Introduction
Breast cancer represents the second most frequent
cause of brain metastases (after lung cancer), diagnosed in
approximately 15% of advanced breast cancer cases (1,2).
However, the appearance of breast cancer metastasis in a meningioma
is extremely rare. Distinguishing between overt breast cancer
intracranial metastasis and metastasis in a meningioma is important
as the prognosis for these two entities could be very diverse. The
terms ‘tumor-to-tumor metastasis’ and ‘collision tumor’ have been
used often interchangeably in literature to describe cases of
intra-meningioma metastasis. The term collision indicates the
presence of two histologically distinct tumors occurring
concurrently in the same anatomic location with some intermingling.
Tumor-to-tumor metastasis definition requires the presence of two
distinct histopathological features and the encasement of the
metastatic focus with a rim of distinct host tumor tissue.
With regards to breast cancer and meningioma, the
majority of previously reported cases have highlighted the
presentation of tumor-to-tumor metastasis or collision tumor in
patients with a previous history of breast cancer. However, our
case has a unique presentation of an intracranial meningioma with
collision breast cancer as the primary presentation leading to the
diagnosis of metastatic breast cancer. There has been only one
other reported case of intrameningioma metastasis as a first
clinical manifestation of occult primary breast carcinoma (3).
Case report
A 57-year-old female presented with a 6-week history
of vertigo and headache. Her Glasgow coma scale was 15/15. She had
no medical comorbidities and her World Health Organization (WHO)
performance status was 1. Magnetic Resonance Imaging (MRI) scan
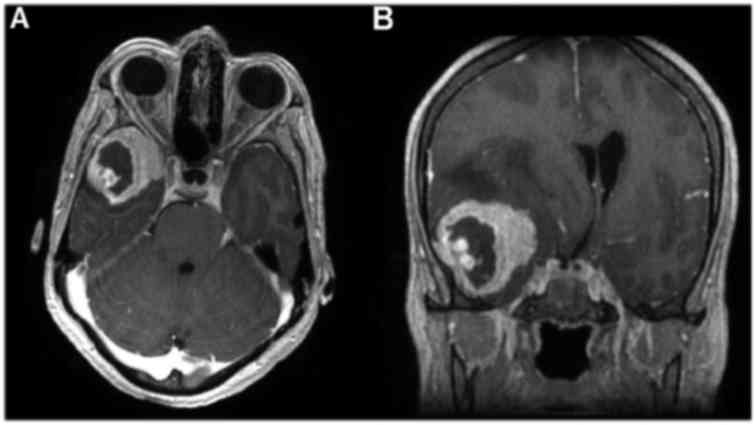
revealed an extra-axial dural-based mass overlying the right
lateral sphenoid wing with intense enhancement, dural tailing and
perilesional oedema consistent with a meningioma (Fig. 1). She underwent craniotomy and
sub-total resection of the suspected right sphenoid wing
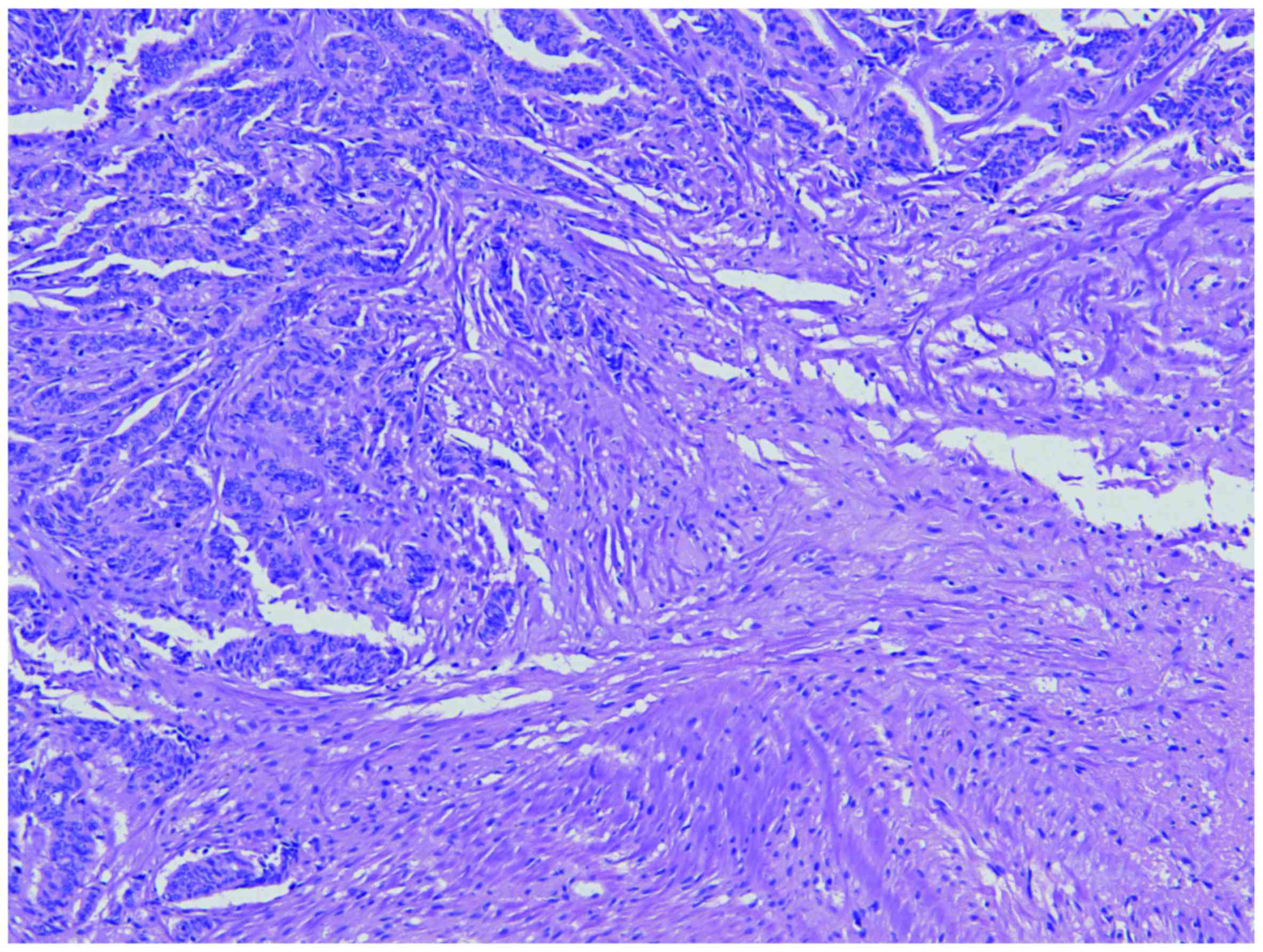
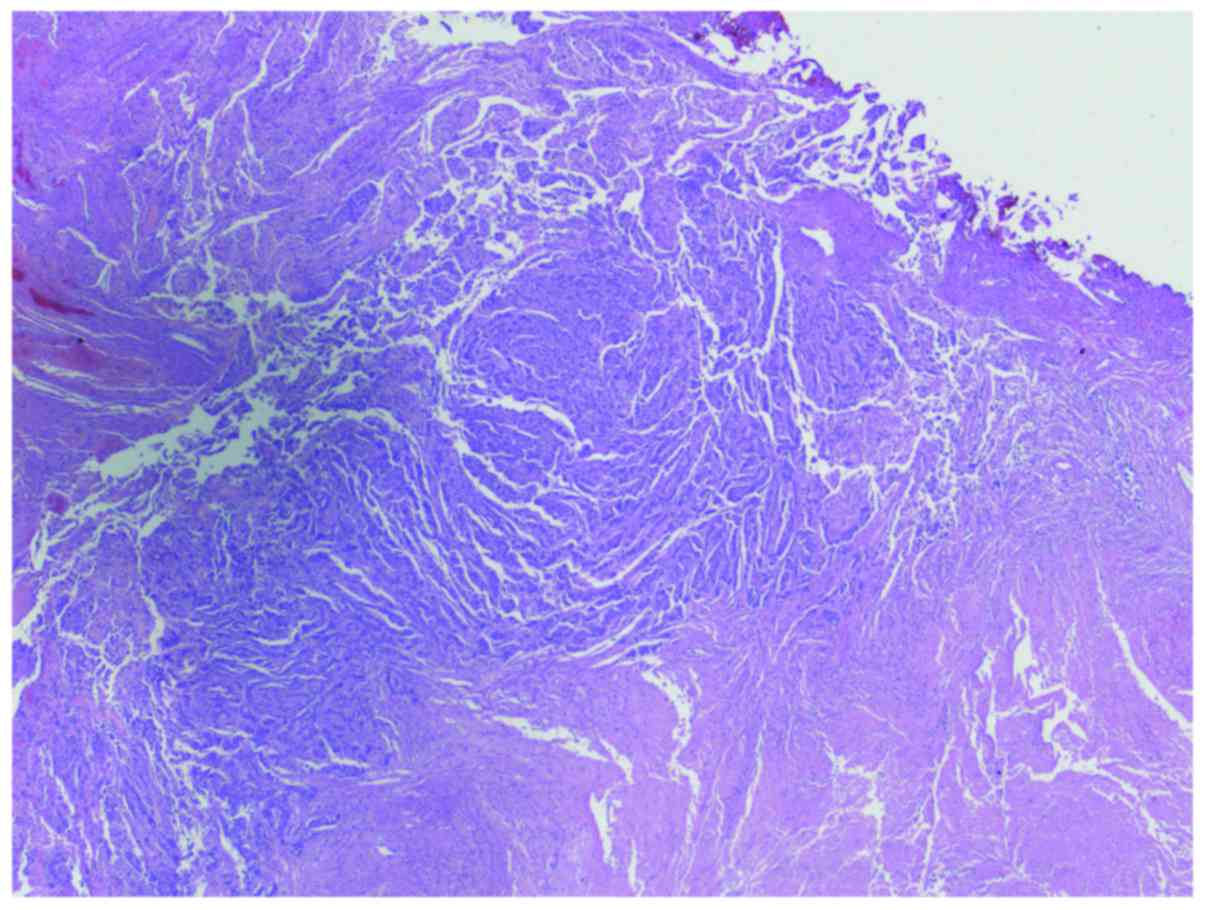
meningioma. Histopathology revealed two distinct neoplastic
processes (Figs. 2 and 3). The first was a WHO grade I meningioma
of transitional type with low mitotic activity [<1 mitoses/10
high power field (hpf)] and low Ki-67 proliferation index (<2%).
The second neoplastic process consisted of a malignant tumor
composed of corded epithelioid cells with extensive necrosis, very
high mitotic activity (>30 mitoses/10 hpf) and a high Ki-67
proliferation index (>40%). Immunohistochemical (IHC) studies
for the grade I meningioma component showed positive staining for
epithelial membrane antigen (EMA) and progesterone receptor (PR)
nuclear expression. IHC studies for the malignant tumor showed
positive staining for EMA, but negative oestrogen Receptor (ER), PR
and pan-cytokeratin. HER 2 Fluorescence in situ hybridization
(FISH) was negative. The findings favoured the likely diagnosis of
WHO grade 3 meningioma, however, alluded to the possibility of a
metastatic collision tumor provided there was evidence of a
concomitant malignancy.
Post-operative clinical examination revealed a
suspicious right breast mass. Mammography and breast ultrasound
revealed a highly suspicious lesion in the right breast associated
with axillary lymphadenopathy. Tru-cut biopsy from the suspicious
right breast lesion showed mucinous carcinoma of the breast with
positive IHC for ER (>90%) and PR (15%). Staging isotope bone
scan and computed tomography (CT) scan for chest, abdomen and
pelvis showed multiple bone metastases but no visceral metastases.
MRI spine showed multiple spinal metastases with spinal cord
compression at thoraco-lumbar spine (T12-L1 level). She underwent
posterior decompression and spinal fixation followed by
post-operative palliative spinal radiotherapy 20 Gray in 5
fractions. Histopathology from the bone biopsy confirmed the
presence of metastatic adenocarcinoma cells consistent with
metastatic breast cancer. IHC profile was negative for ER, PR and
cytokeratin (CK)-20 but was positive for CK-7. She was considered
for cranial irradiation but following a discussion at the
multidisciplinary tumor board meeting it was decided that further
re-resection of the cranial lesion should be the preferred approach
if the lesion increased in size or the patient became symptomatic
during follow-up.
She was commenced on systemic endocrine therapy with
oral letrozole along with monthly zoledronic acid infusions. Seven
months later, she developed disease progression with worsening
axillary lymphadenopathy and new bone metastases. MRI brain showed
increase in the size and extent of the previously noted right
middle cranial fossa extra-axial dural-based space occupying
lesions along with oedema and midline shift suggestive of disease
progression. She underwent craniotomy and complete resection of the
right fronto-temporal lesion. Histopathology revealed a malignant
neoplasm with features similar to the patient's previous breast
tumor biopsies, although the mucinous component was lacking, and
the IHC panel showed negative expression for ER, PR and
pan-cytokeratin. Overall features were suggestive of a collision
metastatic breast cancer involving a grade 1 meningioma. She was
offered whole brain radiotherapy but she declined this as she was
concerned regarding hair loss and possibility of cognitive
deterioration. She was treated with capecitabine chemotherapy for
10 months followed by second-line endocrine-based treatment with a
combination of everolimus and letrozole on further disease
progression. Follow-up MRI brain performed recently has not shown
any evidence of disease recurrence. She remains clinically and
radiologically stable on her current systemic treatment 3 years on
from her initial presentation.
In summary, this lady's presentation with a
collision breast tumor involving a low-grade meningioma led to the
diagnosis of metastatic breast cancer. Following surgical treatment
of her intra-cranial disease and spinal cord decompression, she
remains stable on systemic endocrine therapy 3-years on following
her initial presentation.
Discussion
Several cases of tumor-to-tumor metastases and
collision tumors have been reported previously (4–9). Based
on case series and retrospective studies, the most frequent donor
tumor appears to be lung carcinoma and the most common malignant
recipient is renal cell carcinoma (10,11).
Meningioma appears to be the most common benign recipient tumor
(12). The process of
epithelial-mesenchymal transition (EMT) is thought to enable cancer
cells to acquire less adhesion and more motility enhancing their
ability to migrate leading to tumor metastases and progression
(13). Primary tumor-derived
components, tumor-mobilized bone-marrow-derived cells (BMDCs), and
the local stromal microenvironment of the host are the three major
factors crucial for the formation of the pre-metastatic niche. The
pre-metastatic niche can be defined as the supportive and receptive
microenvironment in the host tissue that undergoes a series of
molecular and cellular changes to help for the subsequent seeding
and colonization of tumor cells (14).
The association between breast cancer and meningioma
is controversial. Early reports observed a strong epidemiological
association between breast cancer and meningioma suggesting that
women with either condition had a higher risk of being diagnosed
with the other condition (15).
Meningiomas are twice as common in women than men, and, like breast
cancer, have a predilection for the fifth or sixth decades of life
and similarly tend to grow during pregnancy (16). However, several retrospective cohort
studies have shown conflicting results regarding the association
between breast cancer and meningioma (17–19).
More recently, it has also been questioned whether the presumed
association between breast cancer and meningioma could simply be
related to the increased frequency of cranial imaging for staging
and/or follow-up, particularly among women with advanced stage
breast cancer (20). Several factors
may contribute to the development of metastases in a meningioma
including, high vascularity, slow growth and hormonal influences
(21). The highly collagenous and
vascular histology of meningiomas, combined with its slow growth
rate for a prolonged duration provides a fertile environment for
development of intra-tumoral metastases (22). The mutual expression of E-cadherin
may facilitate the seeding of one tumor by another (23). Amplification of c-myc oncogene may
play a role in estrogen-induced proliferation and in the
pathogenesis of both breast cancer and meningioma (24).
Certain criteria were proposed for the diagnosis of
tumor-to-tumor metastasis. Mainly, there must be an evidence of at
least two primary tumors and the recipient tumor must be a true
neoplasm. Direct contiguous growth or tumor emboli from an adjacent
tumor are excluded; and the recipient cannot be a lymph node
involved by leukemia or lymphoma (25). In addition, Pamphlett et al
proposed additional criteria for the diagnosis of true
tumor-to-meningioma metastasis: the metastatic focus must at least
be partially enclosed by a rim of histologically distinct host
tumor tissue; and the existence of the metastasizing primary
carcinoma must be proven and compatible with the metastasis
(26).
Routine radiological imaging techniques such as CT
or MRI cannot reliably exclude the presence of metastasis within a
meningioma, however, perfusion MRI and MR spectroscopy provide
additional functional assessment and are likely to provide
additional diagnostic information (27). The limitations of radiological
diagnosis of this unusual lesion underscore the importance of
careful pathologic analysis as the diagnosis of breast carcinoma-
to-meningioma metastasis can be missed if the entire tumor is not
systematically sampled. This merits careful coordination between
surgeons and pathologists in cases where tumor- to-tumor metastasis
is possible, given its potential implications for patient prognosis
and subsequent management (28).
In our case, the diagnosis of the breast cancer
collision tumor in the meningioma led to further assessment and
diagnosis of high-risk metastatic breast cancer with spinal cord
compression. Prompt diagnosis of breast cancer followed by surgery
and radiotherapy prevented catastrophic and debilitating
consequences such as paraplegia.
In conclusion, this case highlights the rare
presentation of a metastatic collision breast carcinoma with
primary presentation mimicking a high-grade meningioma. It is
important to be aware about this unusual condition as careful
pathologic analysis of the resected meningioma, high index of
suspicion for breast cancer, and prompt intervention prevented
significant morbidity in this case.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used in the current article are
available from the corresponding author on request.
Authors' contributions
AF, JA, conception and design. AF, JA, MA, GS, HK,
AH wrote, reviewed and gave final approval of the manuscript to be
published. JA and AH were study supervisors.
Competing Interests
The authors declare they have no competing
interests.
Ethics approval and consent to
participate
Not applicable
Consent for publication
Written informed consent was obtained.
References
|
1
|
Lin NU, Bellon JR and Winer EP: CNS
metastases in breast cancer. J Clin Oncol. 22:3608–3617. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Barnholtz-Sloan JS, Sloan AE, Davis FG,
Vigneau FD, Lai P and Sawaya RE: Incidence proportions of brain
metastases in patients diagnosed (1973 to 2001) in the Metropolitan
Detroit Cancer Surveillance System. J Clin Oncol. 22:2865–2872.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Caroli E, Salvati M, Giangaspero F,
Ferrante L and Santoro A: Intrameningioma metastasis as first
clinical manifestation of occult primary breast carcinoma.
Neurosurg Rev. 29:49–54. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Fadare O, Parkash V, Fiedler PN, Mayerson
AB and Asiyanbola B: Tumor-to-tumor metastasis to a thyroid
follicular adenoma as the initial presentation of a colonic
adenocarcinoma. Pathol Int. 55:574–579. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Sayegh ET, Burch EA, Henderson GA, Oh T,
Bloch O and Parsa AT: Tumor-to-tumor metastasis: Breast carcinoma
to meningioma. J Clin Neurosci. 22:268–274. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Bohn OL, De las Casas LE and Leon ME:
Tumor-to-tumor metastasis: Renal cell carcinoma metastatic to
papillary carcinoma of thyroid-report of a case and review of the
literature. Head Neck Pathol. 3:327–330. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Takei H and Powell SZ: Tumor-to-tumor
metastasis to the central nervous system. Neuropathology.
29:303–308. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Basil I, Ru K, Pu C, Silverman J and
Jasnosz K: A collision tumor: Primary central nervous system B-cell
lymphoma and anaplastic astrocytoma. Lab Med. 42:324–328. 2011.
View Article : Google Scholar
|
|
9
|
Greer WS, Gardner JM and Montgomery CO:
Collision tumor of bone: Primary chondrosarcoma of bone as a rare
recipient of tumor-to-tumor metastasis from metastatic breast
carcinoma. Case Rep Clin Pathol. 2:25–29. 2015.
|
|
10
|
Ichijima K, Yamabe H, Kobashi Y and Iwata
T: Metastasis of cancer to cancer. Acta Pathol Jpn. 30:293–300.
1980.PubMed/NCBI
|
|
11
|
Sella A and Ro JY: Renal cell cancer: Best
recipient of tumor-to-tumor metastasis. Urology. 30:35–38. 1987.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Honma K, Hara K and Sawai T:
Tumour-to-tumour metastasis. A report of two unusual autopsy cases.
Virchows Arch A Pathol Anat Histopathol. 416:153–157. 1989.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Thiery JP: Epithelial-mesenchymal
transitions in tumour progression. Nat Rev Cancer. 2:442–454. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Liu Y and Cao X: Characteristics and
Significance of the Pre-metastatic Niche. Cancer Cell. 30:668–681.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Schoenberg BS, Christine BW and Whisnant
JP: Nervous system neoplasms and primary malignancies of other
sites. The unique association between meningiomas and breast
cancer. Neurology. 25:705–712. 1975. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Smith FP, Slavik M and MacDonald JS:
Association of breast cancer with meningioma: Report of two cases
and review of the literature. Cancer. 42:1992–1994. 1978.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Custer BS, Koepsell TD and Mueller BA: The
association between breast carcinoma and meningioma in women.
Cancer. 94:1626–1635. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Criscitiello C, Disalvatore D, Santangelo
M, Rotmensz N, Bazolli B, Maisonneuve P, Goldhirsch A and
Curigliano G: No link between breast cancer and meningioma: Results
from a large monoinstitutional retrospective analysis. Cancer
Epidemiol Biomarkers Prev. 23:215–217. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Cea-Soriano L, Blenk T, Wallander MA and
Rodríguez LA: Hormonal therapies and meningioma: Is there a link?
Cancer Epidemiol. 36:198–205. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Milano MT and Grossman CE: Meningioma in
breast cancer patients: Population-based analysis of
clinicopathologic characteristics. Am J Clin Oncol. 40:11–16. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Okada E, Nakamura M, Koshida Y, Mukai K,
Toyama Y and Matsumoto M: Breast carcinoma metastasis to meningioma
in the thoracic spine: A case report and review of the literature.
J Spinal Cord Med. 38:231–235. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Watanabe T, Fujisawa H, Hasegawa M,
Arakawa Y, Yamashita J, Ueda F and Suzuki M: Metastasis of breast
cancer to intracranial meningioma: Case report. Am J Clin Oncol.
25:414–417. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Shimada S, Ishizawa K and Hirose T:
Expression of E-cadherin and catenins in meningioma: Ubiquitous
expression and its irrelevance to malignancy. Pathol Int. 55:1–7.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Leeman DJ, Chandrasekhar SS, Brackmann DE
and Poletti BJ: Collision tumors at the cerebellopontine angle:
Case report with literature review. Otolaryngol Head Neck Surg.
117:S76–S80. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Campbell LV Jr, Gilbert E, Chamberlain CR
Jr and Watne AL: Metastases of cancer to cancer. Cancer.
22:635–643. 1968. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Pamphlett R: Carcinoma metastasis to
meningioma. J Neurol Neurosurg Psychiatry. 47:561–563. 1984.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Jun P, Garcia J, Tihan T, McDermott MW and
Cha S: Perfusion MR imaging of an intracranial collision tumor
confirmed by image-guided biopsy. AJNR Am J Neuroradiol. 27:94–97.
2006.PubMed/NCBI
|
|
28
|
Sayegh ET, Henderson GA, Burch EA, Reis
GF, Cha S, Oh T, Bloch O and Parsa AT: Intrameningioma metastasis
of breast carcinoma. Rare Tumors. 6:53132014. View Article : Google Scholar : PubMed/NCBI
|