Introduction
Bladder cancer (BC) is the ninth most commonly
diagnosed cancer in the world (1).
Approximately 75% of cases are non-muscle invasive bladder cancer
(NMIBC), including Ta, carcinoma in situ (CIS; Tis), and T1
tumors. NMIBC is associated with favorable cancer-specific survival
compared to muscle invasive bladder cancer (MIBC) (2,3). Among
NMIBC, T1 tumor, which invades subepithelial connective tissue, has
a high risk for recurrence and/or progression. Approximately
one-third of T1 tumors develop recurrence and one-third eventually
progress to MIBC (4,5).
Bladders with T1 tumors may have CIS, which is a
flat, high-grade, often multifocal, non-invasive urothelial
carcinomatous lesion. Thus, random bladder biopsy, in which tissue
is taken from the normal looking bladder mucosa, may be needed to
detect CIS. CIS lesions are usually macroscopically
indistinguishable from non-cancerous mucosa and can exist far away
from the visible tumors. The European Association of Urology (EAU)
guidelines recommend random bladder biopsy in patients with
positive urine cytology (5).
However, clinical significance of random bladder biopsy in primary
NMIBC has not been fully evaluated. In this study, we investigated
the significance of positive random bladder biopsy in primary T1
NMIBC.
Patients and methods
This study was approved by the Institutional Review
Board of University of Tokyo Hospital (Tokyo, Japan; no. 3124).
Written informed consent was obtained from each patient before
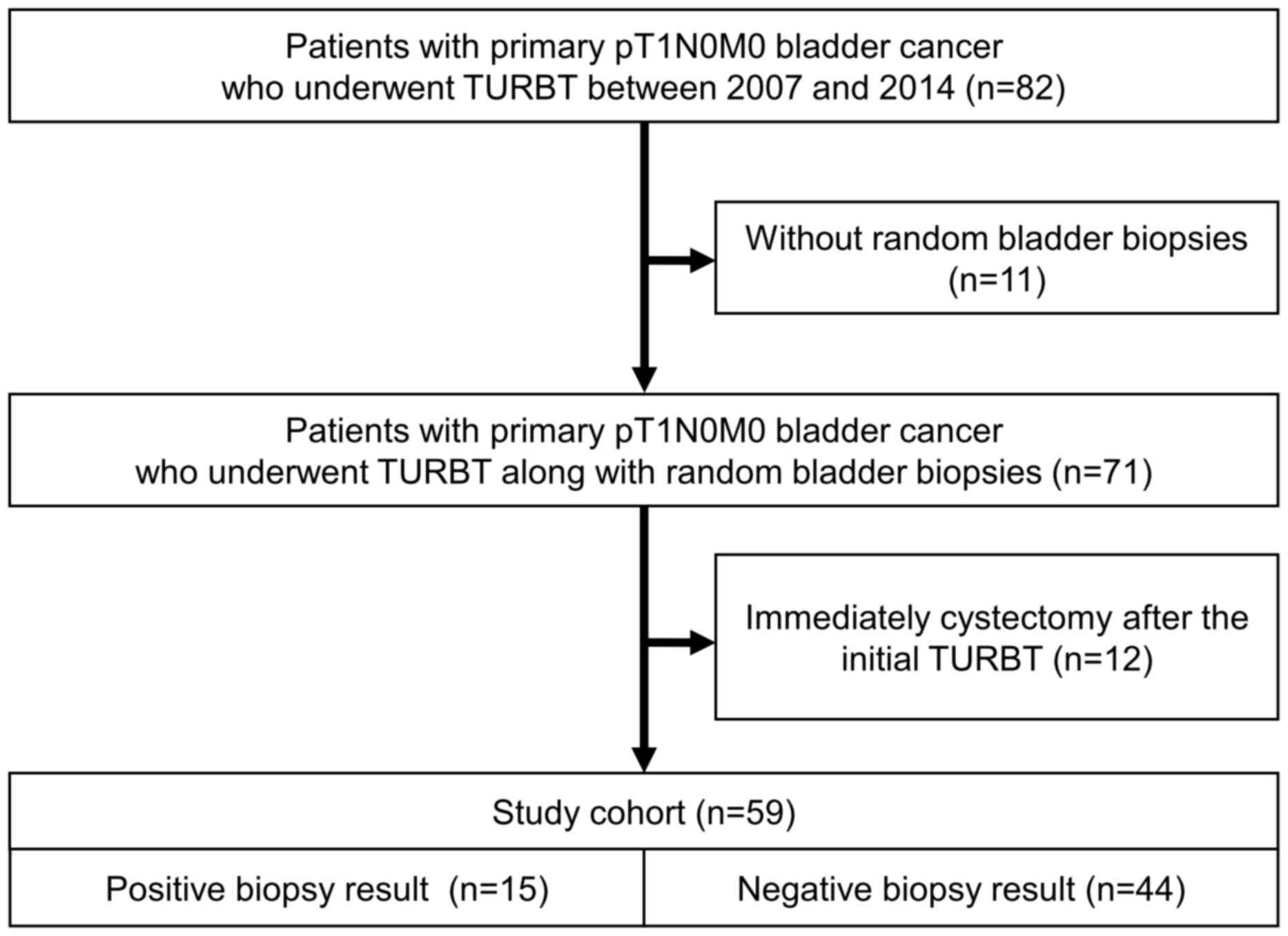
surgery. We retrospectively reviewed medical records of 82 patients
with primary pT1N0M0 bladder cancer who underwent transurethral
resection of the bladder tumor (TURBT) at The University of Tokyo
Hospital between January 2007 and December 2014. Amongst these 82
patients, random bladder biopsy was performed along with TURBT in
71 patients. After excluding 12 patients who received radical
cystectomy immediately (within 3 months) after the initial TURBT,
we included the remaining 59 patients in the present study cohort
(Fig. 1).
Random bladder biopsy was defined as cold-cup biopsy
of normal-looking tissues. Although this biopsy is designated as
‘random’, the biopsy samples were systematically obtained from
eight pre-specified areas: The bladder trigone, right wall, left
wall, posterior wall, dome, anterior wall, bladder neck, and
prostatic urethra of both sides. The urethra was not sampled in
female patients. Biopsy targeting suspicious (i.e. reddish) tissues
was not regarded as random biopsy. All biopsy specimens were
reviewed by a single pathologist (T.M.). Histological diagnosis was
performed according to the World Health Organization (WHO) 2016
classification system (6).
Pirarubicin (THP) 20 mg was routinely instilled
immediately after TURBT as intravesical chemotherapy. A second
TURBT was carried out for T1 bladder tumors, if the specimen lacked
adequate muscle layer for histological examination. Bacillus
Calmette-Guerin (BCG) instillation for 6 to 8 consecutive weeks
of Immunobladder® (Tokyo 172 strain) or
ImmuCyst® (Connaught strain) was indicated. However, the
attending physician and/or patient sometimes decided against BCG
because of the risk of side effects. Post-surgical follow-up
constituted cystoscopy and urine cytology, every 3 months for the
first 2 years, then every 6 months until 5 years, and annually
thereafter.
The primary endpoint was recurrence-free survival
(RFS). Recurrence was defined as histologically proven intravesical
recurrence. The secondary endpoint was progression-free survival
(PFS). Progression was defined as appearance of MIBC and/or nodal
or distant metastasis. Recurrence-free interval and
progression-free interval were defined as the time from TURBT to
recurrence or progression.
The correlation of the random biopsy result with age
was evaluated using Mann-Whitney U test, and the correlation with
sex, urine cytology, grade, concomitant CIS, multifocality, tumor
size, intravesical chemotherapy, and BCG instillation was evaluated
using Pearson's chi-square test.
RFS and PFS were estimated by the Kaplan-Meier
method and compared using the Log-rank test. For multivariate
analysis, Cox's proportional hazards regression model was used with
a backward stepwise procedure (entry, 0.05; removal, 0.10). All
statistical analyses were performed using JMP® 11 (SAS
Institute Inc., Cary, NC, USA). Probability P-values <0.05 were
considered statistically significant.
Results
The study cohort comprised 48 males and 11 females,
with a median age of 74 years [interquartile range (IQR), 64–81
years]. CIS lesions were detected in random biopsy samples in 15
(25%) patients (Table I). Positive
random biopsy completely overlapped with concomitant CIS, and was
significantly correlated with positive cytology (P=0.011) and main
tumor size less than 3 cm (P=0.008).
 | Table I.Patient characteristics. |
Table I.
Patient characteristics.
|
|
| Random bladder
biopsy |
|
|---|
|
|
|
|
|
|---|
| Variables | Total (n=59) | Positive (n=15) | Negative (n=44) | P-value |
|---|
| Age, years, median
(IQR) | 74 (64–81) | 79 (69–84) | 72 (64–79) | 0.2161a |
| Sex |
|
|
| 0.8766b |
| Male | 48 (81%) | 12 (80%) | 36 (82%) |
|
|
Female | 11 (19%) | 3 (20%) | 8 (18%) |
|
| Urine cytology |
|
|
| 0.0111b,c |
|
Negative | 28 (47%) | 3 (20%) | 25 (57%) |
|
|
Positive | 31 (53%) | 12 (80%) | 19 (43%) |
|
| Grade |
|
|
| 0.0789b |
| Low | 5 (8%) | 0 (0%) | 5 (11%) |
|
| High | 54 (92%) | 15 (100%) | 39 (89%) |
|
| Concomitant CIS |
|
|
|
<0.0001b,c |
|
Negative | 44 (75%) | 0 (0%) | 44 (100%) |
|
|
Positive | 15 (25%) | 15 (100%) | 0 (0%) |
|
| Multifocality |
|
|
| 0.7076b |
|
Solitary | 30 (51%) | 7 (47%) | 23 (52%) |
|
|
Multiple | 29 (49%) | 8 (53%) | 21 (48%) |
|
| Tumor size, cm |
|
|
| 0.0084b,c |
|
<3 | 35 (59%) | 13 (87%) | 22 (50%) |
|
| ≥3 | 24 (41%) | 2 (13%) | 22 (50%) |
|
| 2nd TURBT |
|
|
| 0.6798b |
| No | 21 (36%) | 6 (40%) | 15 (34%) |
|
| Yes | 38 (64%) | 9 (60%) | 29 (66%) |
|
| Instillation of
intravesical chemotherapy |
|
|
| 0.7076b |
| No | 29 (49%) | 8 (53%) | 21 (48%) |
|
|
Yes | 30 (51%) | 7 (47%) | 23 (52%) |
|
| BCG
instillation |
|
|
| 0.0555b |
| No | 19 (32%) | 2 (13%) | 17 (39%) |
|
|
Yes | 40 (68%) | 13 (87%) | 27 (61%) |
|
During the median follow-up of 32 months (IQR, 18–51
months), 15 (25%) patients had recurrence at a median time of 24
months (IQR, 12.5–41.5 months), and five (8%) patients developed
progression at a median time of 31 months (IQR, 16.5–49.5
months).
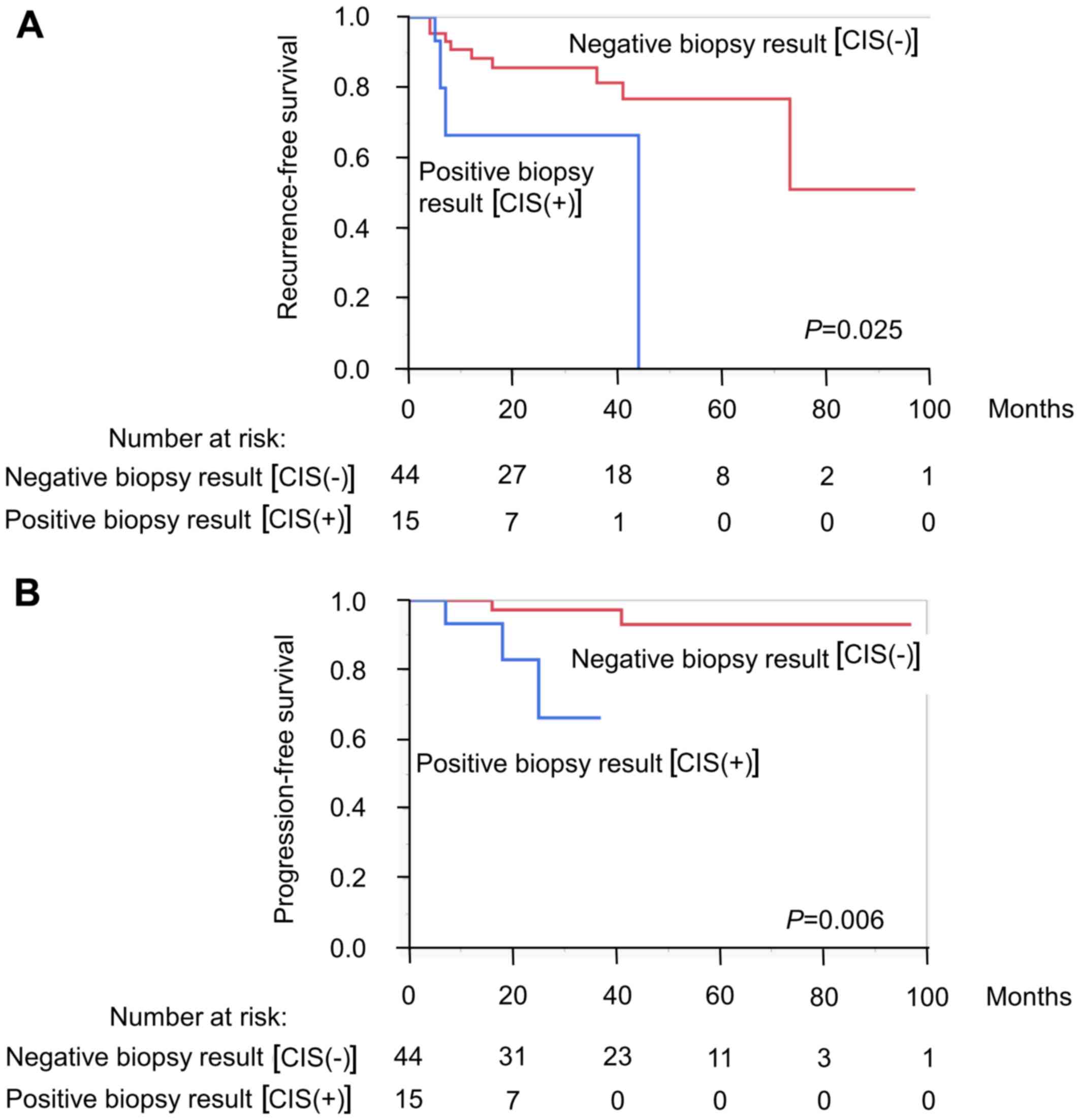
The estimated RFS rate at 3 years in patients with
positive random biopsy (67%) was significantly lower than that in
patients with negative biopsy (81%) (P=0.025, Fig. 2A); so was the PFS rate at 3 years
(66% for positive biopsy and 97% for negative biopsy, respectively;
P=0.006, Fig. 2B). Risk factors that
were analyzed in the study included patient age (<74 vs. ≥74
years), sex, urine cytology (negative vs. positive), random biopsy
result (negative vs. positive), tumor grade (low vs. high),
multifocality, tumor size (<3 vs. ≥3 cm), intravesical
chemotherapy, and BCG instillation (Table II). On the multivariate analysis,
positive random biopsy was found to be an independent poor
prognostic factor for recurrence (P=0.014, hazard ratio=4.69, 95%
confidence interval 1.40–15.4). Although positive random biopsy
results were also associated with poor PFS (P=0.006, Fig. 2B), multivariate analysis for PFS
could not be performed because of infrequent events (n=5).
 | Table II.Univariate and multivariate analyses
of risk factors for recurrence. |
Table II.
Univariate and multivariate analyses
of risk factors for recurrence.
|
| Univariate | Multivariate |
|---|
|
|
|
|
|---|
| Factor | HR (95% CI) | P-value | HR (95% CI) | P-value |
|---|
| Age, years |
| 0.1219 |
|
|
|
<74 | Reference |
|
|
|
|
≥74 | 2.30
(0.80–7.46) |
|
|
|
| Sex |
| 0.4524 |
|
|
|
Male | Reference |
|
|
|
|
Female | 1.58
(0.44–4.66) |
|
|
|
| Urine cytology |
| 0.9563 |
|
|
|
Negative | Reference |
|
|
|
|
Positive | 1.03
(0.35–3.03) |
|
|
|
| Random biopsy
result |
| 0.0431a |
| 0.0136a |
|
Negative | Reference |
| Reference |
|
|
Positive | 3.25
(1.04–9.72) |
| 4.69
(1.40–15.4) |
|
| Grade |
| 0.8007 |
|
|
|
Low | Reference |
|
|
|
|
High | 1.29
(0.25–23.5) |
|
|
|
| Multifocality |
| 0.8708 |
|
|
|
Solitary | Reference |
|
|
|
|
Multiple | 1.09
(0.37–3.20) |
|
|
|
| Tumor size |
| 0.8698 |
|
|
| <3
cm | Reference |
|
|
|
| ≥3
cm | 0.92
(0.31–2.56) |
|
|
|
| Instillation of
intravesical chemotherapy |
| 0.0756 |
|
|
| No | Reference |
|
|
|
|
Yes | 0.37
(0.10–1.11) |
|
|
|
| BCG
instillation |
| 0.1896 |
| 0.0536 |
| No | Reference |
| Reference |
|
|
Yes | 0.49
(0.17–1.44) |
| 0.32
(0.10–1.02) |
|
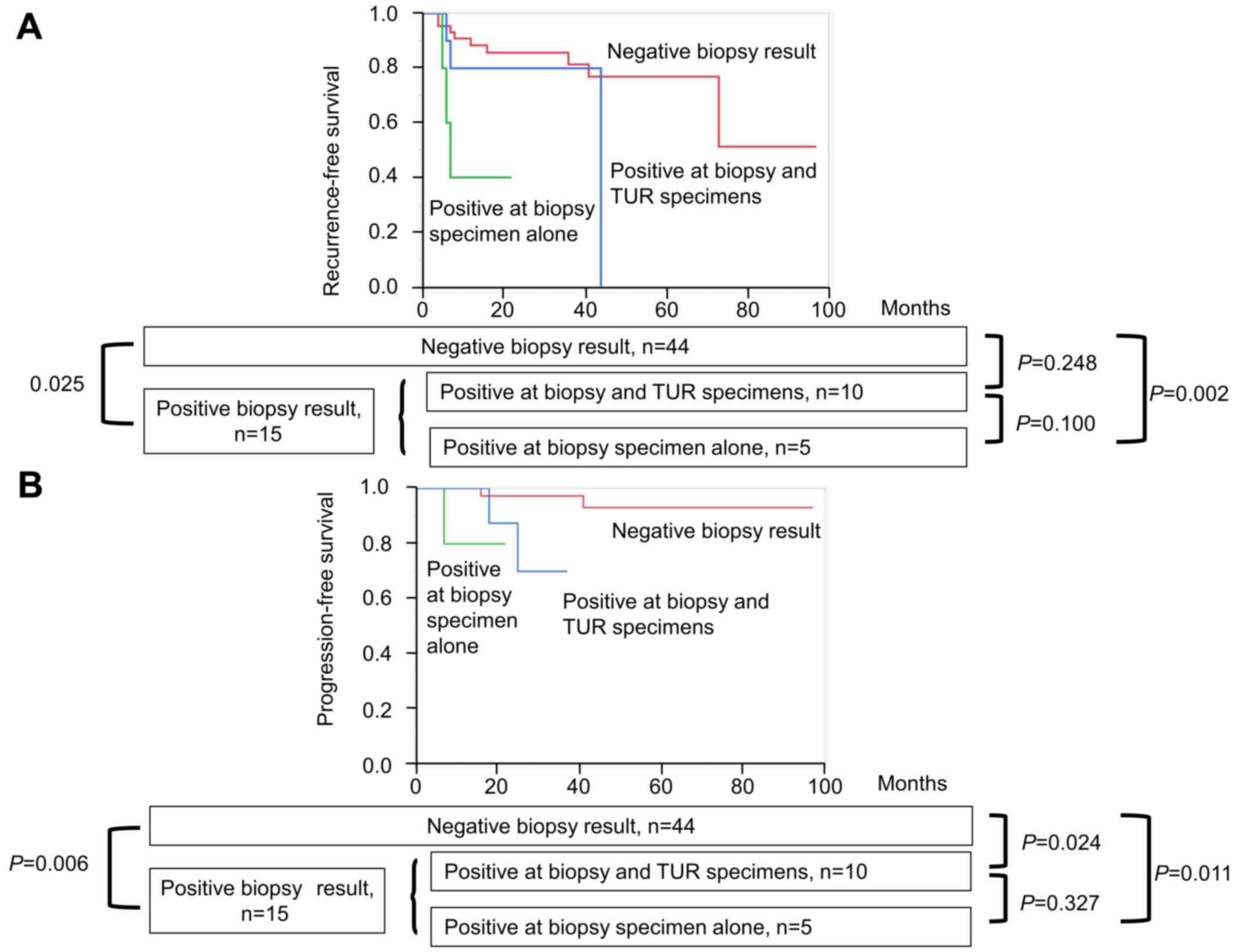
When the 15 patients with positive random biopsy
were divided into those with positive results at biopsy sites alone
(n=5) and those with positive results at both biopsy sites and
adjacent to visible tumors of TUR samples (n=10), the former showed
non-significantly lower RFS and PFS than the latter (P=0.100,
P=0.327, respectively; Fig. 3). The
former five patients had significantly lower RFS and PFS than those
without CIS (P=0.002 and P=0.011, respectively), while the latter
10 patients had non-significantly lower RFS and significantly lower
PFS than those without CIS (P=0.248 and P=0.024, respectively).
Discussion
In the present study, CIS was detected by random
bladder biopsy in 25% of the patients with primary pT1 bladder
cancer. The presence of CIS in one-third of these patients could
not have been proved without random biopsy. Positive result at
random biopsy, equivalent to the presence of CIS, was an
independent predictor of recurrence.
The EAU guidelines recommend that all suspicious
areas in the bladder should be biopsied. On the other hand, random
bladder biopsies are not recommended for all patients with NMIBC
but only for the patients with positive urine cytology or with
high-risk non-papillary exophytic tumors (5). Bladder biopsy carries risks of
bleeding, infection, and even the possible implantation of tumor
cells at the biopsied mucosa (7).
Thus, the indications of random bladder biopsies needs to be
carefully optimized. Previous studies have reported that random
bladder biopsy has demonstrated positive results in 5 to 30% of the
patients with all-risk NMIBC, and in as high as 60% of the patients
with high-risk NMIBC (8–12). However, prognostic significance of
random bladder biopsy in primary NMIBC has not been well
defined.
In this study, 15 (25%) of the 59 patients
demonstrated carcinomatous lesions by random bladder biopsy, all of
which were CIS. Conversely, all 15 cases with CIS were positive on
random biopsy. In more detail, five (33%) patients had CIS only in
biopsy specimens, whereas the remaining 10 (67%) had CIS in both
biopsy and TUR specimens. The existence of CIS, equivalent to
positive random biopsy result, was shown to be an independent poor
prognostic factor for recurrence and was associated with disease
progression in univariate analysis. Although these results are in
line with previous reports assessing the prognostic significance of
CIS (13), it should be noted that
one-third of CIS lesions could not have been detected without
random bladder biopsy. Moreover, patients who yielded positive
results only in biopsy specimens had worse RFS and PFS than the
remaining two-third patients who yielded positive results in both
biopsy and TUR specimens; however, the differences were not
statistically significant. Our results suggested that random
bladder biopsy might be justified for patients with T1 tumors.
T1 NMIBC generally carries high risk of recurrence
and progression. Early cystectomy should be considered for
carefully selected patients with T1 NMIBC (14). Several studies have identified
potential prognostic factors in T1 NMIBC, including sex, age, tumor
diameter, CIS, tumor grade, multifocality, lymphovascular invasion,
lamina propria invasion, solid tumor pattern, and
immunohistochemical detection of p53 in the tumor-cell nuclei
(13,15–20).
However, optimal prediction of recurrence and progression of tumor
is still under debate. Our results have provided a rationale for
the precise detection of CIS by random bladder biopsy.
The formation of tumors in multiple foci throughout
the entire urinary tract is one of the most important features of
urothelial cancer. CIS is a flat, intraurothelial neoplasm and
believed to be a precursor of invasive bladder cancer. The
detection of CIS was traditionally performed with combination of
urine cytology, cystoscopy, and random bladder biopsy. Although
experienced urologists may be able to point out possible CIS areas
on cystoscopy, these lesions may be overlooked without random
bladder biopsy. Recent advances in fluorescence cystoscopy and
narrow-band imaging may aid in detecting flat CIS lesions, and
their results need to be compared with those of random bladder
biopsy (21,22).
The present study has several limitations. This is a
retrospective analysis of a relatively small study cohort at a
single center. Treatment scheme including second TUR and BCG
administration had not been standardized. There were only 5
patients with positive results in biopsy specimens alone, and only
5 of 59 patients developed progression. Despite that the
differences were statistically significant and that their
implications were clinically meaningful, the results need to be
interpreted cautiously. Thus, a large-scale multicenter study would
be necessary to validate the findings of our study.
In conclusion, Positive bladder random biopsy,
equivalent to the presence of CIS, was an independent predictor of
recurrence in primary T1 bladder cancer. Only 11% of the patients
with negative urine cytology had CIS, and therefore the results of
random biopsy affect only limited fraction of patients. However,
given that one-third of CIS lesions could not have been detected
without biopsy, random bladder biopsy may be considered for
patients with T1 tumors.
Acknowledgements
The authors would like to thank all staff in the
Department of Urology of the University of Tokyo for their help and
support.
Funding
No funding was received.
Availability of data and materials
The datasets analyzed during the current study are
not publicly available due to the regulations of the Institutional
Review Board (IRB) of University of Tokyo Hospital, however are
available from the corresponding author on reasonable request and
after approval by IRB.
Authors' contributions
MO, ST, TN, conception and design. MO, ST, TM, SM,
JM, acquisition of data. MO, ST, TN, TM, SM, JM, AM, HM, TF, HF,
HK, YI, YH, analysis and interpretation of data, final approval of
the version to be published, sufficient participation in the work
to take public responsibility for appropriate portions of the
content and have agreed to be accountable for all aspects of the
work in ensuring that questions related to the accuracy or
integrity of any part of the work are appropriately investigated
and resolved.
MO and TN, drafting of the manuscript. ST, TM, SM,
JM, AM, HM, TF, HF, HK, YI, YH, critical revision of the manuscript
for important intellectual content. YI also contributed
administrative support and YH as supervisor.
Ethics approval and consent to
participate
The present study was approved by the Institutional
Review Board of University of Tokyo Hospital (Tokyo, Japan; no.
3124). Written informed consent was obtained from each patient
prior to surgery.
Consent for publication
Written informed consent was obtained from each
patient prior to surgery.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Ferlay J, Shin HR, Bray F, Forman D,
Mathers C and Parkin DM: Estimates of worldwide burden of cancer in
2008: GLOBOCAN 2008. Int J Cancer. 127:2893–2917. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Burger M, Catto JW, Dalbagni G, Grossman
HB, Herr H, Karakiewicz P, Kassouf W, Kiemeney LA, La Vecchia C,
Shariat S and Lotan Y: Epidemiology and risk factors of urothelial
bladder cancer. Eur Urol. 63:234–241. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Steinmaus C, Ferreccio C, Acevedo J, Yuan
Y, Liaw J, Durán V, Cuevas S, García J, Meza R, Valdés R, et al:
Increased lung and bladder cancer incidence in adults after in
utero and early-life arsenic exposure. Cancer Epidemiol Biomarkers
Prev. 23:1529–1538. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Van Rhijn BW, Burger M, Lotan Y, Solsona
E, Stief CG, Sylvester RJ, Witjes JA and Zlotta AR: Recurrence and
progression of disease in non-muscle invasive bladder cancer: From
epidemiology to treatment strategy. Eur Urol. 56:430–442. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Babjuk M, Burger M, Zigeuner R, Shariat
SF, van Rhijn BW, Compérat E, Sylvester RJ, Kaasinen E, Böhle A,
Redorta Palou J, et al: EAU guidelines on non-muscle-invasive
urothelial carcinoma of the bladder: update 2013. Eur Urol.
64:639–653. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Moch H, Cubilla AL, Humphrey PA, Reuter VE
and Ulbright TM: The 2016 WHO Classification of Tumours of the
urinary system and male genital organs-part A: Renal, penile, and
testicular tumours. Eur Urol. 70:93–105. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Soloway MS and Masters S: Urothelial
susceptibility to tumor cell implantation. Influence of
cauterization. Cancer. 46:1158–1163. 1980. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Fradet Y, Grossman HB, Gomella L, Lerner
S, Cookson M, Albala D and Droller MJ: PC B302/01 Study Group: PC
B302/01 study group. A comparison of hexaminolevulinate
fluorescence cystoscopy and white light cystoscopy for the
detection of carcinoma in situ in patients with bladder cancer: A
phase III, multicenter study. J Urol. 178:68–73. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
May F, Treiber U, Hartung R and Schwaibold
H: Significance of random bladder biopsies in superficial bladder
cancer. Eur Urol. 44:47–50. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Taguchi I, Gohji K, Hara I, Gotoh A,
Yamada Y, Yamanaka K, Okada H, Arakawa S and Kamidono S: Clinical
evaluation of random biopsy of urinary bladder in patients with
superficial bladder cancer. Int J Urol. 5:30–34. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Fujimoto N, Harada S, Terado M, Sato H and
Matsumoto T: Multiple biopsies of normal-looking urothelium in
patients with superficial bladder cancer: Are they necessary? Int J
Urol. 10:631–635. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Hara T, Takahashi M, Gondo T, Nagao K,
Ohmi C, Sakano S, Naito K and Matsuyama H: Risk of concomitant
carcinoma in situ determining biopsy candidates among primary
non-muscle-invasive bladder cancer patients: Retrospective analysis
of 173 Japanese cases. Int J Urol. 16:293–298. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Denzinger S, Otto W, Fritsche HM, Roessler
W, Wieland WF, Hartmann A and Burger M: Bladder sparing approach
for initial T1G3 bladder cancer: Do multifocality, size of tumor or
concomitant carcinoma in situ matter? A long-term analysis of 132
patients. Int J Urol. 14:995–999. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Herr HW and Sogani PC: Does early
cystectomy improve the survival of patients with high risk
superficial bladder tumors? J Urol. 166:1296–1299. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Palou J, Sylvester RJ, Faba OR, Parada R,
Peña JA, Algaba F and Villavicencio H: Female gender and carcinoma
in situ in the prostatic urethra are prognostic factors for
recurrence, progression and disease-specific mortality in T1G3
bladder cancer patients treated with Bacillus
Calmette-Guérin. Eur Urol. 62:118–125. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Fernandez-Gomez J, Solsona E, Unda M,
Martinez-Piñeiro L, Gonzalez M, Hernandez R, Madero R, Ojea A,
Pertusa C, Rodriguez-Molina J, et al: Club Urológico Español de
Tratamiento Oncológico CUETO). Prognostic factors in patients with
non-muscle-invasive bladder cancer treated with Bacillus
Calmette-Guérin: Multivariate analysis of data from four
randomized CUETO trials. Eur Urol. 53:992–1001. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Fukumoto K, Kikuchi E, Mikami S, Miyajima
A and Oya M: Lymphovascular invasion status at transurethral
resection of bladder tumors may predict subsequent poor response of
T1 tumors to Bacillus Calmette-Guérin. BMC Urol. 16:52016.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Orsola A, Trias I, Raventos CX, Español I,
Cecchini L, Búcar S, Salinas D and Orsola I: Initial high-grade T1
urothelial cell carcinoma: Feasibility and prognostic significance
of lamina propria invasion microstaging (T1a/b/c) in BCG-treated
and BCG-non-treated patients. Eur Urol. 48:231–238. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Andius P, Johansson SL and Holmang S:
Prognostic factors in stage T1 bladder cancer: Tumor pattern (solid
or papillary) and vascular invasion more important than depth of
invasion. Urology. 70:758–762. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Esrig D, Elmajian D, Groshen S, Freeman
JA, Stein JP, Chen SC, Nichols PW, Skinner DG, Jones PA and Cote
RJ: Accumulation of nuclear p53 and tumor progression in bladder
cancer. N Engl J Med. 331:1259–1264. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Drejer D, Béji S, Oezeke R, Nielsen AM,
Høyer S, Johansen Bjerklund TE, Lam GW and Jensen JB: Comparison of
white light, photodynamic diagnosis and narrow-band imaging in
detection of carcinoma in situ or flat dysplasia at transurethral
resection of the bladder: The DaBlaCa-8 study. Urology.
102:138–142. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Schmidbauer J, Witjes F, Schmeller N,
Donat R, Susani M and Marberger M: Hexvix PCB301/01 study group:
Improved detection of urothelial carcinoma in situ with
hexaminolevulinate fluorescence cystoscopy. J Urol. 171:135–138.
2004. View Article : Google Scholar : PubMed/NCBI
|