Introduction
Squamous cell carcinoma (SqCC) is a malignant
neoplasm of epidermal keratinocytes that arises most commonly on
the skin and organs lined with squamous cells. Squamous cell
carcinoma is a rare form of metaplastic carcinoma in breast that
accounts <0.1% (1). The precursor
of pure squamous carcinoma is a benign squamous metaplasia that
occurs in the epithelium of cysts, fibroadenomas ana phyllodes
tumors. It can be also associated with ductal and lobular
hyperplasia or papillomas. Recent studies confirmed that benign
squamous metaplasia of ductal and lobular epithelial cells can be
linked with fat necrosis and infracted ademonas. Squamous cell
carcinoma should be differentiated between lesions of keratinizing
squamous carcinoma and squamous metaplasia associated to mammary
carcinoma (2). The characteristic
features of metaplastic cell carcinoma include: i) primary
carcinoma without other neoplastic components such as ductal or
mesenchymal elements, ii) the tumor origin is independent of the
overlying skin and nipple and iii) absence of primary epidermoid
tumors present in other site (oral cavity, bronchus, esophagus,
bladder, cervix ect.) (3). However,
squamous metaplastic carcinoma should be also differentiated with
pure squamous cell carcinoma of skin in the breast. The extensive
infiltrate of squamous cancer cells to the skin can make it
difficult to confirm the cutaneous origin of tumor and exclude the
presence of malignant metaplastic changes as a primary breast. In
the present study, cases of cutaneous squamous cell carcinoma and
primary metaplastic squamous cell carcinoma of the breast are
described. To our knowledge, such cases are great diagnostic
challenge in pathomorphology and should be carefully analyzed in
daily practice.
The present study was performed in conformity with
the Declaration of Helsinki for Human Experimentation and the
protocol was approved by the Bioethics Committee of the Medical
University of Bialystok. Written informed consent was obtained from
both participants.
Case report
Case 1
History
A 72-year-old female was admitted to the Department
of Surgical Oncology in Bialystok (Poland) for planned surgery.
There was no family history of malignant neoplasms. Patient had
used some medication against hypertension. She complained of
abdominal pain with normal peristalsis and had normal stools. She
was postmenopausal and had given birth to 3 children. Previously,
the histological examination of the biopsy material, obtained
during a fine-needle aspiration (FNA), confirmed the presence of
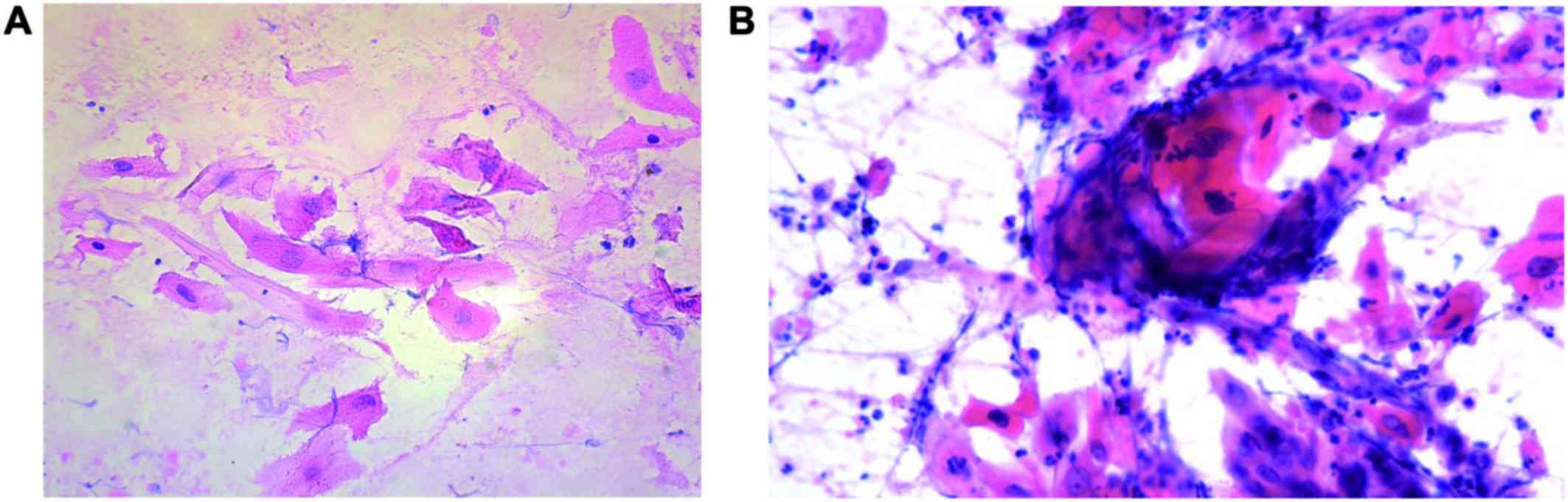
the cancer cell infiltrate in the left breast (Fig. 1A). The description of preoperative,
physical examination demonstrated a large mass with ulceration
localized on the left breast. Tumor took the three quarters of the
breast and raised several years. The skin and the nipple-areola
complex were involved. The tumor was attached underlying chest
wall. No abnormalities were observed in the opposite breast nor
axilla. Local lymph nodes were not enlarged on palpation
examination. Chest X-ray was performed preoperatively and
demonstrated the heterogenic, rounded area of increased density,
1.3 cm diameter in size, located in the right supraclavival field
of the left lung. In addition, diaphragm was normal. The
preoperative blood parameters showed the low levels of red blood
cells, hemoglobin and hematocrit and the increased levels of CA125
and CA15.3. Following normalization of anemia with the concentrate
of red blood cells, patient was qualified for scheduled surgery.
Due to large size tumor that infiltrated the chest wall, the left
radical mastectomy was performed according to the Halsted's method
(1). The surgical procedure included
the removal of the breast tumor with a greater pectoral muscle and
axilla with local lymph nodes. The left breast was reconstructed
with the latissimus dorsi musculocutaneous flap. There was no
postoperative morbidity. The clinical staging was T4 N0 MX.
Histopathology
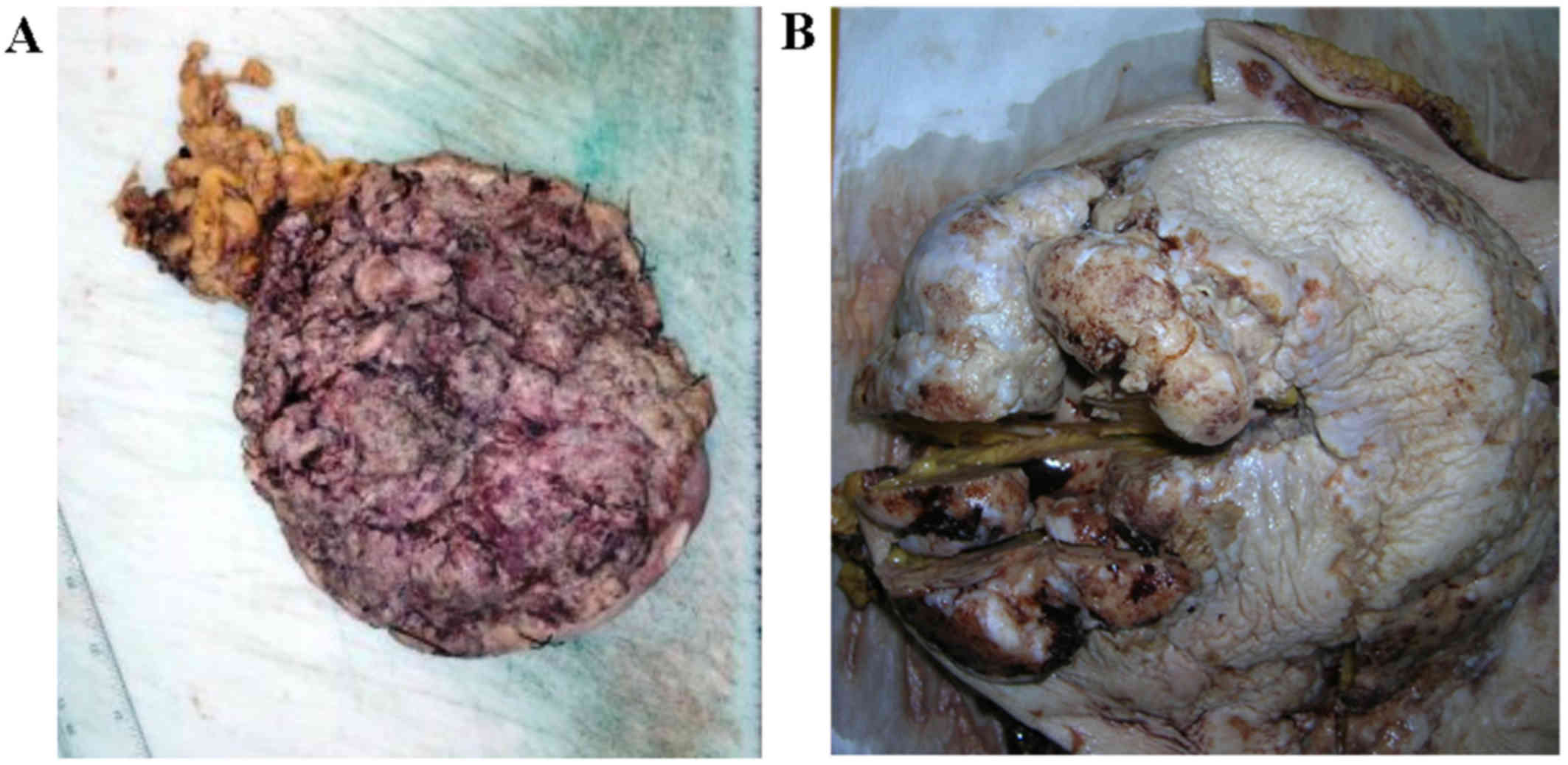
Macroscopically, the postoperative formalin-fixed
material of the left breast 17.0×16.5 cm in size, showed a huge,
ulcerative tumor with cauliflower-like appearance, 16.8×16.2 cm in
size, of gray-brown surface with a narrow margin at the periphery
of the skin without the presence of nipple. On cross-sections,
tumor was solid, gray-brown color with gray-white foci of necrosis,
coming near this deep incision line. Fourteen local lymph nodes
were dissected with no evidence of metastases. The biggest lymph
node was 3.1×1.8 cm in size. Optimal surgical margins were obtained
(Fig. 2A).
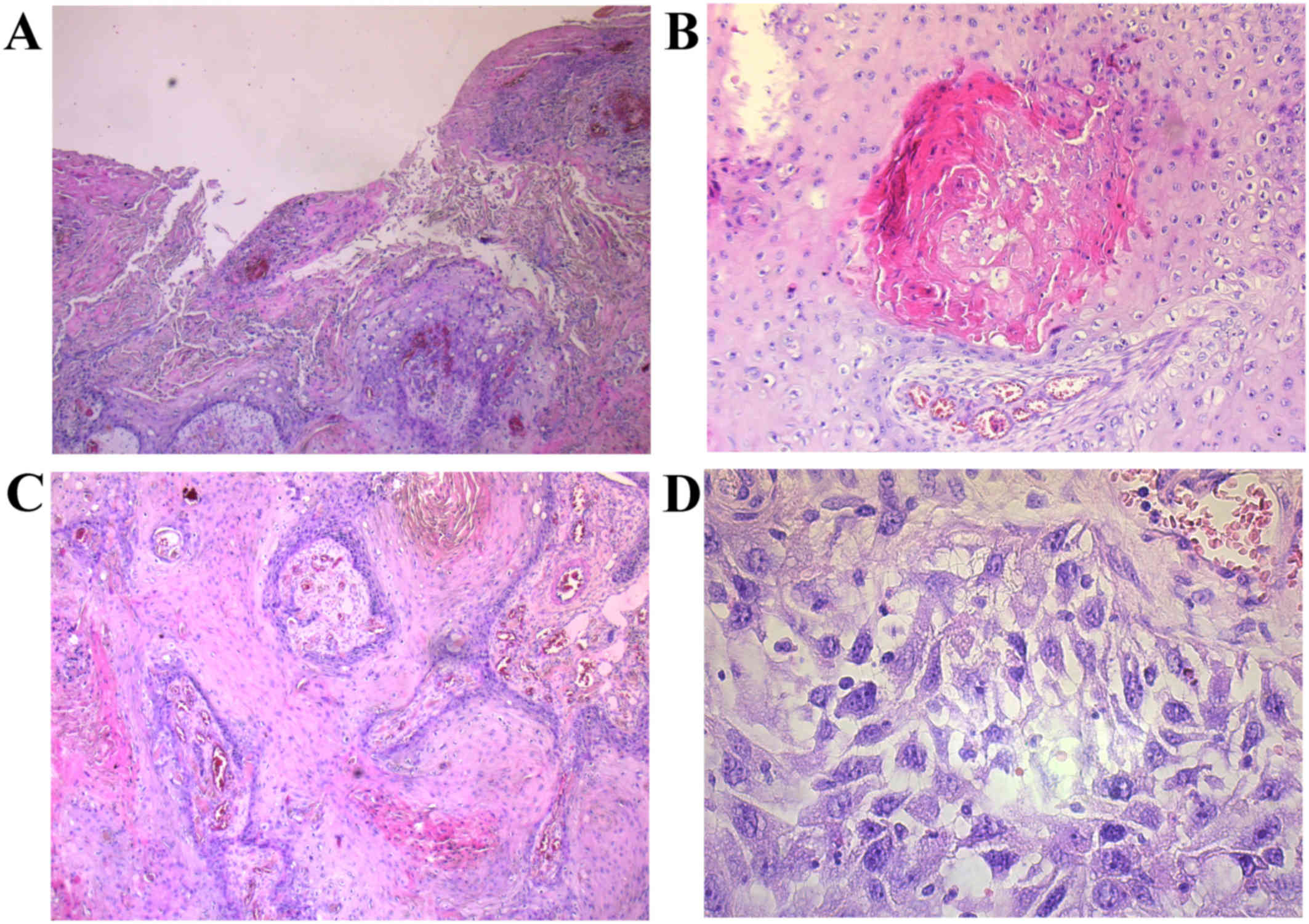
The microscopic examination of the surgical specimen
revealed a moderately differentiated (G2) squamous cell carcinoma
according to WHO Classification of the Skin Tumours (4). Cancer cells have abundant eosiphilic
cytoplasm with large vesicular nucleus. The prominent intracellular
brigdes, central keratinization and pearl formation were observed.
SqCC does not infiltrate along nerve sheaths and lymphovascular
vessels. Cancer cells proliferated from the stratified squamous
epithelium covering the breast into 2.5 cm deeper layers of the
organ. No other primary site and metastatic disease to breast were
ruled out (Fig. 3A and B).
Immunohistochemical analysis confirmed negative nuclear expression
of progesterone and estrogen receptors, and HER2. Moreover, we
observed a positive membrane reaction of adhesion
protein-E-cadherin. The diagnosis of the tumor was established as
triple negative SqCC of breast skin with moderate proliferation
index (Ki67 positive in 20% cancer cells).
Follow-up
Patient was discharged home in good general
condition and received five courses of chemotherapy with Taxotere +
Cisplatin (110 mg i.v.). After 1.5 month following the surgery,
patient visited the Surgical Outpatient Clinic and was directed to
USG of abdomen and retroperitoneal space, and the FNA of right
breast. Imaging studies confirmed the presence of a heterogeneous
good well-isolated area with dimensions 27.0×22.0 mm, probably
metastatic changes. The material obtained from the FNA cytology of
right breast showed the presence of poorly differentiated cancer
cells, probably originated from squamous cancer. The change was
treated as a distant metastases. One month later, imaging studies
of PET-CT confirmed an active metabolic process in the left side of
nasopharynx. Patient was qualified for surgery under local
anesthesia. Macroscopically, it was visualized a smooth mucosa of
the nasopharynx without significant changes. The histopathological
study of the nasopharynx did not show a presence of cancer cells
but only morphological features of chronic inflammation markers.
Another CT scan showed numerous secondary changes within both
lungs, the largest diameter of 35.0 mm and a single focus in the
segment V of the liver diameter of 24.0 mm. We did not detect
secondary changes in the skeletal system. Disease progression of
cancer was revealed.
Case 2
History
A 59-years-old woman evaluated in our hospital for
recent onset of pain and tenderness in the left breast. Physical
examination revealed a palpable well circumscribed mass in the left
upper lateral quadrant. The right breast appeared normal. There was
no evidence of supraclavicular or axillary lympadenopathy. The
overlying skin was unremarkable. An ultrasound examination of the
left breast revealed a defined 3.0 cm mass with reduced central
echogenicity, consistent with a cystic space. Mammography showed a
round, high-density mass (without microcalcifications) with almost
regular margins, measuring approximately 3.0 cm, which was
classified as BIRADS 4. A fine-needle aspiration biopsy was
performed and yielded 0.5 ml of white dense fluid material.
Cytological preparations revealed markedly atypical squamous cells
arranged in sheets, clusters and as a single cell as well as
numerous neutrophils. Several cells were keratinized and some
showed degenerative changes (Fig.
1B).
Histopathology
The patient had an ultrasound-guided core biopsy of
the left breast mass at the local anesthesia. Patient underwent
radical mastectomy with axillary lymph nodes dissection. Gross
examination revealed a 6.0 cm tumor with central cystic space
containing necrotic material (Fig.
2B). Microscopically, large polygonal cells with keratinizing
eosinophilic cytoplasm were presented. Individual cells have
abundant eosinophilic cytoplasm and hyperchromatic pleomorphic
nuclei with abortive squamous pearl formation (Fig. 3C and D). Histopathological analysis
revealed metaplastic carcinoma (a pure primary squamous cell
carcinoma of the breast) according to WHO Classification of the
Breast (2). The breast tumor profile
was negative for estrogen receptors, progesterone receptors and
HER2/neu expression.
Follow-up
Patient had adjuvant chemotherapy based on cisplatin
and 5-fluorouracil. She had no evidence of recurrence 6 months
after surgery.
Discussion
Squamous cell carcinoma is one of the most common
skin cancer that was developed in association with prolonged
exposure to sunlight. However, not all squamous cell cancers are
directly related to UV radiation. They can grow in the shores of
chronic ulcers, within the scars burn of skin or as a result of
damage to the epidermis the chemical or radiation therapy. Also
patients with immune suppression have an increased risk of squamous
cancer (4). We reported a very
unusual location-skin overlying the breast in first case. To our
knowledge, first patient described in our paper is the one third of
cases of breast or axilla reported in the literature. Miranda et
al (5) confirmed the presence of
three small changes of SqCC in the axillary skin. Moreover, we
noted histological type of metaplastic cancer of the breast, which
probably evolved directly from glandular tubes or is developed in
the basis of squamous metaplasia in second case (3). This type of breast carcinoma accounts
for ~1% of all malignancies in this location and its presence was
described in several reports in the literature (6–11). The
incidence of PSqCC falls to 5–6 decade of life compared to SqCC of
skin that occurred usually in patients aged above 70 years
(12,13).
Squamous cell carcinoma of the skin develops in the
form of initially small, hard lumps, often in the middle ulcers,
modified necrotic or excessively keratinizing (4). The development of tumor takes years, as
observed in our first case. The lesion was large, a diameter of
16,0 cm, and within the ulcerated surface. In evaluated tissue
material, it was found a tumor ulcerous and necrotic surface with
the presence of inflammatory granulation tissue. Macroscopically,
tumor occupied the outer skin surface of the breast and
nipple-areola complex. In contrast, metaplastic cancer of the
breast formed tumors with rapid growth, involving several months
(14,15). These lesions are accompanied by
inflamed multicystes or abscesses in 50% of cases (16–18). In
the second described case, the gross examination revealed the
presence of grayish-white, solid tumor located in the middle part
of the breast in size 6 cm. Tumor has a central cystic space
containing necrotic material. The overlying skin was unremarkable.
In both cases, we ruled out the presence of suspected cancer
focuses in other locations.
The large size of the tumor and advanced process of
necrosis caused a difficulty of confirmation whether macroscopic
tumor derived from the squamous epithelium of the skin or are
created in the basis of abnormal glandular epithelium metaplasia.
First tumor was built with bands of moderately differentiated
squamous cancer cells with large vesicular nucleus. We observed
prominent intracellular bridges, central keratinization and pearl
formation. SqCC does not infiltrate along nerve sheaths and
lymphovascular vessels. Cancer cells proliferated from the
stratified squamous epithelium covering the breast into deeper
layers of the body. Morphological image of metaplastic breast
cancer is very similar. They also may have focal anaplastic
component or focal clear cell changes (19). Squamous cell carcinomas showed
morphological similarity regardless of location. However, the
differentiating feature of our case was the way of cancer spread.
In the case of skin, we observed a cancer infiltration that was
continuous and formed in ‘icicles’ from the skin into the tissue of
the breast. Therefore, metaplastic cancer had irregular growth of
squamous cell that creates cystic-solid tumor confined to the
breast parenchyma.
Moreover, because of the similar changes in both
histogenesis, we can not use immmunophenotype methods to
differentiated cancer cells. Immunohistochemical analysis also does
not allow to determine whether it is a primary lesion or metastatic
one. In both of our cases, we recorded a positive expression of
pancytokeratin which confirms the presence of cells differentiated
towards squamous cell carcinoma. In case 1, the characteristics of
immunophenotyping were: Cytokreatyna7 (−), cytokeratin 20 (−) and
triple negative receptor status suggests that this lesion will
probably not derive from the mammary gland. However, in the
majority of metaplastic breast cancers also observed a lack of
expression of estrogen, progesterone, and HER2 (17). A positive response to these antigens
were reported in few percentage of cases (19,20).
Only one of case described in literature was confirmed as a SqCC of
breast with HER2-basal phenotype (21).
Treatment of squamous cell carcinoma of the skin and
metaplastic breast cancer are depends mainly on the stage of the
cancer. Typically, the patients are undergoing surgery in the first
stage of therapy then adjuvant treatment such as radio- or
chemotherapy was used (22–24). In our two cases, the total mastectomy
was performed that included removing of breast with local lymph
nodes. Chemotherapy was used as a second part of treatment. In SqCC
case of skin, we recorded metastases located in the second breast
after 1.5 months. In the following months, there was a further
progression of the disease manifested by the presence of multiple
metastatic lesions in the lungs and a single metastatic focus in
the segment V of the liver. In the literature it has been shown
that the risk of developing metastatic squamous cell carcinoma of
the skin, which is accompanied by changes in sun-damaged (0.5%) is
lower than in patients in places not exposed to sunlight (25). Moreover, the ability of squamous
cancer cells of the skin metastasize is dependent on the depth of
invasion, tumor size, and involvement of vascular and lymphatic
vessels (26). In case 2, we did not
notice relapse within 6 months after surgery. Time progression-free
survival in patients with metaplastic cell carcinoma of the breast
is 2–36 months (18,20,24).
Overall survival after 2 years was 80%, and after 5 years of 35–67%
(19,20,27). It
has been shown that tumors with the presence of >10% of spindle
cell component have a worse prognosis, however, the focal length of
keratinization determines longer overall survival (27). It seems that despite of the similar
morphology of cancer cells, the determination of the originality of
the tumor may be important in identification of prognostic
factors.
In conclusion, cutanous squamous cell carcinoma and
metaplastic cell carcinoma of breast are extremely rare lesions
that should be differentiated from primary squmanous cell carcinoma
in this localization and should be treated with special clinician's
and pathomorpgologist's attention. In our opinion, such cases are
great diagnostic challenge in pathomorphology and should be more
carefully analyzed in daily practice.
References
|
1
|
Hennessy BT, Krishnamurthy S, Giordano S,
Buchholz TA, Kau SW, Duan Z, Valero V and Hortobagyi GN: Squamous
cell carcinoma of the breast. J Clin Oncol. 23:7827–7835. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Tavassoli FA and Devilee P: Metaplastic
carcinomasPathology and Genetics. Tumours of the Breast and Female
Genital Organs. World Organization Classification of Tumors. IARC
Press; Lyon: pp. 37–38. 2003
|
|
3
|
Rosen PR: Chapter 21. Rosen's Breast
Pathology. Lippincott-Raven; Philadelphia, New York: pp. 397–404.
1997
|
|
4
|
Leboit PE, Burg G, Weedon D and Sarasin A:
Squamous Cell CarcinomaPathology and Genetics. Skin Tumors. World
Organization Classification of Tumors. IARC Press; Lyon: pp. 20–21.
2007
|
|
5
|
Miranda BH, Malahias M, El-Said TF and
Fahmy FS: Axillary skin malignancy: A rare breast cancer
presentation. Ann Plast Surg. 72:513–514. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Badge SA, Gangane NM, Shivkumar VB and
Sharma SM: Primary squamous cell carcinoma of the breast. Int J
Appl Basic Med Res. 4:53–55. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Mitra B, Pal M, Debnath S, Paul B, Saha TN
and Maiti A: Primary squamous cell carcinoma of breast with
ipsilateral axillary lymph node metastasis: An unusual case. Int J
Surg Case Rep. 2:194–197. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Flikweert ER, Hofstee M and Liem MS:
Squamous cell carcinoma of the breast: A case report. World J Surg
Oncol. 6:1352008. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Tsung SH: Primary pure squamous cell
carcinoma of the breast might be sensitive to Cisplatin-based
chemotherapy. Case Rep Oncol. 5:561–565. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Bhosale SJ, Kshirsagar AY, Deshmukh SJ,
Jagtap SV and Langade YB: Squamous cell carcinoma of the breast. Am
J Case Rep. 14:188–190. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Wang J, Zhang X, He J, Yang M, Tang J, Li
X, Tang H and Xie X: Co-expression of EGFR and CK5/6 in primary
squamous cell carcinoma of the breast. Med Oncol. 31:1722014.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Porzio R, Cordini C, Orsi N, Brigati F,
Paties CT and Cavanna L: Primary squamous cell carcinoma of the
breast after cured bilateral breast cancer. In Vivo. 28:1155–1158.
2014.PubMed/NCBI
|
|
13
|
Mokhtar GA: Squamous cell carcinoma of the
breast. Saudi Med J. 30:1346–1349. 2009.PubMed/NCBI
|
|
14
|
Sharma R, Usmani S and Siegel R: Primary
squamous cell carcinoma of breast in background of phyllodes
tumor-a case report. Conn Med. 73:341–343. 2009.PubMed/NCBI
|
|
15
|
Cichon P, Drucis K and Piotrkowski J:
Primary squamous cell carcinoma of the breast-a case report. Pol
Merkur Lekarski. 38:140–143. 2015.(In Polish). PubMed/NCBI
|
|
16
|
Samuels TH, Miller NA, Manchul LA,
DeFreitas G and Panzarella T: Squamous cell carcinoma of the
breast. Can Assoc Radiol J. 47:177–182. 1996.PubMed/NCBI
|
|
17
|
Salemis NS: Breast abscess as the initial
manifestation of primary pure squamous cell carcinoma: A rare
presentation and literature review. Breast Dis. 33:125–131. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Nair VJ, Kaushal V and Atri R: Pure
squamous cell carcinoma of the breast presenting as a pyogenic
abscess: A case report. Clin Breast Cancer. 7:713–715. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Liu J, Yu Y, Sun JY, He SS, Wang X, Yin J
and Cao XC: Clinicopathologic characteristics and prognosis of
primary squamous cell carcinoma of the breast. Breast Cancer Res
Treat. 149:133–140. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Behranwala KA, Nasiri N, Abdullah N, Trott
PA and Gui GP: Squamous cell carcinoma of the breast:
Clinico-pathologic implications and outcome. Eur J Surg Oncol.
29:386–389. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Shui R, Li A, Yang F, Zhou X, Yu B, Xu X
and Yang W: Primary squamous cell carcinoma of the breast with
unusual basal-HER2 phenotype. Int J Clin Exp Pathol. 7:5203–5209.
2014.PubMed/NCBI
|
|
22
|
Lebbe C, Becker JC, Grob JJ, Malvehy J,
Del Marmol V, Pehamberger H, Peris K, Saiag P, Middleton MR,
Bastholt L, et al: Diagnosis and treatment of merkel cell
carcinoma. European consensus-based interdisciplinary guideline.
Eur J Cancer. 51:2396–2403. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Kurpad V, Prabhu KC, Ramesh MK and
Harindranath HR: Primary denovo squamous cell carcinoma breast
masquerading as breast abscess. Indian J Surg Oncol. 4:48–51. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Murialdo R, Boy D, Musizzano Y, Tixi L,
Murelli F and Ballestrero A: Squamous cell carcinoma of the breast:
A case report. Cases J. 2:73362009.PubMed/NCBI
|
|
25
|
Rowe DE, Carroll RJ and Day CL Jr:
Prognostic factors for local recurrence, metastasis, and survival
rates in squamous cell carcinoma of the skin, ear, and lip.
Implications for treatment modality selection. J Am Acad Dermatol.
26:976–990. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Schmults CD, Karia PS, Carter JB, Han J
and Qureshi AA: Factors predictive of recurrence and death from
cutaneous squamous cell carcinoma: A 10-year, single-institution
cohort study. JAMA Dermatol. 149:541–547. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Nayak A, Wu Y and Gilcrease MZ: Primary
squamous cell carcinoma of the breast: Predictors of locoregional
recurrence and overall survival. Am J Surg Pathol. 37:867–873.
2013. View Article : Google Scholar : PubMed/NCBI
|