Introduction
During the last 20 years, novel therapies for lung
cancer have led to the prevention of more than 1.7 million deaths
(1). Even with this optimistic news,
lung cancer remains the leading cause of death in USA (1). Approximately 50% of lung cancer
patients have widespread metastatic disease at presentation, most
frequently with cerebral, hepatic or adrenal involvement. The
gastrointestinal tract is only rarely involved (2). The prognosis of gastric metastases with
origin in lung cancer is very poor, survival rates being estimated
at 20% at 1 year and 1% at 5 years (3,4).
Although gastric metastases of lung cancer were believed to be
exceptionally rare, autopsy studies report an incidence ranging
from 0.19 to ≥11% (5–9).
Gastric metastases are rarely symptomatic and
therefore easily overlooked when investigating lung cancer patients
(10). Nevertheless,
gastrointestinal bleeding and perforation have been reported and
are often fatal. In light of these findings, an increased use of
esophagogastroduodenoscopy may be needed to achieve a higher
detection of secondary gastric tumors. For the early detection of
gastric tumors one can enhance the performances of
esophagogastroduodenoscopy by associating narrow band imaging (NBI)
technology and in vivo staining examination techniques
(11,12). The most frequent staining agents used
for the assessment of digestive tract tumors are methylene blue,
toluidine blue and Lugol iodine (13–15). The
NBI technology improves specificity, sensitivity and accuracy of
the methylene blue video staining method providing essential
information regarding the superficial vascular network and the
features of the cellular field (11). A thorough evaluation of tumor
cell-specific characteristics utilizing ELISA assay and flow
cytometry can improve the diagnostic and treatment protocol
(16).
In the present study, we report a case of advanced
lung cancer presenting as a symptomatic gastric tumor. This is an
infrequent situation, only a few similar cases being reported in
the past decades, to the best of our knowledge. We discuss the
clinicopathological features of secondary gastric cancer with
emphasis on the differential diagnosis and review the available
treatment options.
Case report
A 66-year-old male patient, with a history of
smoking, was admitted in August 2015 in the Department of
Cardiology Elias Emergency Hospital (Bucharest, Romania) for mild
pain in the sternum and upper abdominal area for >4 h. The
physical examination revealed the presence of a right parietal
skull tumor, the rest of the general examination being
unremarkable. All cardiac causes for the pain were excluded and the
patient was referred to the Department of Gastroenterology.
Abdominal ultrasound was not relevant due to the patient's lack of
compliance. Upper gastrointestinal endoscopy showed the presence of
an ulcerated tumor 80×70 mm in size located in the gastric
fundus.
Multiple endoscopic biopsies were performed as well
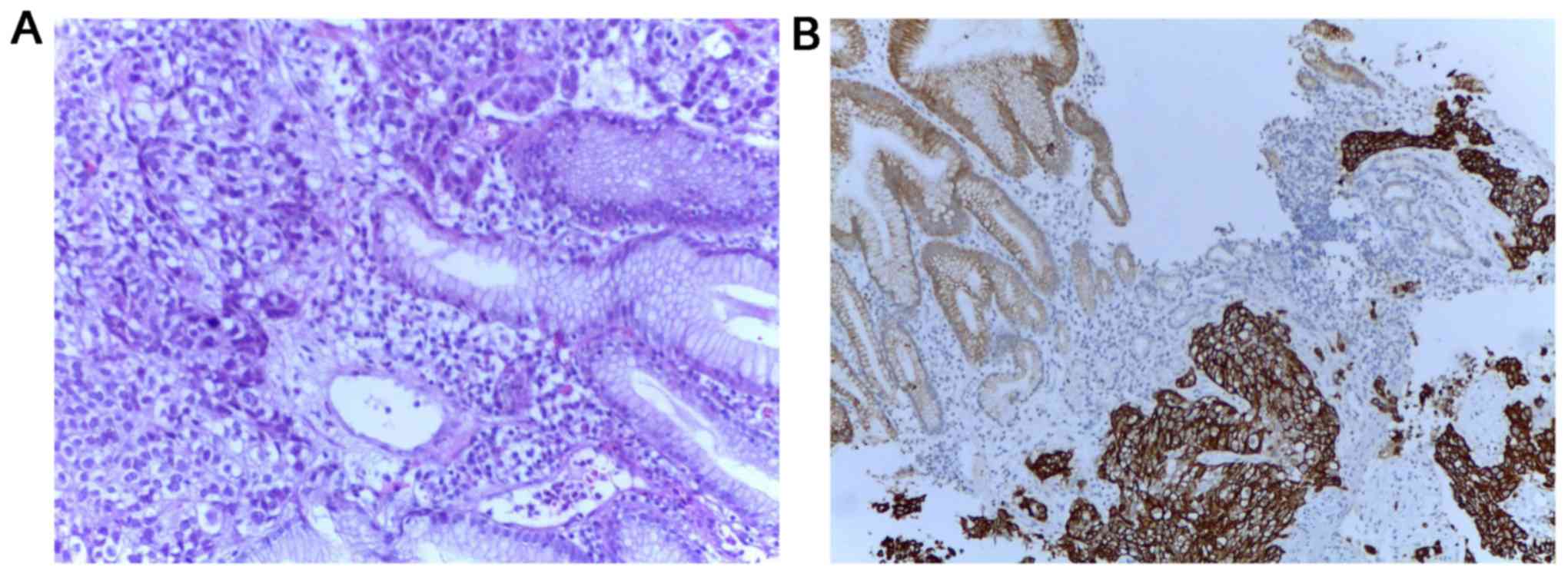
as a histopathologic examination, followed by hematoxylin and eosin
and immunohistochemical staining. Positive tumor protein 63 (P63),
positive cytokeratin 34βE12 (CK34βE12), weakly positive thyroid
transcriptional factor-1 (TTF-1), negative cytokeratin 7 (CK7) and
negative carcinoembryonic antigen (CEA) (Fig. 1) confirmed the diagnosis of gastric
metastasis of pulmonary origin.
Whole body computed tomography (CT) scan identified
multiple tumors: One in the gastric fundus, one in the left lung
and numerous tumors in the liver, kidneys, bones and brain, the
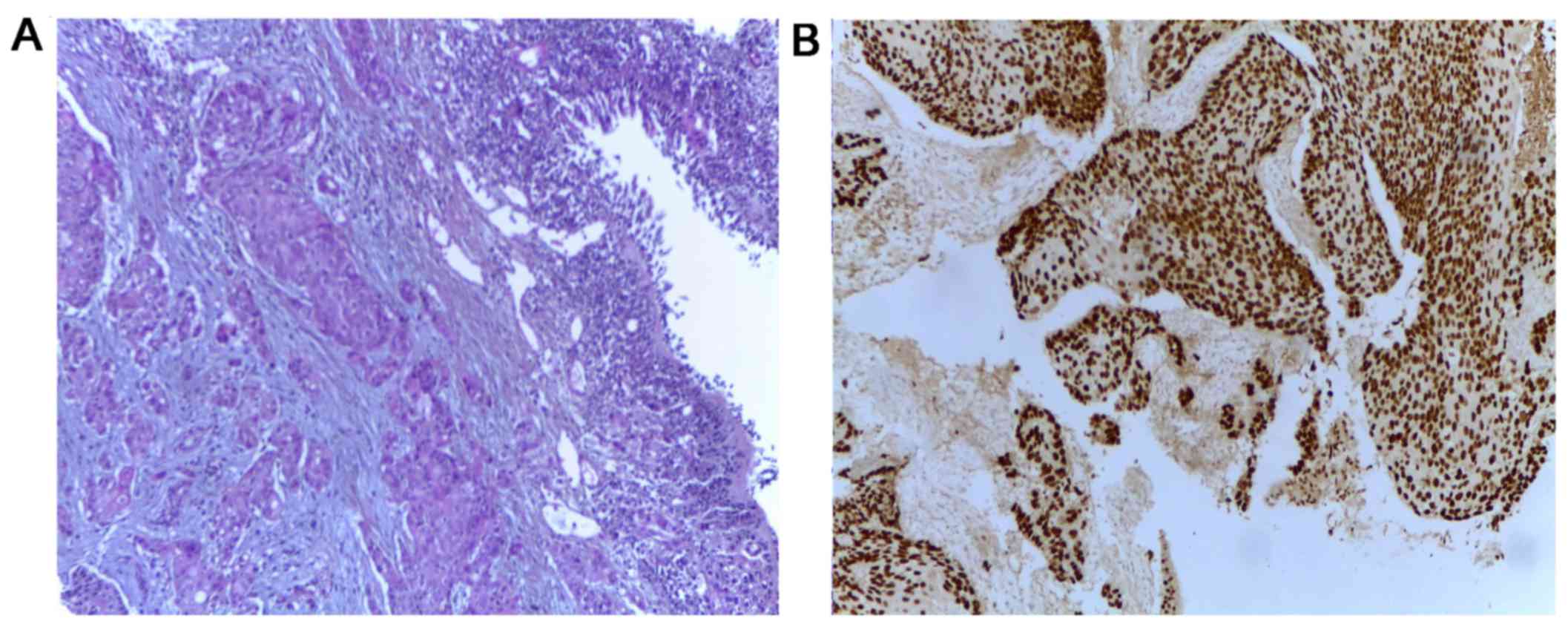
clinical stage being cT2N3M1. Bronchoscopy and transbronchial
biopsy of the left lung tumor were carried out to establish the
origin of the lung tumor (Fig. 2)
leading to the diagnosis of squamous cell carcinoma (SCC) or
non-small cell lung cancer. As the lung and gastric tumor had
identical immunohistochemical profiles, it was considered the
origin of the metastases. The tissue tested negatively for EGFR
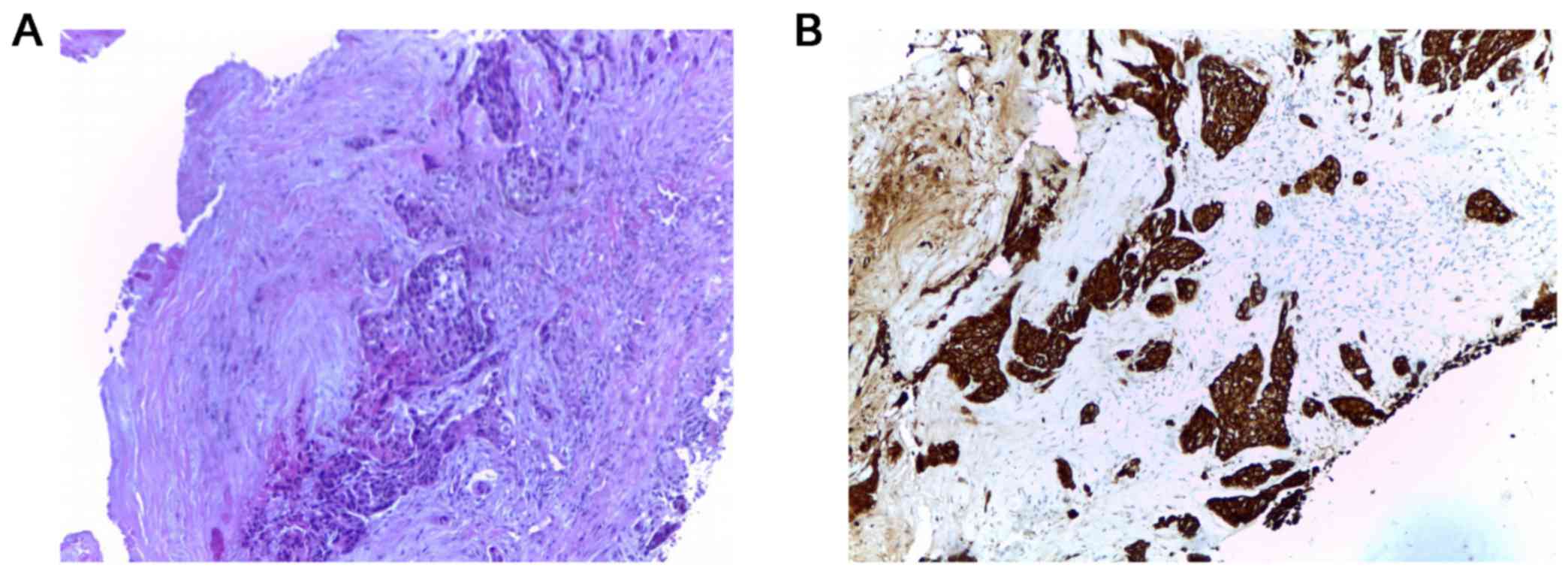
mutation and no ALK rearrangement was reported. Biopsy of the skull
tumor also showed the same histologic pattern (Fig. 3).
Laboratory findings showed no specific changes.
Whole brain radiotherapy was performed for the brain metastases
(total dose, 30 Gy). It was well tolerated, with no serious adverse
events. Following radiotherapy, the patient underwent chemotherapy
with paclitaxel 200 mg/m2 and carboplatin AUC6 and
osteoclast inhibitors (zoledronic acid), measured as q3w. After the
second cycle, grade II aplastic anemia and mild elevation of liver
enzymes occurred. Nevertheless, the oncology board, comprising a
medical oncologist, gastroenterologists and hematologists continued
the aggressive treatment, with daily monitoring of the hemogram and
liver enzymes.
The patient received 6 cycles of chemotherapy, with
stable disease, according to response evaluation criteria in solid
tumors (RECIST). Due to grade IV anemia and hepatotoxicity from
chemotherapy, oncological treatment was ceased and the patient
offered best supportive care. The patient succumbed four weeks
later to multiple organ failure.
Discussion
Gastric site metastasis from primary lung cancer was
rarely reported, portending an inferior prognosis even with
aggressive oncologic treatment (17). Breast cancer, esophageal cancer,
melanoma, as well as testicular seminoma, hepatocellular carcinoma,
choriocarcinoma and Merkel cell carcinoma can also be the origin of
secondary gastric tumors and should be included in the differential
diagnosis (18–21).
In 1993, large cell lung cancer was reported to be
the most common histologic type of primary lung cancer associated
with gastric metastases (22).
Nevertheless, according to more recent reports, pulmonary
adenocarcinoma has become the leading disease, followed by SCC,
small cell lung cancer, and pleomorphic carcinoma of the lung
(23). The distinction between
subtypes is critical for the selection of appropriate targeted
therapies (erlotinib/crizotinib/bevacizumab/pemetrexed).
Lung cancer cells spread hematogenously to the
gastric submucosa (17). The tumor
is thus clinically silent until it reaches considerable sizes.
Although non-specific, symptoms and signs such as epigastric pain,
vomiting, anorexia and chronic upper gastrointestinal bleeding
manifesting as melena and anemia occurring in cancer patients
should alert the clinician and prompt endoscopic evaluation
(17,24). However, such complaints are generally
misinterpreted as side-effects of chemotherapy. Severe
complications including acute bleeding and perforation may also
occur and are usually lethal.
Endoscopic findings can mimic other gastric tumors,
but a volcano-like or umbilicated mass (‘the bull's eye sign’) is
considered a classic endoscopic appearance of metastatic gastric
cancer, as was first suggested by Pomerantz and Margolin in 1962
(25). Nodules, solitary or
multiple, sometimes ulcerated polypoid submucosal masses, as well
as infiltrating constricting tumors have also been described
(25–28). The most frequent locations of gastric
metastases are reported to be the middle or upper third of the
stomach. Usually, the greater curvature is involved (29,30).
Given the variable morphology and the lack of characteristic
appearances on endoscopy, metastases to the stomach may also be
confused with gastric ulcer, ectopic pancreas, eosinophilic
granuloma, lymphoma, carcinoid and Kaposi's sarcoma (31).
The histopathologic examination of gastric tumors in
lung cancer patients raises yet another diagnostic challenge given
the difficulty to distinguish between a metastasis from primary
lung cancer and an initial gastric cancer, especially when
considering adenocarcinoma. Hence, immunohistochemical tests are
the most important step in the diagnostic algorithm of these cases
and are crucial for optimal management (26). The common markers used for subtyping
non-small cell lung cancer include TTF-1, CK7, Napsin-A for
adenocarcinoma and p63, CK5/6, and CK34βE12/CK903 for SCC (32). In our patient, the positive P63 and
CK34βE12 staining and the weakly positive TTF-1 staining support
the lung origin of the metastasis.
Serum tumor markers were tested in order to complete
the diagnosis: CEA and SCC levels were 3-fold higher than normal
values, CA 19-9 was within reasonable limits. It is known that,
each serum tumor marker has its specificity and sensitivity and can
be used mainly to establish the prognosis of the disease. Non-small
cell lung cancer also demonstrated the overexpression of endocan
(33). This marker of angiogenesis
was studied in several types of cancer and was used as a possible
prognostic factor in non-small cell lung cancer and
gastrointestinal cancer (34–36).
Gastrointestinal metastases secondary to lung cancer
represent a late stage of disease, usually being associated with
metastases in other organs. Patients rarely survive longer than 16
weeks after diagnosis of gastrointestinal metastases (9).
The therapeutic management of such patients includes
surgery, chemotherapy with or without radiotherapy, molecularly
targeted therapies and supportive care. Surgical intervention in
patients with lung cancer metastatic to the stomach remains
controversial as some authors report longer survival rates in the
best supportive care approach (37),
while others support metastasectomy in patients with non-small cell
lung cancer and unique metastasis not involving the brain or the
adrenal gland (38,39). However, life-threatening
complications such as massive hemorrhage or perforation can be
prevented by surgical intervention (37,40).
Therefore, treatment should be personalized. Our patient did not
undergo surgery given the presence of multiple other extrathoracic
metastases. We managed to control the disease and patient's
symptoms for 22 weeks by offering 6 cycles of chemotherapy,
radiation therapy and best supportive care when his performance
status did not allow us to continue active oncological
treatment.
In conclusion, although non-specific,
gastrointestinal signs and symptoms occurring in lung cancer
patients should alert the clinicians as to the possibility of
gastrointestinal metastases and prompt endoscopic evaluation.
Distinguishing between primary and secondary gastric tumors can be
challenging and requires immunohistochemical tests. Early detection
of gastric metastases and initiation of appropriate treatment can
improve the patient's quality of life and prolong patient
survival.
References
|
1
|
Siegel RL, Miller KD and Jemal A: Cancer
statistics, 2016. CA Cancer J Clin. 66:7–30. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
van Meerbeeck JP, Fennell DA and De
Ruysscher DK: Small-cell lung cancer. Lancet. 378:1741–1755. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Yang CJ, Hwang JJ, Kang WY, Chong IW, Wang
TH, Sheu CC, Tsai JR and Huang MS: Gastro-intestinal metastasis of
primary lung carcinoma: Clinical presentations and outcome. Lung
Cancer. 54:319–323. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Greenlee RT, Hill-Harmon MB, Murray T and
Thun M: Cancer statistics, 2001. CA Cancer J Clin. 51:15–36. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Green LK: Hematogenous metastases to the
stomach. A review of 67 cases. Cancer. 65:1596–1600. 1990.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Kim MS, Kook EH, Ahn SH, Jeon SY, Yoon JH,
Han MS, Kim CH and Lee JC: Gastrointestinal metastasis of lung
cancer with special emphasis on a long-term survivor after
operation. J Cancer Res Clin Oncol. 135:297–301. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Kim SY, Ha HK, Park SW, Kang J, Kim KW,
Lee SS, Park SH and Kim AY: Gastrointestinal metastasis from
primary lung cancer: CT findings and clinicopathologic features.
AJR Am J Roentgenol. 193:W197–W201. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Hillers TK, Sauve MD and Guyatt GH:
Analysis of published studies on the detection of extrathoracic
metastases in patients presumed to have operable non-small cell
lung cancer. Thorax. 49:14–19. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
McNeill PM, Wagman LD and Neifeld JP:
Small bowel metastases from primary carcinoma of the lung. Cancer.
59:1486–1489. 1987. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Antler AS, Ough Y, Pitchumoni CS, Davidian
M and Thelmo W: Gastrointestinal metastases from malignant tumors
of the lung. Cancer. 49:170–172. 1982. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Stefanescu DC, Ceachir O, Zainea V,
Hainarosie M, Pietrosanu C, Ionita IG and Hainarosie R: Methilene
blue video contact endoscopy enhancing methods. Rev Chim.
67:1558–1559. 2016.
|
|
12
|
Hainarosie R, Ceachir O, Zainea V,
Hainarosie M, Pietrosanu C, Zamfir C and Stefanescu DC: The test of
lugol iodine solution associated with NBI examination in early
diagnostic of tongue carcinoma. Rev Chim. 68:226–227. 2017.
|
|
13
|
Stefanescu DC, Ceachir O, Zainea V,
Hainarosie M, Pietrosanu C, Ionita IG and Hainarosie R: The use of
methylene blue in assessing disease free margins during
CO2 LASER assisted direct laryngoscopy for glottis
cancer. Rev Chim. 67:1327–1328. 2016.
|
|
14
|
Hainarosie R, Zainea V, Ceachir O,
Hainarosie M, Pietrosanu C and Stefanescu DC: The use of methylene
blue in early detection of the vocal fold cancer. Rev Chim.
68:16–17. 2017.
|
|
15
|
Stefanescu DC, Ceachir O, Zainea V,
Hainarosie M, Pietrosanu C, Ionita IG and Hainarosie R: The value
of toluidine blue staining test in assessing disease free margins
of oral cavity carcinomas. Rev Chim. 67:1255–1256. 2016.
|
|
16
|
Petrică-Matei GG, Iordache F, Hainăroşie R
and Bostan M: Characterization of the tumor cells from human head
and neck cancer. Rom J Morphol Embryol. 57:791–799. 2016.PubMed/NCBI
|
|
17
|
Menuck LS and Amberg JR: Metastatic
disease involving the stomach. Am J Dig Dis. 20:903–913. 1975.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Okazaki R, Ohtani H, Takeda K, Sumikawa T,
Yamasaki A, Matsumoto S and Shimizu E: Gastric metastasis by
primary lung adenocarcinoma. World J Gastrointest Oncol. 2:395–398.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
McLemore EC, Pockaj BA, Reynolds C, Gray
RJ, Hernandez JL, Grant CS and Donohue JH: Breast cancer:
Presentation and intervention in women with gastrointestinal
metastasis and carcinomatosis. Ann Surg Oncol. 12:886–894. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Lin CP, Cheng JS, Lai KH, Lo GH, Hsu PI,
Chan HH, Hsu JH, Wang YY, Pan HB and Tseng HH: Gastrointestinal
metastasis in hepatocellular carcinoma: Radiological and endoscopic
studies of 11 cases. J Gastroenterol Hepatol. 15:536–541. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Senadhi V and Dutta S: Testicular seminoma
metastasis to the gastrointestinal tract and the necessity of
surgery. J Gastrointest Cancer. 43:499–501. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Hasegawa N, Yamasawa F, Kanazawa M,
Kawashiro T, Kikuchi K, Kobayashi K, Ishihara T, Kuramochi S and
Mukai M: Gastric metastasis of primary lung cancer. Nihon Kyobu
Shikkan Gakkai Zasshi. 31:1390–1396. 1993.(In Chinese). PubMed/NCBI
|
|
23
|
Huang Q, Su X, Bella AE, Luo K, Jin J,
Zhang S, Luo G, Rong T and Fu J: Clinicopathological features and
outcome of gastric metastases from primary lung cancer: A case
report and systematic review. Oncol Lett. 9:1373–1379. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Casella G, Di Bella C, Cambareri AR, Buda
CA, Corti G, Magri F, Crippa S and Baldini V: Gastric metastasis by
lung small cell carcinoma. World J Gastroenterol. 12:4096–4097.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Pomerantz H and Margolin HN: Metastases to
the gastrointestinal tract from malignant melanoma. Am J Roentgenol
Radium Ther Nucl Med. 88:712–717. 1962.PubMed/NCBI
|
|
26
|
Sileri P, D'Ugo S, Del Vecchio Blanco G,
Lolli E, Franceschilli L, Formica V, Anemona L, De Luca C and
Gaspari AL: Solitary metachronous gastric metastasis from pulmonary
adenocarcinoma: Report of a case. Int J Surg Case Rep. 3:385–388.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Scobie BA: Malignant gastric ulcer due to
metastasis. Australas Radiol. 10:119–123. 1966. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Hsu CC, Chen JJ and Changchien CS:
Endoscopic features of metastatic tumors in the upper
gastrointestinal tract. Endoscopy. 28:249–253. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Wu MH, Lin MT and Lee PH:
Clinicopathological study of gastric metastases. World J Surg.
31:132–136. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Oda KH, Kondo H, Yamao T, Saito D, Ono H,
Gotoda T, Yamaguchi H, Yoshida S and Shimoda T: Metastatic tumors
to the stomach: Analysis of 54 patients diagnosed at endoscopy and
347 autopsy cases. Endoscopy. 33:507–510. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Kim YI, Kang BC and Sung SH: Surgically
resected gastric metastasis of pulmonary squamous cell carcinoma.
World J Gastrointest Surg. 5:278–281. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Reis-Filho JS, Carrilho C, Valenti C,
Leitão D, Ribeiro CA, Ribeiro SG and Schmitt FC: Is TTF1 a good
immunohistochemical marker to distinguish primary from metastatic
lung adenocarcinomas? Pathol Res Pract. 196:835–840. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Grigoriu BD, Depontieu F, Scherpereel A,
Gourcerol D, Devos P, Ouatas T, Lafitte JJ, Copin MC, Tonnel AB and
Lassalle P: Endocan expression and relationship with survival in
human non-small cell lung cancer. Clin Cancer Res. 12:4575–4582.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Waldner MJ, Wirtz S, Jefremow A, Warntjen
M, Neufert C, Atreya R, Becker C, Weigmann B, Vieth M, Rose-John S,
et al: VEGF receptor signaling links inflammation and tumorigenesis
in colitis-associated cancer. J Exp Med. 207:2855–2868. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Hatfield KJ, Lassalle P, Leiva RA, Lindås
R, Wendelboe Ø and Bruserud Ø: Serum levels of endothelium-derived
endocan are increased in patients with untreated acute myeloid
leukemia. Hematology. 16:351–356. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Voiosu T, Bălănescu P, Benguş A, Voiosu A,
Baicuş CR, Barbu M, Ladaru A, Nitipir C, Mateescu B, Diculescu M
and Voiosu R: Serum endocan levels are increased in patients with
inflammatory bowel disease. Clin Lab. 60:505–510. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Lee PC, Lo C, Lin MT, Liang JT and Lin BR:
Role of surgical intervention in managing gastrointestinal
metastases from lung cancer. World J Gastroenterol. 17:4314–4320.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Aokage K, Yoshida J, Ishii G, Takahashi S,
Sugito M, Nishimura M, Ochiai A and Nagai K: Long-term survival in
two cases of resected gastric metastasis of pulmonary pleomorphic
carcinoma. J Thorac Oncol. 3:796–799. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Salah S, Tanvetyanon T and Abbasi S:
Metastatectomy for extra-cranial extra-adrenal non-small cell lung
cancer solitary metastases: Systematic review and analysis of
reported cases. Lung Cancer. 75:9–14. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Goh BK, Yeo AW, Koong HN, Ooi LL and Wong
WK: Laparotomy for acute complications of gastrointestinal
metastases from lung cancer: Is it a worthwhile or futile effort?
Surg Today. 37:370–374. 2007. View Article : Google Scholar : PubMed/NCBI
|