | 239 | 668 | Complete | 1 | DOD | 18.2 |
| 9 | LMS | Pelvis | 2 | Met, Col | 420 | 650 | Complete | 1 | NED | 43.2 |
The re-recurrence rate and PFI following SCS are
presented in Table IV. Although the
patient number was limited, relatively good disease control was
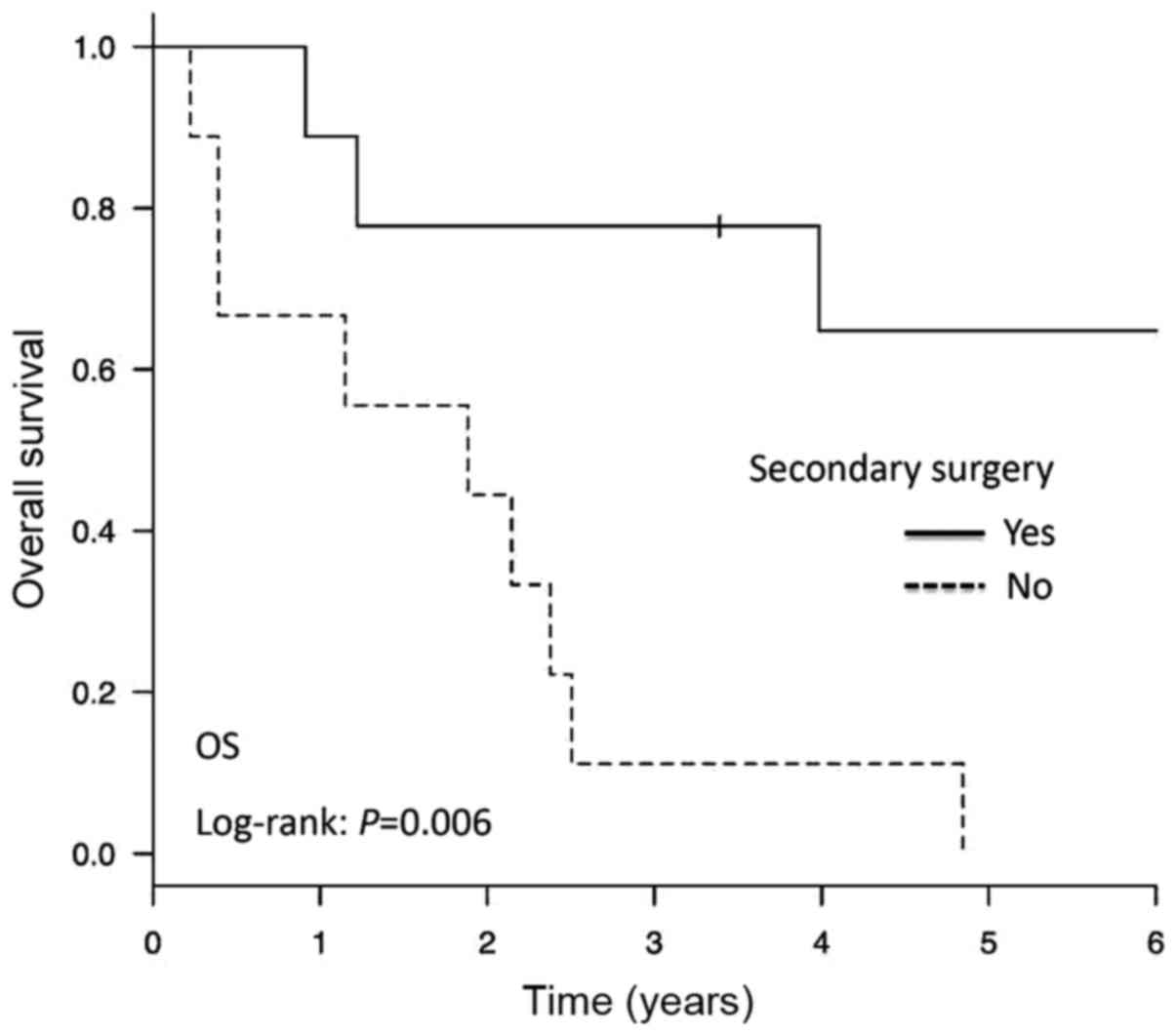
observed in patients with LMS and CS. The 3-year OS rates were 77.8
and 11.1% in the SCS and non-SCS groups, respectively. As presented
in Fig. 1, patients who underwent
SCS experienced a significantly longer OS than those who did not
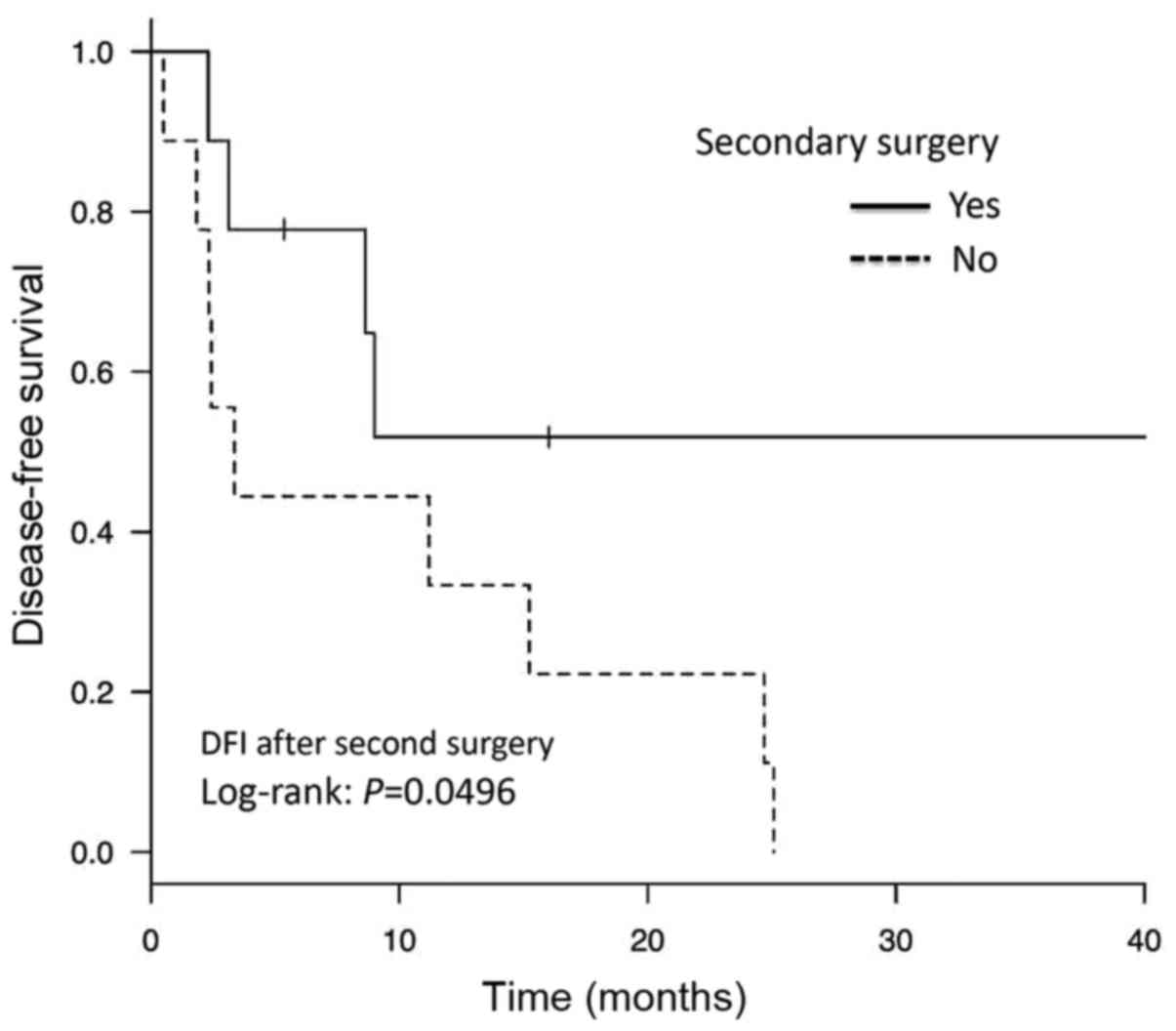
receive SCS (P=0.006). Additionally, the PFI post-SCS was
significantly longer in the SCS group compared with that in the
non-SCS group (P=0.0496; Fig.
2).
 | Table IV.Frequency of recurrence after
secondary cytoreduction surgery. |
Table IV.
Frequency of recurrence after
secondary cytoreduction surgery.
| Histology | Frequency of
recurrence, n (%) | Median PFI
(months) |
|---|
| LMS | 1/3 (33.3) | 8.6 |
| CS | 2/2 (100) | 12.5
(9.0–16.0) |
| ESS | 2/3 (66.7) | 3.8 (2.3–5.4) |
| Other | 1/1 (100) | 3.1 |
| Total | 6/9 (66.7) | 9.7 (2.3–16.0) |
Discussion
Due to the rarity and heterogeneity of uterine
sarcomas, optimal management strategies have not yet been
established (12). Furthermore,
despite the application of appropriate primary therapy, uterine
sarcomas often behave in an aggressive and malignant fashion with a
high risk of recurrence and poor prognosis. The optimal treatment
for recurrent uterine sarcomas remains unclear. These tumors are
relatively chemo- and/or radio-resistant, and certain studies have
previously evaluated the effectiveness of SCS in patients with
recurrent uterine sarcomas (11,13,14).
In the present study, it was demonstrated that
patients with recurrent uterine sarcomas who underwent the SCS had
longer overall survival times and disease-free intervals following
the initial relapse, when compared with those treated with
chemotherapy or radiotherapy alone. SCS for patients who have
pulmonary recurrent disease has been reported to lead to improved
survival, particularly in those with unilateral disease, a smaller
number of resected metastases (<3), a smaller tumor (<3 cm)
and a longer DFI (<6, or 12 months) (8–10).
However, studies on SCS for extra-pulmonary recurrences of uterine
sarcomas are limited, and have only small sample sizes (4,11,14–17).
Leitao et al (14) reported
that optimal surgical resection may improve the survival of
patients with either pulmonary or extra-pulmonary metastases, after
retrospectively surveying 41 patients with recurrent LMS (including
28 patients who had non-thoracic recurrences). Yoshinaga et
al (17) concluded that patient
survival was improved by the resection of pulmonary or intrapelvic
metastases of uterine sarcomas. Nevertheless, this report included
only 8 patients, 3 of whom had extra-pulmonary recurrences.
According to a review by Giuntoli et al (4) that analyzed 208 patients with recurrent
LMS, SCS is associated with the improvement of disease-specific
survival from the first relapse, unlike chemotherapy or
radiotherapy.
In the current study, 8/9 patients in the SCS group
had extra-pulmonary metastases. Although 4 of these had multiple
metastases (the number of metastases, 2–14), SCS may contribute to
prolonged survival. Hoang et al (11) reported that single tumor recurrence
was significantly correlated with a longer survival time, when
compared with that of patients with >2 tumors. Based on the
current examination, the resection of recurrent tumors may improve
survival, even in patients with multiple extra-pulmonary diseases
derived from uterine sarcomas. As aforementioned, the optimal
indication regarding the number of metastases in candidate has yet
to be clarified. However, in this study, no severe complications of
multiple metastasectomy were observed. Therefore, SCS may be
considered even for patients with multiple recurrences, if there
are no other favorable alternatives and optimal resection is
considered possible.
In the current study, the re-recurrence rate was 67%
(6/9) in patients treated with SCS, while all patients (9/9; 100%)
treated without SCS experienced relapse. It may be considered that
SCS is a superior management procedure to chemo- or radiotherapy
for recurrent uterine sarcoma. Nevertheless, the limitations of the
current study are those associated with any retrospective study:
insufficient patient numbers, the possibility of selection bias and
treatment heterogeneity. Thus, it is possible the results reflect
the fact that patients with a favorable condition for SCS
demonstrated a better oncologic outcome. The largest limitation of
the present study was that only 18 patients with recurrent sarcoma
were available. This small-scale patient cohort was based on the
actual clinical situation that recurrent uterine sarcomas are rare.
A large-scale additional study verifying the current findings is
desirable, involving more patients from multiple institutions.
Further limitations of the current report are those associated with
any retrospective study. Indeed, the treatment protocol for uterine
sarcomas, including the regimen of salvage chemotherapy, the number
of courses, doses, criteria of surgery, diagnostic and surgical
procedures were heterogeneous. In particular, the development of
surgical devices and perioperative management is frequent and
evident currently. Furthermore, the advancement of imaging
modalities, including MRI, new-generation ultrasound and PET-CT,
has contributed to surgical decisions. The possibilities that these
factors may influence the treatment and disease outcomes of
patients with recurrent uterine sarcomas must be considered. It is
possible the current findings provide essential information on how
to develop better treatments for this type of tumor.
In conclusion, the results of the current
investigation support the consideration of SCS for patients with
recurrent uterine sarcomas. Several reports have recently
demonstrated that new-generation anticancer agents, including
bevacizumab, pazopanib, eribulin and trabectedin, are effective for
recurrent LMS (18–21). Comparison of SCS with these
treatments could be useful. Irrespective of this, the rates of
mortality and relapse remain high and further studies are
required.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
SCS
|
secondary cytoreductive surgery
|
|
PFI
|
progression-free interval
|
|
LMS
|
leiomyosarcoma
|
|
ESS
|
endometrial stromal sarcoma
|
|
CS
|
carcinosarcoma
|
References
|
1
|
Park JY, Kim DY, Suh DS, Kim JH, Kim YM,
Kim YT and Nam JH: Prognostic factors and treatment outcomes of
patients with uterine sarcoma: Analysis of 127 patients at a single
institution, 1989–2007. J Cancer Res Clin Oncol. 134:1277–1287.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Burghaus S, Halmen S, Gass P, Mehlhorn G,
Schrauder MG, Lux MP, Renner SP, Beckmann MW, Hein A and Thiel FC:
Outcome and prognosis in uterine sarcoma and malignant mixed
Mullerian tumor. Arch Gynecol Obstet. 294:343–351. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Fujita H, Adachi S, Kigawa J, Sugiyama T
and Takeuchi S: A clinicopathlogical study of uterine sarcoma in
last decade-a retrospective study of KCOG/USSG inter group study.
Adv Obstet Gynecol. 56:463–465. 2004.
|
|
4
|
Giuntoli RL II, Garrett-Mayer E, Bristow
RE and Gostout BS: Secondary cytoreduction in the management of
recurrent uterine leiomyosarcoma. Gynecol Oncol. 106:82–88. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Mayerhofer K, Obermair A, Windbichler G,
Petru E, Kaider A, Hefler L, Czerwenka K, Leodolter S and Kainz C:
Leiomyosarcoma of the uterus: A clinicopathologic multicenter study
of 71 cases. Gynecol Oncol. 74:196–201. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Leath CA III, Huh WK, Hyde J Jr, Cohn DE,
Resnick KE, Taylor NP, Powell MA, Mutch DG, Bradley WH and Geller
MA: A multi-institutional review of outcomes of endometrial stromal
sarcoma. Gynecol Oncol. 105:630–634. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Callister M, Ramondetta LM, Jhingran A,
Burke TW and Eifel PJ: Malignant mixed mullerian tumors of the
uterus: Analysis of patterns of failure, prognostic factors and
treatment outcome. Int J Radiat Oncol Biol Phys. 58:786–796. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Anraku M, Yokoi K, Nakagawa K, Fujisawa T,
Nakajima J, Akiyama H, Nishimura Y and Kobayashi K; Metastatic Lung
Tumor Study Group of Japan, : Pulmonary metastases from uterine
malignancies: Results of surgical resection in 133 patients. J
Thorac Cardiovasc Surg. 127:1107–1112. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Anderson TM, McMahon JJ, Nwogu CE, Pombo
MW, Urschel JD, Driscoll DL and Lele SB: Pulmonary resection in
metastatic uterine and cervical malignancies. Gynecol Oncol.
83:472–476. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Levenback C, Rubin SC, McCormack PM,
Hoskins WJ, Atkinson EN and Lewis JL Jr: Resection of pulmonary
metastases from uterine sarcomas. Gynecol Oncol. 45:202–205. 1992.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Hoang HL, Ensor K, Rosen G, Leon Pachter H
and Raccuia JS: Prognostic factors and survival in patients treated
surgically for recurrent metastatic uterine leiomyosarcoma. Int J
Surg Oncol. 2014:9193232014.PubMed/NCBI
|
|
12
|
Reed NS: The management of uterine
sarcomas. Clin Oncol (R Coll Radiol). 20:470–478. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Burt BM, Ocejo S, Mery CM, Dasilva M,
Bueno R, Sugarbaker DJ and Jaklitsch MT: Repeated and aggressive
pulmonary resections for leiomyosarcoma metastases extends
survival. Ann Thorac Surg. 92:1202–1747. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Leitao MM, Brennan MF, Hensley M, Sonoda
Y, Hummer A, Bhaskaran D, Venkatraman E, Alektiar K and Barakat RR:
Surgical resection of pulmonary and extrapulmonary recurrences of
uterine leiomyosarcoma. Gynecol Oncol. 87:287–294. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Chen H, Pruitt A, Nicol TL, Gorgulu S and
Choti MA: Complete hepatic resection of metastases from
leiomyosarcoma prolongs survival. J Gastrointest Surg. 2:151–155.
1998. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Korets SB and Curtin JP: Surgical options
for recurrent uterine sarcomas. Am Soc Clin Oncol Educ Book. 1–366.
2012.
|
|
17
|
Yoshinaga M, Togami S, Tsuji T, Fukamachi
N, Kamio M, Yanagi M and Douchi T: Clinical outcome of metastatic
uterine leiomyosarcoma and carcinosarcoma in a single institute. J
Obstet Gynaecol Res. 33:818–822. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Benson C, Ray-Coquard I, Sleijfer S,
Litière S, Blay JY, Le Cesne A, Papai Z, Judson I, Schöffski P,
Chawla S, et al: Outcome of uterine sarcoma patients treated with
pazopanib: A retrospective analysis based on two European
organisation for research and treatment of cancer (EORTC) soft
tissue and bone sarcoma group (STBSG) clinical trials 62043 and
62072. Gynecol Oncol. 142:89–94. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Han Y, Li S, Holt HK and Wu L: Curative
effect of bevacizumab combined with chemotherapy in advanced or
recurrent uterine sarcoma. Mol Clin Oncol. 4:245–248. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Martin-Broto J, Pousa AL, de Las Peñas R,
García Del Muro X, Gutierrez A, Martinez-Trufero J, Cruz J, Alvarez
R, Cubedo R, Redondo A, et al: Randomized Phase II study of
trabectedin and doxorubicin compared with doxorubicin alone as
first-line treatment in patients with advanced soft tissue
sarcomas: A spanish group for research on sarcoma study. J Clin
Oncol. 34:2294–2302. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Schöffski P, Chawla S, Maki RG, Italiano
A, Gelderblom H, Choy E, Grignani G, Camargo V, Bauer S, Rha SY, et
al: Eribulin versus dacarbazine in previously treated patients with
advanced liposarcoma or leiomyosarcoma: A randomised, open-label,
multicentre, phase 3 trial. Lancet. 387:1629–1637. 2016. View Article : Google Scholar : PubMed/NCBI
|