Introduction
Cervical cancer is the second most common type of
cancer worldwide and the leading cause of cancer-related mortality
among women in developing countries (1). Although surgery is reserved for
early-stage disease whereas locally advanced cervical cancer (LACC)
is treated by chemoirradiation (CRT) (2–7), there
is an increasing number of studies on multimodal treatments of
LACC, including preoperative radiotherapy (RT) and chemotherapy
followed by surgery (8–11). Different multimodality regimens have
been suggested, including preoperative pelvic RT along with
cisplatin and 5-fluorouracil (8–10),
preoperative low-dose rate uterovaginal brachytherapy (9) and external pelvic CRT with low-dose
cisplatin-based chemotherapy followed by brachytherapy and
subsequent surgery (11). However,
despite the promising results, severe side effects and a delay in
definitive treatment remain a major concern. The aim of the present
study was to review our experience with neoadjuvant treatment
consisting of brachytherapy with one course of chemotherapy, which
may be completed within 1–2 weeks, immediately followed by radical
surgery. The short- and long-term outcomes of this regimen were
compared with those of standard CRT in stage IB2 and IIA
patients.
The present study was approved by the institutional
Ethics Review Board of Tianjin Central Hospital of Obstetrics and
Gynecology
Patients and methods
Patients and treatments
The present study retrospectively reviewed the
medical records of all patients clinically staged as IB2 or IIA
according to the International Federation of Gynecology and
Obstetrics (FIGO) 2000 criteria (12) at Tianjin Central Hospital of
Obstetrics and Gynecology (Nankai, China) over a 4-year period
(June 2006-June 2010). All the patients were clinically staged by
two gynecological oncologists and cervical biopsies were taken for
histological confirmation. The patients were allocated to either
the CRT group or the operation (OT) group, mainly based on age and
overall medical condition. Patients in the CRT group were treated
with external pelvic RT and brachytherapy with concomitant
chemotherapy. A dose of 50 Gy in 26–30 fractions was delivered to
the pelvis by 60Co external-beam RT (ERT).
192Ir intracavity brachytherapy was delivered once a
week during ERT to a total dose of 30–50 Gy at point A (defined as
the point of crossing of the uterine artery and ureter). ERT was
omitted on the day of intracavity brachytherapy. Chemotherapy was
started at the beginning of ERT. Patients either received 3–4
cycles of three-weekly cisplatin at 50 mg/m2 together
with 5-fluorouracil 20 mg/kg for 4 consecutive days, or cisplatin
at 50 mg/m2 and one dose of paclitaxel (135–175
mg/m2), based on the gynecologist's preference and the
financial situation of the patient. Patients in the OT group first
underwent preoperative vaginal brachytherapy twice within 1 week.
The median delivered dose to the clinical target volume (1 cm from
the radioactive source) was 20–24 Gy. One cycle of chemotherapy
with bleomycin, vincristine and cisplatin (2 mg vincristine on the
first day and a total of 50 mg/m2 cisplatin in five
doses over 5 consecutive days, and 15 mg bleomycin on alternate
days administered in three doses) was administered either during
the brachytherapy week or immediately thereafter. After neoadjuvant
treatment, the patients were examined by the same two gynecologists
to assess clinical response. Patients with a tumour size of <4
cm and clinical tumor improvement underwent radical hysterectomy
(RH) and systematic pelvic lymph-node dissection within 3 weeks of
neoadjuvant treatment. Patients not fulfilling these criteria were
re-assigned to the CRT group. Postoperatively, patients with
high-risk factors, including pelvic lymph node metastasis, positive
resection margin or parametrial invasion, received adjuvant
concurrent CRT. Patients who had ≥2 intermediate risk factors,
including deep stromal invasion, defined as invasion into the
cervical wall by more than half of its thickness, lymphovascular
space invasion, or a tumor size of ≥4 cm, received postoperative RT
alone. After completion of treatment, patients were followed up
every 3 months for the first 2 years, then every 6 months for the
following 3 years, and once a year thereafter, with clinical
examination, cervical or vault smear, and assessment of squamous
cell carcinoma antigen (SCC) antigen levels.
Statistical analysis
SPSS software version 13.0 (SPSS, Inc., Chicago, IL,
USA) was used for all statistical analyses. The χ2 test
or Fisher's exact test were used to compare discrete variables
among groups. Disease-free survival (DFS) was calculated from the
date of surgery or the date of completed CRT to the date of relapse
or the date of the last follow-up, and overall survival (OS) was
calculated from the date of diagnosis to the date of death or the
date of the last follow-up. Medians and life tables were computed
using the product limit estimate by the Kaplan-Meier method, and
the log-rank test was used to assess statistical significance.
Results
Patient characteristics
Between June 2006 and June 2010, a total of 70
patients were diagnosed with stage IB2 or IIA cervical cancer at
Tianjin Central Hospital of Obstetrics and Gynecology (Nankai,
China). The OT group included 50 patients and the CRT group 20
patients. The median follow-up period was 48 months (range, 30–72
months) for the CRT group and 54 months (range, 11–84 months) for
the OT group.
The patient and tumor characteristics, including
histological type, stage and grade, are summarized in Table I. There were no significant
differences in age, histology or grade between the two groups;
however, the OT group included a higher number of stage IB2
patients compared with the CRT group.
 | Table I.Characteristics of patients in the two
groups. |
Table I.
Characteristics of patients in the two
groups.
| Characteristics | CRT, n (n=20) | OT, n (n=50) | P-value |
|---|
| Median age
(years) | 47.5 | 43.8 | 0.09 |
| Histology |
|
| 0.19 |
| Squamous
cell carcinoma | 20 | 37 |
|
|
Adenocarcinoma | 0 | 9 |
|
|
Adenosquamous carcinoma | 0 | 2 |
|
|
Other | 0 | 2 |
|
| Stage |
|
| <0.01 |
| IB2 | 3 | 33 |
|
| IIA | 17 | 17 |
|
| Grade |
|
| 0.10 |
| I | 0 | 9 |
|
| II | 12 | 21 |
|
|
III | 8 | 20 |
|
Surgery
A total of 50 patients underwent Piver type III RH
and pelvic lymphadenectomy. Bilateral salpingo-oophorectomy was
performed in 9 patients with adenocarcinoma of the cervix and in 16
patients with SCC (menopause), 2 patients with adenosquamous
carcinoma and 2 patients with sarcoma. Ovarian transposition was
performed in 21 patients with SCC. Pelvic lymphadenectomy included
the external iliac, internal iliac, obturator and common iliac
lymph nodes.
The median interval between preoperative therapy and
surgery was 25.8 days (range, 15–50 days). Intra- and postoperative
complications were evaluated in all surgical patients. The median
operative time was 195 min (range, 110–285 min) and the median
blood loss was 583 ml (range, 200–1,400 ml). Of the 50 patients, 14
(28%) suffered an estimated blood loss of >800 ml during surgery
and were transfused. The median duration of hospital stay was 7
days (range, 4–18 days). The surgical specimens of 7 patients (14%)
contained no tumor following neoadjuvant CRT, while residual tumors
were found in the surgical specimens of 43 patients (86%). Among
patients with residual tumors, 42 (98%) had a residual tumor in the
cervix and 1 patient (2%) had a residual tumor in the vagina alone.
Among all patients in the OT group, 12 (24%) had pelvic lymph node
involvement, of whom 2 (4%) also had parametrial invasion. The
pathological results of the patients in the OT group are summarized
in Table II.
 | Table II.Pathology results of patients in the
operation group (n=50). |
Table II.
Pathology results of patients in the
operation group (n=50).
| Pathology
result | n (%) |
|---|
| Residual tumor | 43 (86) |
|
Squamous cell carcinoma | 31 |
|
Adenocarcinoma | 8 |
|
Adenosquamous carcinoma | 2 |
| Other
(sarcoma) | 2 |
| Parametrial
invasion | 2 (4) |
| Positive lymph
nodes | 12 (24) |
| Positive vaginal
margin | 1 (2) |
| ≥2 intermediate
risk factors | 23 (46) |
| Deep stromal
invasion | 20 (40) |
| Lymphovascular
space invasion | 13 (26) |
| Tumor size ≥4
cm | 42 (84) |
Postoperative therapy
A total of 26 patients received postoperative
therapy. A total of 13 patients (26%) with pelvic lymph node
involvement, parametrial invasion and positive vaginal margin
received concurrent CRT, among whom 6 received cisplatin (50
mg/m2) with 5-fluorouracil (20 mg/kg) daily for 4 days,
repeated twice with a 21-day interval, 5 received paclitaxel
(135–175 mg/m2) and cisplatin (50–60 mg/m2),
and 2 patients were treated with docetaxel (60 mg/m2)
and carboplatin (area under the curve 4) for 4–6 cycles.
Standard-field ERT was administered for 4–6 weeks after surgery to
a total dose of 40 Gy. Among these patients, 1 (2%) received a
parametrial boost to a total of 50 Gy due to a positive vaginal
margin and 2 patients (4%) received extended-field ERT due to
common iliac lymph node involvement. All the patients completed
their prescribed treatment.
Additional ERT was administered to 13 patients (26%)
with ≥2 intermediate risk factors. The median time interval between
surgery and RT was 40 days (range, 30–46 days). The median
delivered dose to the pelvis was 40 Gy (range, 30–50 Gy) for the
standard field; 2 of those patients did not complete the treatment
against doctors' recommendations.
Complications
Early complications, defined as those occurring
within 2 months after surgery, developed in 9 patients (18%). Among
those, 4 patients had fever >38°C for >2 days, 4 patients had
urine retention requiring catheterization for >2 weeks, and 1
patient had thrombophlebitis. As regards late complications, the
CRT group exhibited a higher frequency of symptomatic vaginal
stenosis, as well as proctitis and cystitis (35 vs. 4% and 20 vs.
2%, respectively) compared with the OT group. Grade 3–4
myelosuppression and lymphedema were similar between the two
groups. The late complications of the CRT and OT groups are listed
in Table III.
 | Table III.Late complications in the
chemoradiotherapy (CRT) and operation (OT) groups. |
Table III.
Late complications in the
chemoradiotherapy (CRT) and operation (OT) groups.
| Late
complications | CRT, n (%)
(n=20) | OT, n (%)
(n=50) | P-value |
|---|
| Grade 3–4
myelosuppression | 2 (10) | 2 (4) | 0.572 |
| Proctitis and
cystitis | 5 (20) | 1 (2) | 0.006 |
|
Ureterohydronephrosis | 0 (0) | 1 (2) | 1.000 |
| Lymphedema | 2 (10) | 3 (6) | 0.619 |
| Symptomatic vaginal
stenosis | 7 (35) | 2 (4) | 0.002 |
A subgroup analysis of stage IIA patients (n=17 in
each group) revealed that the rate of complications in the CRT
group was higher compared with that in the OT group (P=0.037;
Table IV).
 | Table IV.Late complications of stage IIA
patients in the chemoradiotherapy (CRT) and operation (OT)
groups. |
Table IV.
Late complications of stage IIA
patients in the chemoradiotherapy (CRT) and operation (OT)
groups.
| Late
complications | CRT, n (%)
(n=17) | OT, n (%)
(n=17) | P-value |
|---|
| Grade 3–4
myelosuppression | 1 (5.9) | 1 (5.9) | 0.037 |
| Proctitis and
cystitis | 3 (17.6) | 0 (0) |
|
|
Ureterohydronephrosis | 0 (0) | 0 (0) |
|
| Lymphedema | 1 (5.9) | 2 (11.8) |
|
| Symptomatic vaginal
stenosis | 5 (29.4) | 1 (5.9) |
|
Follow-up
As of June 2013, the median duration of follow-up
was 52 months (range, 11–84 months) in the entire patient cohort,
49 months (range, 30–72 months) in the CRT group and 54 months
(range, 11–84 months) in the OT group. All the patients were
followed up for at least 3 years. In the OT group, 5 patients (10%)
developed recurrence. Among them, 1 patient had a vaginal
recurrence after 29 months. One patient with cervical sarcoma had
pelvic metastasis within 2 years after treatment. Another patient
had parametrial recurrence 26 months later and succumbed to disease
progression; this patient exhibited a poor response to neoadjuvant
CRT and did not complete the postoperative ERT. One patient
suffered a metastatic relapse 6 months later, affecting the pelvis
and lung, and succumbed to the disease within 1 year; this patient
exhibited parametrial and pelvic lymph node involvement. Another
patient developed recurrence near the ureter and sigmoid colon
after 5 months and succumbed to the disease; this patient had not
received any postoperative therapy due to the absence of risk
factors. The 3-year overall and disease-free survival rates in the
OT group were 90% [95% confidence interval (CI): 72.93–83.07] and
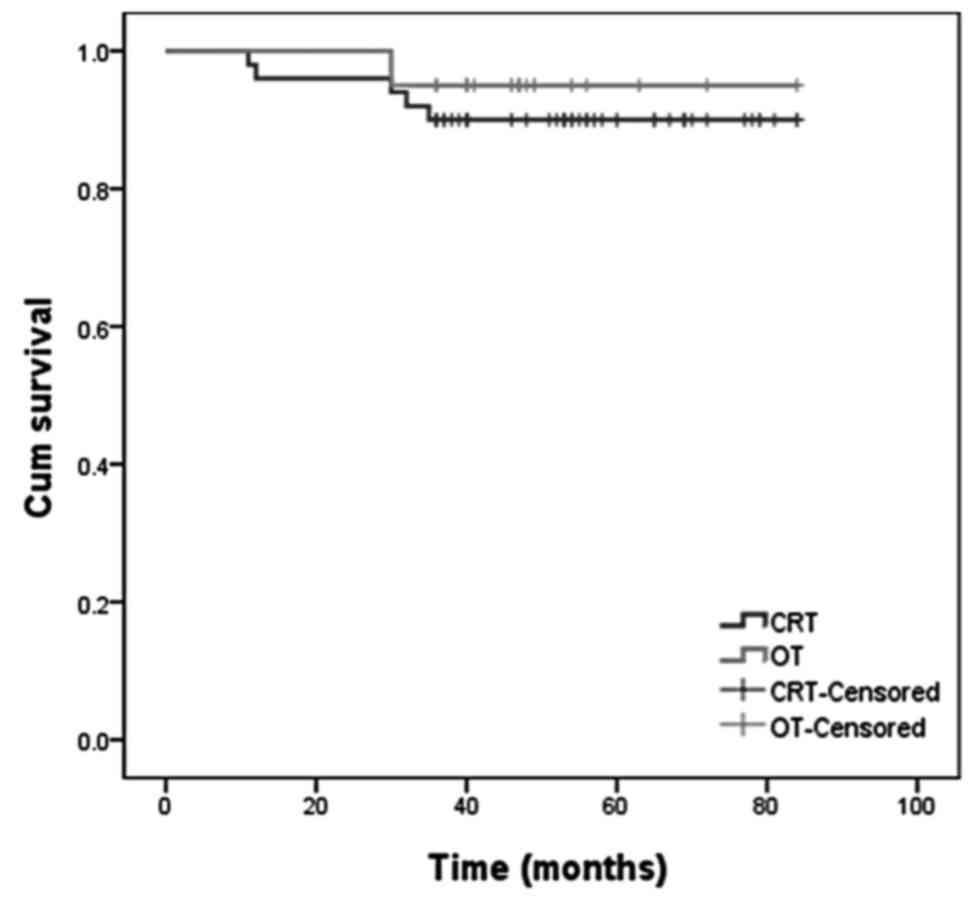
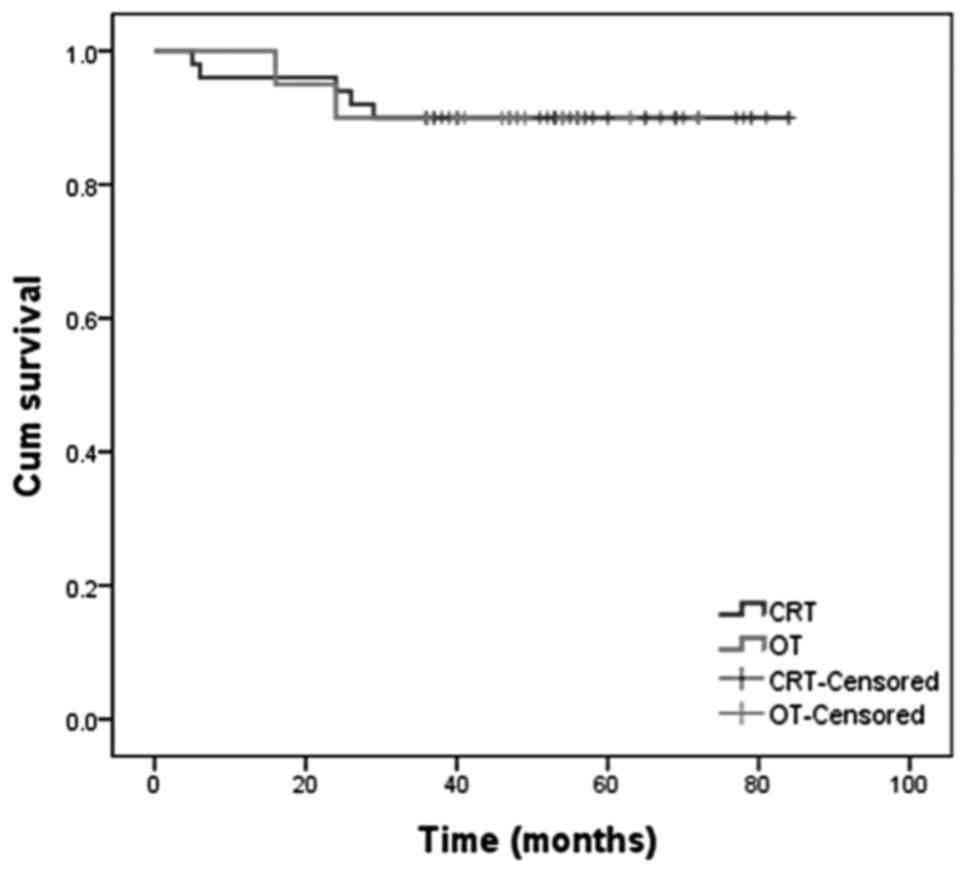
90% (95% CI: 71.84–82.96), respectively (Figs. 1 and 2).
In the CRT group, 2 patients (10%) suffered a
relapse: In 1 patient the relapse affected the pelvic lymph nodes,
while the other patient developed extensive disease (affecting the
pelvic lymph nodes, lung and supraclavicular lymph nodes). In the
CRT group, the 3-year overall survival and disease-free survival
rates were 95% (95% CI: 76.14–86.46) and 90% (95% CI: 59.94–73.66),
respectively (Figs. 1 and 2).
In the subgroup of patients with stage IIA disease,
1 patient in the CRT group and 2 patients in the OT group suffered
a recurrence and succumbed to the disease. There was no
statistically significant difference in survival between the two
groups.
Discussion
Early-stage cervical cancer is commonly treated by
surgery alone. For patients with FIGO stage IB2-IVA disease, also
referred to as LACC, the preferred therapy is concurrent CRT. The
use of a three-modality treatment, including RT, chemotherapy and
surgery, has been reported as early as 1994 (13,14).
Recent studies on preoperative chemotherapy and/or RT have shown
promising results, including better local control and overall
survival, while reducing short- and long-term complications,
resulting in a better quality of life (8,11,15–19).
Another advantage of neoadjuvant treatment is that the response in
the post-neoadjuvant biopsy sample may also identify patients who
are more suitable for definitive CRT rather than surgery. For
example, patients insensitive to neoadjuvant CRT were not
considered suitable for RH due to the higher likelihood of
high-risk factors, as they would have required further adjuvant
chemotherapy or RT, which may have increased the occurrence of
complications.
The present study used a three-modality treatment
strategy comprising RT, chemotherapy and surgery for patients with
stage IB2-IIA cervical cancer in an attempt to improve the overall
and disease-free survival and reduce the requirement for adjuvant
therapy, thereby minimizing complications.
The overall survival and disease-free survival rates
of the present study were similar between the two groups. The
3-year disease-free survival rate of 90% and OS rate of 91%
observed in the entire cohort were encouraging when compared with
the survival rate of 83% reported by previous studies (20–23). The
results of the present study were also comparable to those of
Ferrandina et al (8), who
achieved local and distant control rates of 93 and 92%,
respectively, in patients with stage IB2-II over a similar
follow-up time. The recurrence rate in the present study was 10% in
each group. This result is comparable with that of a previous
study, which reported a local recurrence rate of 16% in patients
with stage IB and II disease treated with RT alone (n=171) or
surgery (n=172) (22). At present,
the study by Landoni et al (22) is the only single randomized
controlled trial on patients with early-stage cervical cancer
comparing RH followed by tailored adjuvant therapy with primary
RT.
With regard to the possible worsening of survival
and recurrence rates with a longer follow-up period, it is
important to consider that 88% of recurrences/progressions in the
present study occurred within the first 2 years, which is in
accordance with previous reports (8,24). In
the present study, all the patients were followed up for at least 3
years and >70% of patients were followed up for >4 years.
The complications were similar between the CRT and
OT groups in terms of grade 3–4 myelosuppression (10 vs. 4%,
respectively), ureterohydronephrosis (0 vs. 2%, respectively) and
lymphedema (10 vs. 6%, respectively). However, in the CRT group,
symptomatic vaginal stenosis, as well as proctitis and cystitis,
were significantly more frequent compared with the OT group (35 vs.
4% and 20 vs. 2%, respectively). Landoni et al (22) reported late complications in 16% of
the patients treated exclusively with RT and in 29% of those
treated with surgery and adjuvant RT. Among the patients with stage
IB2 tumors, 84% required adjuvant RT due to high-risk
characteristics. By contrast, in the present study, only 50% of the
patients in the OT group required adjuvant RT. The lower number of
patients requiring adjuvant RT in the present study may have been
due to the reduction of risk factors by preoperative CRT. In the
present study, preoperative CRT included only one cycle of
cisplatin, vinblastine and bleomycin chemotherapy and vaginal
brachytherapy (20–24 Gy), which had few complications.
Various neoadjuvant treatments, including
chemotherapy alone, RT alone and CRT, have been applied to patients
with LACC (8–11,25–27). In
the present study, neoadjuvant CRT was used. However, instead of
the commonly used ERT, brachytherapy was used in the OT group, as
it has been shown to provide local control with fewer complications
(28–30).
The use of neoadjuvant chemotherapy has also been
reported. Compared with RT treatment alone, the 5-year overall
survival rate of patients subjected to neoadjuvant chemotherapy
followed by surgery was 15% increased (25). A Cochrane meta-analysis revealed that
progression-free survival was significantly improved by neoadjuvant
chemotherapy followed by surgery compared with surgery alone;
however, there was no overall survival benefit (27).
The aim of preoperative brachytherapy and
chemotherapy is to decrease the size of the tumor in order to
improve the feasibility of the operation. RT decreases the size of
most tumors and facilitates resection with clear margins, while
preoperative chemotherapy may decrease high-risk factors, such as
lymphovascular involvement, reducing the requirement for adjuvant
therapy and thereby minimizing the occurrence of complications
(31). In the present study, the
median time from neoadjuvant treatment to surgery was 25 days,
which did not cause any significant delay to definitive
treatment.
Radical surgery after CRT has been shown to reduce
the rate of local recurrence (18,32) and
to improve the prognosis for patients with bulky residual tumors
(33). Pathological assessment of
surgical specimens allows for the identification of patients with
poor response to CRT and also provides important prognostic
information to guide the adjuvant therapy (14,32,33).
In the present study, neoadjuvant CRT with
brachytherapy followed by RH achieved good control of stage IB2 and
IIA cervical cancer accompanied with a low incidence rate of
complications compared with CRT alone. Although the present study
was a retrospective analysis of a relatively small patient cohort
and the chemotherapy regimen was somewhat heterogenous, it is one
of few studies using brachytherapy instead of ERT for neoadjuvant
CRT (9,34,35).
There were no significant differences in survival between the OT
and CRT groups. A possible limitation of the present study was that
the median follow-up duration was only 52 months. However, since
most recurrences occurred within the first 24 months, a longer
follow-up may yield the same outcome. Another limitation of the
present study was the significant difference in stage distribution
between the two groups. A subgroup analysis of stage IIA patients
also demonstrated that the complication rates were lower in the OT
group. However, there was no difference in OS between the OT and
CRT groups.
In conclusion, the present study demonstrated that
neoadjuvant CRT may reduce the tumor volume in a proportion of
stage IB2-IIA patients, enabling them to undergo definitive surgery
rather than definitive CRT, with a reduced incidence of long-term
complications and similar overall survival compared with patients
receiving CRT alone. A larger randomized trial with a standardized
chemotherapy regimen and longer follow-up is required to confirm
these results.
References
|
1
|
Jemal A, Bray F, Center MM, Ferlay J, Ward
E and Forman D: Global cancer statistics. CA Cancer J Clin.
61:69–90. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Keys HM, Bundy BN, Stehamn FB, Muderspach
LI, Chafe WE, Suggs CL III, Walker JL and Gersell D: Cisplatin,
radiation and adjuvant hysterectomy compared with radiation and
adjuvant hysterectomy for bulky stage IB cervical carcinoma. N Engl
J Med. 340:1154–1161. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Morris M, Eifel PJ, Lu J, Grigsby PW,
Levenback C, Stevens RE, Rotman M, Gershenson DM and Mutch DG:
Pelvic radiation with concurrent chemotherapy compared with pelvic
and para-aortic radiation for high risk cervical cancer. N Engl J
Med. 340:1137–1143. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Whitney CW, Sause W, Bundy BN, Malfetano
JH, Hannigan EV, Fowler WC Jr, Clarke-Pearson DL and Liao SY:
Randomized comparison of fluorouracil plus cisplatin versus
hydroxyurea as an adjunct to radiation therapy in stages IIB-IVA
carcinoma of the cervix with negative para-aortic lymph nodes. A
Gynecologic Oncology Group and Southwest Oncology Group Study. J
Clin Oncol. 17:1339–1348. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Rose PG, Bundy BN, Watkins EB, Thigpen JT,
Deppe G, Maiman MA, Clarke-Pearson DL and Insalaco S: Concurrent
cisplatin-based radiotherapy and chemotherapy for locally advanced
cervical cancer. N Engl J Med. 340:1144–1153. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Peters WA III, Liu PY, Barrett RJ II,
Stock RJ, Monk BJ, Berek JS, Souhami L, Grigsby P, Gordon W Jr and
Alberts DS: Concurrent chemotherapy and pelvic radiation therapy
compared with pelvic radiation therapy alone as adjuvant therapy
after radical surgery in high-risk early-stage cancer of the
cervix. J Clin Oncol. 18:1606–1613. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Pearcey R, Brundage M, Drouin P, Jeffrey
J, Johnston D, Lukka H, MacLean G, Souhami L, Stuart G and Tu D:
Phase III trial comparing radical radiotherapy with and without
cisplatin chemotherapy in patients with advanced squamous cell
cancer of the cervix. J Clin Oncol. 20:966–972. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Ferrandina G, Legge F, Fagotti A, Fanfani
F, Distefano M, Morganti A, Cellini N and Scambia G: Preoperative
concomitant chemoradiotherapy in locally advanced cervical cancer:
Safety, outcome and prognostic measures. Gynecol Oncol. 107 1 Suppl
1:S127–S132. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Ngô C, Alran S, Plancher C, Fourchotte V,
Petrow P, Campitelli M, Batwa S, Sastre X, Salmon RJ and de la
Rochefordière A; Institut Curie Gynaecological Cancer Study Group
coordinated by P. Cottu, : Outcome in early cervical cancer
following pre-operative low dose rate brachytherapy: A ten-year
follow up of 257 patients treated at a single institution. Gynecol
Oncol. 123:248–252. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Ferrandina G, Margariti PA, Smaniotto D,
Petrillo M, Salerno MG, Fagotti A, Macchia G, Morganti AG, Cellini
N and Scambia G: Long-term analysis of clinical outcome and
complications in locally advanced cervical cancer patients
administered concomitant chemoradiation followed by radical
surgery. Gynecol Oncol. 119:404–410. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Classe JM, Rauch P, Rodier JF, Morice P,
Stoeckle E, Lasry S and Houvenaeghel G; Groupe des Chirurgiens de
Centre de Lutte Contre le Cancer (GCCLCC), : Surgery after
concurrent chemoradiotherapy and brachytherapy for the treatment of
advanced cervical cancer: Morbidity and outcome: Results of a
multicenter study of the GCCLCC. Gynecol Oncol. 102:523–529. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Benedet JL, Bender H, Jones H 3rd, Ngan HY
and Pecorelli S: FIGO staging classifications and clinical practice
guidelines in the management of gynecologic cancers. FIGO Committee
on Gynecologic Oncology. Int J Gynaecol Obstet. 70:209–262. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Resbeut M, Cowen D, Viens P, Noirclerc M,
Perez T, Gouvernet J, Delpero JR, Gamerre M, Boubli L and
Houvenaeghel G: Concomitant chemoradiation prior to surgery in the
treatment of advanced cervical carcinoma. Gynecol Oncol. 54:68–75.
1994. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Jurado M, Martínez-Monge R,
García-Foncillas J, Azinovic I, Aristu J, López-García G and
Brugarolas A: Pilot study of concurrent cisplatin, 5-fluorouracil,
and external beam radiotherapy prior to radical surgery +/−
intraoperative electron beam radiotherapy in locally advanced
cervical cancer. Gynecol Oncol. 74:30–37. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Mancuso S, Smaniotto D, Benedetti Panici
P, Favale B, Greggi S, Manfredi R, Margariti PA, Morganti AG,
Scambia G, Tortoreto F, et al: Phase I–II trial of preoperative
chemoradiation in locally advanced cervical carcinoma. Gynecol
Oncol. 78:324–328. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Distefano M, Ferrandina G, Smaniotto D,
Margariti AP, Zannoni G, Macchia G, Manfredi R, Mangiacotti MG,
Cellini N and Scambia G: Concomitant radiochemotherapy plus surgery
in locally advanced cervical cancer: Update of clinical outcome and
cyclooxygenase-2 as predictor of treatment susceptibility.
Oncology. 67:103–111. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Mariagrazia D, Anna F, Gabriella F,
Francesco F, Daniela S, Giuseppe D, Alessio M and Giovanni S:
Preoperative chemoradiotherapy in locally advanced cervical cancer:
Long-term outcome and complications. Gynecol Oncol. 99 3 Suppl
1:S166–S170. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Motton S, Houvenaeghel G, Delannes M,
Querleu D, Soulé-Tholy M, Hoff J and Lèguevaque P: Results of
surgery after concurrent chemoradiotherapy in advanced cervical
cancer: Comparison of extended hysterectomy and extrafascial
hysterectomy. Int J Gynecol Cancer. 20:268–275. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Touboul C, Uzan C, Mauguen A, Gouy S, Rey
A, Pautier P, Lhommé C, Duvillard P, Haie-Meder C and Morice P:
Prognostic factors and morbidities after completion surgery in
patients undergoing initial chemoradiation therapy for locally
advanced cervical cancer. Oncologist. 15:405–415. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Quinn MA, Benedet JL, Odicino F, et al:
Carcinoma of the cervix uteri. FIGO 26th annual report on the
results of treatment in gynaecological cancer. Int J Gynaecol
Obstet. 95:S43–S103. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Green JA, Kirwan JM, Tierney JF, Symonds
P, Fresco L, Collingwood M and Williams CJ: Survival and recurrence
after concurrent chemotherapy and radiotherapy for cancer of the
uterine cervix: A systematic review and meta-analysis. Lancet.
358:781–786. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Landoni F, Maneo A, Colombo A, Placa F,
Milani R, Perego P, Favini G, Ferri L and Mangioni C: Randomised
study of radical surgery versus radiotherapy for stage Ib-IIa
cervical cancer. Lancet. 350:535–540. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Keys HM, Bundy BN, Stehman FB, Muderspach
LI, Chafe WE, Suggs CL III, Walker JL and Gersell D: Cisplatin,
radiation and adjuvant hysterectomy compared with radiation and
adjuvant hysterectomy for bulky stage IB cervical cancer. N Engl J
Med. 340:1154–1161. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Houvenaeghel G, Lelievre L,
Gonzague-Casabianca L, Buttarelli M, Moutardier V, Goncalves A and
Resbeut M: Long-term survival after concomitant chemoradiotherapy
prior to surgery in advanced cervical carcinoma. Gynecol Oncol.
100:338–343. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Dueñas-Gonzalez A, Cetina L, Mariscal I
and de la Garza J: Modern management of locally advanced cervical
cancer. Cancer Treat Rev. 29:389–399. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Moore DH: Treatment of stage IB2 (bulky)
cervical carcinoma. Cancer Treat Rev. 29:401–406. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Rydzewska L, Tierney J, Vale CL and
Symonds PR: Neoadjuvant chemotherapy plus surgery versus surgery
for cervical cancer. Cochrane Database Syst Rev: CD007406. 2010.
View Article : Google Scholar
|
|
28
|
Nori D, Dasari N and Allbright RM:
Gynecologic brachytherapy I: Proper incorporation of brachytherapy
into the current multimodality management of carcinoma of the
cervix. Semin Radiat Oncol. 12:40–52. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Nag S, Chao C, Erickson B, Fowler J, Gupta
N, Martinez A and Thomadsen B; American Brachytherapy Society, :
The American Brachytherapy Society for low-dose-rate brachytherapy
for carcinoma of the cervix. Int J Radiat Oncol Biol Phys.
52:33–48. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Logsdon MD and Eifel PJ: Figo IIIB
squamous cell carcinoma of the cervix: An analysis of prognostic
factors emphasizing the balance between external beam and
intracavitary radiation therapy. Int J Radiat Oncol Biol Phys.
43:763–775. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Hu JL, Wu LY, Li N, Li XG, Huang MN and
Zhang R: Comparative analysis of neoadjuvant therapies in stage Ib2
and IIa2 cervical carcinoma. Chin J Obstet Gynecol. 47:452–457.
2012.(In Chinese).
|
|
32
|
Keys HM, Bundy BN, Stehman FB, Okagaki T,
Gallup DG, Burnett AF, Rotman MZ and Fowler WC Jr; Gynecologic
Oncology Group, : Radiation therapy with and without extrafascial
hysterectomy for bulky stage IB cervical carcinoma: A randomized
trial of the Gynecologic Oncology Group. Gynecol Oncol. 89:343–353.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Houvenaeghel G, Lelievre L, Buttarelli M,
Jacquemier J, Carcopino X, Viens P and Gonzague-Casabianca L:
Contribution of surgery in patientswith bulky residual disease
after chemoradiation for advanced cervical carcinoma. Eur J Surg
Oncol. 33:498–503. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
de la Rochefordiere A, Kirova Y, Alran S,
et al: Pre-operative concomitant radio-chemotherapy in Bulky
carcinoma of the cervix: a single institution study. Clin Med
Oncol. 2:227–236. 2008.PubMed/NCBI
|
|
35
|
Pearcey RG, Peel KR, Thorogood J, et al:
The value of pre-operative intracavitary radiotherapy in patients
treated by radical hysterectomy and pelvic lymphadenectomy for
invasive carcinoma of the cervix. Clin Radiol. 39:95–98. 1988.
View Article : Google Scholar : PubMed/NCBI
|