Introduction
Cancer represents a major health concern worldwide.
Although the mortality rate of malignant tumours has been
controlled due to the scientific and technological advances in the
field of oncology, it remains one of the deadliest diseases
(1). The majority of cancer-related
deaths may be attributed to the metastatic spread and invasion of
cancer cells into other vital organs. During this process, cancer
cells degrade the basement membrane and extracellular matrix (ECM)
and migrate to adjacent areas, where they then invade the blood
and/or lymphatic vessels and reach other organs or tissues via the
circulation. Growth factors, ECM proteins, intercellular adhesions,
the cytoskeleton and genes all participate in this process
(2). Therefore, it is of great
significance to further elucidate the molecular mechanisms
underlying cancer development and design effective antitumour drugs
to improve prevention and therapeutic strategies against
cancer.
Epithelial-to-mesenchymal transition (EMT) is a
process by which cells lose their epithelial characteristics and
acquire a mesenchymal cell phenotype. EMT is important in embryonic
development, chronic inflammation, tissue reconstruction, a variety
of fibrotic diseases and cancer metastasis (3,4). During
the process of EMT, the cell-to-cell and cell-to-matrix connections
become weaker and the epithelial polarity acquires mesenchymal
characteristics, promoting cancer cell migration and invasion of
the surrounding matrix or other organs (5–7).
Recently accumulated evidence indicates that EMT contributes to
lymph node metastasis, distant metastasis, prognosis and
chemoresistance of cancers (8,9).
During EMT, the expression of epithelial cell
markers, such as E-cadherin, is downregulated, while mesenchymal
cell markers, such as vimentin and N-cadherin, are upregulated.
Transcription factors, such as Snail, Zeb and Twist, are also
involved in these processes. It was also revealed that certain
signalling pathways are involved in EMT, such as the transforming
growth factor (TGF)-β signalling pathway (10), Wnt signalling pathway (11), Hedgehog signalling pathway (12), and Notch signalling pathway (13). The development of drugs or
interventions to reverse the process of EMT has become an important
target of cancer research (14,15).
Bufalin suppresses cancer metastasis by
inhibiting EMT
Bufalin is a bioactive polyhydroxy steroid isolated
from Venenum Bufonis, also referred to as Chansu, a well-known
traditional Chinese herb. Chansu has been widely used in the
clinical treatment of several malignancies in China (16,17).
Recent studies demonstrated that bufalin exhibits anticancer
activity against various cancers, such as breast cancer (18), osteosarcoma (19), colon cancer (20), lung cancer (21), pancreatic cancer (22), bladder cancer (23) and hepatocellular carcinoma (24). The underlying mechanisms include
inhibiting cell proliferation (19),
promoting cell apoptosis and autophagy (25,26),
blocking the cell cycle (27),
reversing multidrug resistance (28), and inhibiting EMT and cancer
metastasis (24). Bufalin has been
shown to affect several EMT signalling pathways.
Bufalin affects EMT by inhibiting TGF-β
The TGF-β family is a superfamily that regulates
cell proliferation, apoptosis, the cell cycle, ECM transformation
and tumour metastasis (29). The
TGF-β family includes three isoform ligands, namely TGF-β1, TGF-β2
and TGF-β3, and two receptors, TβRI and TβRII. When the TGF-β
ligand binds to the receptor, it leads to the phosphorylation of
SMAD2 and SMAD3. Subsequently, SMAD4 is translocated into the
nucleus and leads to the activation of target genes (30). Recent studies have found that the
activation of TGF-β is involved in the EMT process and increases
the potential of cancer metastasis. The EMT transcription factors
targeted by TGF-β include Snail, two-handed zinc finger factors
ZEB1 and ZEB2, and the basic helix-loop-helix Twist1 and Twist2
(31–33).
It was reported that bufalin can inhibit EMT in
cancer cells via the TGF-β pathway. Zhao et al (34) reported that bufalin downregulates the
expression of TGF-β receptors and inhibits TGF-β-induced EMT and
the migration of A549 lung cancer cells. That study demonstrated
that, following treatment with TGF-β, the migratory ability of lung
cancer cells increased, and the cell morphology resembled that of
mesenchymal cells. When treated with bufalin, these phenomena were
markedly suppressed. The expression of Twist2, ZEB2, SMAD2 and
SMAD3 was also downregulated following treatment with bufalin.
Further analysis revealed that the TGF-β receptors TβRI and TβRII
were also downregulated in the presence of bufalin. When the
phosphorylation of TβRI was blocked by the inhibitor SB431542, the
TGF-β-induced EMT was attenuated, similar to treatment with
bufalin. It was also demonstrated that bufalin inhibited the
migration and invasion of human colon cancer HCT116 cells and
increased the ability of cell adhesion. Following treatment with
bufalin, the expression of TGF-β1, SMAD4 and E-cadherin increased,
while the expression of phospho-SMAD3 decreased. These proteins may
be associated with bufalin and can induce the nuclear translocation
of β-catenin and prevent cancer cell EMT (35,36).
Bufalin affects EMT through the PI3K/AKT
signalling pathway
The phosphoinositide 3-kinase (PI3K)/AKT signalling
pathway is involved in cell proliferation, cell cycle regulation
and cell apoptosis. In addition, abnormal activation of AKT is
associated with tumour occurrence and development. Overexpression
of PI3K/AKT inhibits the apoptosis of cancer cells, and some
chemotherapeutic drugs induce cell apoptosis by inhibiting the
activation of AKT (37,38). It was also demonstrated that
activated PI3K/AKT cell signalling may promote the degradation of
ECM and reduce cell-cell adhesions, inducing EMT and thereby
promoting the metastasis and invasion of malignant tumour cells
(39). The overexpression of AKT
promotes the expression of Twist and Snail, which are important in
the activation of EMT. Treatment with an AKT inhibitor can restore
the epithelial cell morphology to a polygonal shape and suppress
the migration ability of cancer cells. While the expression of
Twist and Snail was downregulated, E-cadherin expression was
upregulated (40).
Wang et al (24) reported that bufalin suppresses liver
cancer invasion and metastasis by downregulating the expression of
the PI3K/AKT/mammalian target of rapamycin (mTOR) signalling
pathway. That study demonstrated that, after treatment with
bufalin, the liver/lung metastases were significantly reduced in
mice with transplanted tumours. Furthermore, EMT was inhibited by
bufalin, the expression of E-cadherin was upregulated, and the
expression of N-cadherin, vimentin and Snail was downregulated.
Moreover, the expression of TGF-β1 was inhibited after treatment
with bufalin. It was also observed that bufalin can inhibit the
expression of hypoxia-inducible factor-1α (HIF-1α) by suppressing
the activation of the PI3K/AKT/mTOR pathway (41). Another study reported that bufalin
can prevent hepatocellular carcinoma invasion and metastasis.
Functional studies demonstrated that bufalin can inhibit matrix
metalloproteinase (MMP)2 and MMP9 expression, as well as the
expression of PI3K and the phosphorylation of AKT, thereby
attenuating the expression of nuclear factor (NF)-κB (42).
Bufalin was also found to inhibit endometrial
carcinoma cell migration and invasion. The expression of MMP9 and
Snail was downregulated following treatment with bufalin, while the
expression of E-cadherin was upregulated. Functional studies
demonstrated that bufalin inhibits cervical tumourigenesis and
metastasis by modulating α2/β5/FAK cell signalling and affecting
the expression of PI3K/AKT. Integrins α2/β5 and downstream related
genes FAK, pFAK, pGSK3β, AKT1 and p-AKT1 were all downregulated
following treatment with bufalin (43).
Bufalin affects EMT through the
Wnt/β-catenin signalling pathway
Wnt signalling comprises a group of signal
transduction pathways consisting of proteins that transmit signals
into the cell through cell surface receptors. There are three
characterized Wnt signalling pathways: The canonical Wnt pathway,
the non-canonical planar cell polarity pathway, and the
non-canonical Wnt/calcium pathway (44). The Wnt/β-catenin pathway is crucial
for cellular maintenance and development, such as cell cycle
progression, apoptosis, differentiation, migration and
proliferation (45). The
overstimulation of these pathways is closely associated with cancer
development. During the development of cancer, the phosphorylation
and/or nuclear localization of β-catenin increases, and the
Wnt/β-catenin pathway is activated, increasing the migration and
invasion ability of cancer cells (46). Furthermore, the Wnt/β-catenin pathway
induces EMT by activating Twist1, ZEB1, Snail and Slug (47).
Gai et al (48) observed that bufalin inhibited the
proliferation, migration and invasion of the hepatocellular
carcinoma cell line BEL-7402. Upon investigating the underlying
mechanism, they found that bufalin suppressed the phosphorylation
of the GSK-3β Ser9 site in BEL-7402 cells and decreased the
expression of cyclin D1, MMP7 and cyclooxygenase-2. They also found
that bufalin inhibited the transfer of β-catenin to the nucleus,
which is a key step in the Wnt/β-catenin signalling pathway.
Bufalin inhibits EMT through the Hedgehog
signalling pathway
The Hedgehog (Hh) signalling pathway is highly
conserved and is essential for the development of the normal
embryo, particularly during early embryogenesis and morphogenesis
of specific organs and tissues (49). The Hh pathway is usually silenced in
most adult tissues; however, after injury, the pathway is
reactivated to promote the repair and regeneration of cells.
Furthermore, aberrant Hh signalling has been detected in a number
of human cancers and has been implicated in tumourigenesis
(50,51). When Hh signalling is silenced, the
receptor Patched (PTCH1) binds the receptor Smoothened (SMO),
inhibiting SMO and its downstream pathway activity. When PTCH1
binds its ligand, Sonic Hedgehog (SHH), SMO is activated and,
subsequently, the glioma-associated oncogene Gli transcription
factor is activated (52). Studies
have found that Gli family members are associated with cancer cell
EMT and metastasis, and Gli is also associated with the Hh
signalling pathway (51,53).
Sheng et al (54) reported that bufalin was able to
inhibit liver cancer cell EMT and ECM degradation by affecting the
Gli1 and Gli3 expression of the Hh signalling pathway. Bufalin
inhibited the expression of MMP2, MMP9, β-catenin and vascular
endothelial growth factor in liver cancer cells and upregulated
E-cadherin expression. Another study also demonstrated that bufalin
was able to suppress cancer stem-like cells in
gemcitabine-resistant pancreatic cancer via Hh signalling. Bufalin
was also shown to inhibit the colony formation of pancreatic cancer
cells and reduce tumourigenesis in nude mice. Western blotting and
immunohistochemical results revealed that CD24 and
epithelial-specific antigen levels were downregulated after
treatment with bufalin. Bufalin was also shown to inhibit
metastasis in nude mice injected with tumour cells via the tail
vein. Moreover, Hh signalling was found to be suppressed in
bufalintreated cells (22).
Other antimetastatic effects of bufalin
Bufalin inhibits NF-κB activation. NF-κB was first
identified by Dr Ranjan Sen via its interaction with an 11-base
pair sequence in the immunoglobulin light-chain enhancer in B cells
(55). NF-κB plays an important role
in immune response, as well as in cancer initiation and
progression. When NF-κB is activated, preneoplastic and malignant
cells exhibit increased anti-apoptotic ability. NF-κB is also
implicated in tumour angiogenesis and invasiveness. Thus, NF-κB and
its pathway are promising targets in anticancer therapy (56). It was reported that bufalin can
inhibit hepatocellular carcinoma invasion and metastasis.
Functional studies demonstrated that bufalin inhibited the
expression of MMP2, MMP9 and PI3K and suppressed the
phosphorylation of AKT, which was associated with a reduction in
the level of NF-κB (42).
Bufalin modulates the activation of the
mitogen-activated protein kinase (MAPK)/extracellular
signal-regulated kinase (ERK) signalling pathway. MAPKs are
serine-threonine kinases that are involved in diverse biological
activities, such as cell proliferation, differentiation, survival,
death and transformation (57). The
MAPK family includes ERK, p38 and c-Jun NH2-terminal kinase (JNK).
Both extracellular and intracellular stimuli can promote the
activation of MAPK pathways, such as peptide growth factors,
cytokines, hormones, oxidative stress and endoplasmic reticulum
stress (58). A number of studies
have demonstrated that the abnormal expression of MAPK signalling
pathways is important in tumour development. The ERK pathway also
increases the expression of MMPs, promotes the degradation of ECM
proteins, and enhances the invasion ability of cancer cells
(59,60).
Hong et al (23) reported that bufalin attenuated the
migratory and invasive abilities of bladder cancer cells. Following
treatment with bufalin, the expression of transepithelial
electrical resistance increased, which promoted the expression of
tight junctions. Bufalin also inhibits the expression of MMP2 and
MMP9, while increasing the expression of metalloproteinase
inhibitor. All these molecules are associated with the activation
of the ERK pathway. Chueh et al (61) also found that bufalin inhibited the
migration and invasion of human osteosarcoma U-2 OS cells and
reduced the levels of MAPKs, such as JNK1/2 and ERK1/2.
Bufalin prevents cell migration by regulating the
expression of miRNAs. MicroRNAs (miRNAs) are non-coding RNAs, ~22
nucleotides in length and highly conserved. miRNAs can regulate
gene expression by binding to target sequences in mRNAs to induce
mRNA degradation or suppress translation. miRNAs regulate various
cellular processes, such as metastasis, proliferation and
chemosensitivity. Studies have demonstrated that a number of
malignant tumours have disrupted regulation of miRNAs that exert
anticancer or tumour-promoting effects (62–65).
miRNA profiling has become a diagnostic and prognostic marker as
well as a therapeutic target for cancer (66). Qiu et al (67) demonstrated that bufalin can inhibit
the migration of human colon cancer cells (HCT116) and human
umbilical vein endothelial cells (HUVECs). They observed that
miR-497 was upregulated in human colorectal cancer HCT116 cells
treated with different concentrations of bufalin. In addition,
bufalin was found to enhance the expression of the tumour
suppressor miR-497 in nude mice.
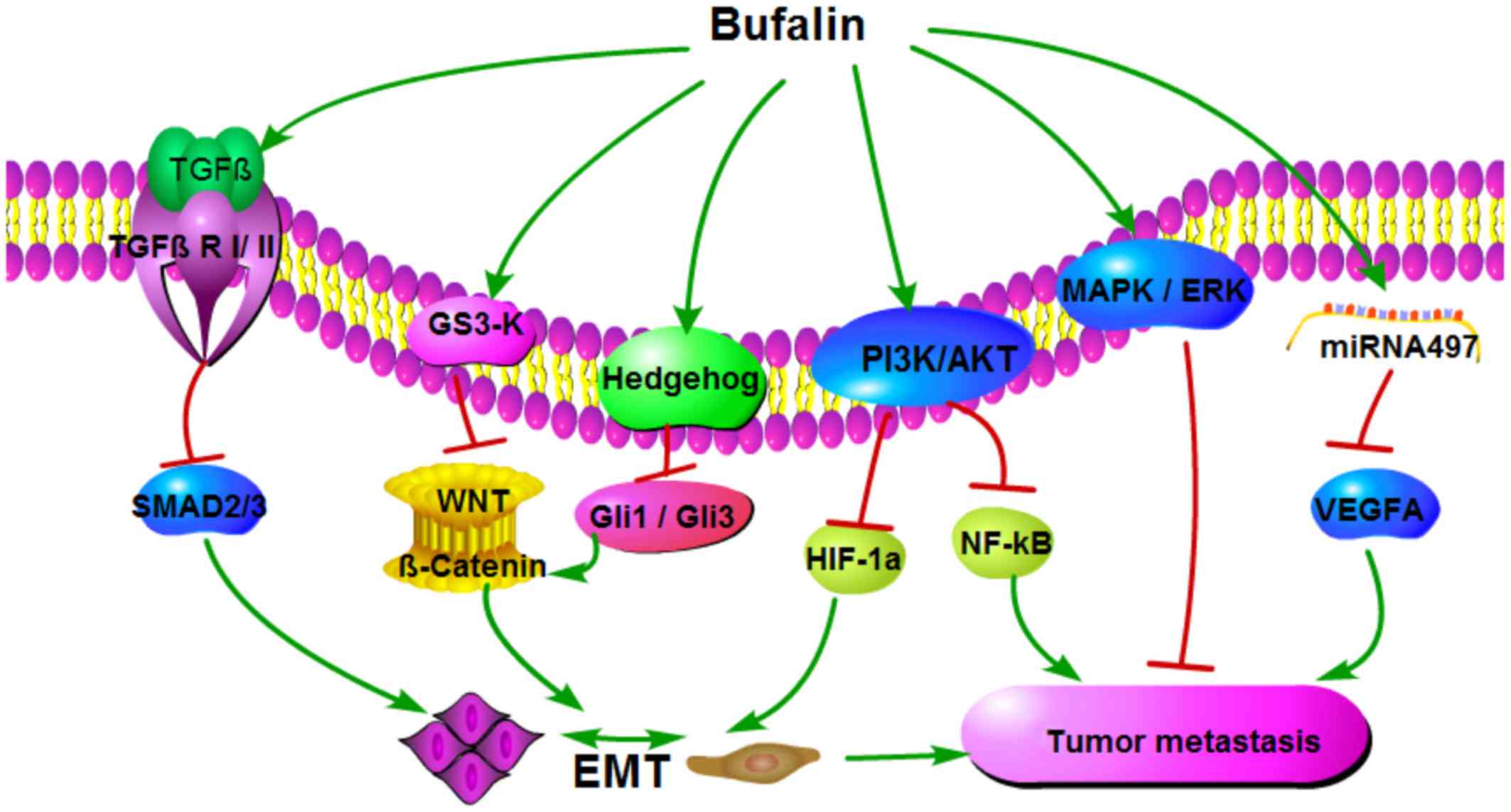
In conclusion, bufalin is a potential polygenic and
multi-target anticancer agent (Fig.
1) that can inhibit EMT by regulating the expression of TGF-β,
PI3K/AKT, Hh and Wnt/β-catenin signalling pathways, thereby
preventing metastasis. Bufalin also inhibits cancer metastasis by
suppressing NF-ĸB activation and regulating the MAPK/ERK signalling
pathway, as well as the expression of certain miRNAs. All these
signalling pathways are interrelated; for example, bufalin
modulates the PI3K/AKT signalling pathway, and PI3K/AKT activity in
turn regulates the expression of NF-ĸB in hepatocellular carcinoma
(42). However, the study of the
anticancer and antimetastatic properties of bufalin is in its
initial phases, and the underlying molecular mechanism remains
unknown. Studies are currently limited to fundamental research, and
several more studies are required before bufalin can be used in a
clinical setting. However, with additional studies investigating
the effect of bufalin on oncogenic cell signalling pathways or
targets, it has the potential to become a drug for targeting cancer
metastasis.
Acknowledgements
Not applicable.
Funding
This study was supported by the Health System
Independent Innovation Science Foundation of Shanghai Putuo
District (grant no. 2013PTKW002), the Foundation of Key Clinical
Specialty of Shanghai Putuo District (grant no. 2016PTZK01), and
the Scientific Research Funds of Sixth People's Hospital of
Shanghai Medical Group (grant no. 15-LY-01).
Availability of data and materials
The analyzed data sets generated during the study
are available from the corresponding author on reasonable
request.
Authors' contributions
JW and TC made substantial contributions to the
conception and design of the study, and wrote the manuscript, JW,
YX and Q-SZ performed literatures search regarding bufalin
anti-cancer activity. The final version of the manuscript has been
read and approved by all authors.
Ethics approval and consent to
participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Siegel RL, Miller KD and Jemal A: Cancer
Statistics, 2017. CA Cancer J Clin. 67:7–30. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Coghlin C and Murray GI: Current and
emerging concepts in tumour metastasis. J Pathol. 222:1–15. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Low WHH, Seet W, A S R, Ng KK, H J, Dan
SP, Teng CL, Lee VKM, Chua SS, My FA, et al: Community-based
cardiovascular Risk Factors Intervention Strategies (CORFIS) in
managing hypertension: A pragmatic non-randomised controlled trial.
Med J Malaysia. 68:129–135. 2013.PubMed/NCBI
|
|
4
|
Zeisberg M and Neilson EG: Biomarkers for
epithelial-mesenchymal transitions. J Clin Invest. 119:1429–1437.
2009. View
Article : Google Scholar : PubMed/NCBI
|
|
5
|
Kalluri R and Weinberg RA: The basics of
epithelial-mesenchymal transition. J Clin Invest. 119:1420–1428.
2009. View
Article : Google Scholar : PubMed/NCBI
|
|
6
|
Lee MY, Chou CY, Tang MJ and Shen MR:
Epithelial-mesenchymal transition in cervical cancer: Correlation
with tumor progression, epidermal growth factor receptor
overexpression, and snail up-regulation. Clin Cancer Res.
14:4743–4750. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Garg M: Epithelial, mesenchymal and hybrid
epithelial/mesenchymal phenotypes and their clinical relevance in
cancer metastasis. Expert Rev Mol Med. 19:e32017. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Piva F, Giulietti M, Santoni M, Occhipinti
G, Scarpelli M, Lopez-Beltran A, Cheng L, Principato G and
Montironi R: Epithelial to Mesenchymal Transition in Renal Cell
Carcinoma: Implications for Cancer Therapy. Mol Diagn Ther.
20:111–117. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Okubo K, Uenosono Y, Arigami T, Yanagita
S, Matsushita D, Kijima T, Amatatsu M, Uchikado Y, Kijima Y,
Maemura K, et al: Clinical significance of altering
epithelial-mesenchymal transition in metastatic lymph nodes of
gastric cancer. Gastric Cancer. 20:802–810. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
He H, Kuriyan AE, Su CW, Mahabole M, Zhang
Y, Zhu YT, Flynn HW, Parel JM and Tseng SC: Inhibition of
Proliferation and Epithelial Mesenchymal Transition in Retinal
Pigment Epithelial Cells by Heavy Chain-Hyaluronan/Pentraxin 3. Sci
Rep. 7:437362017. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Yu M, Ting DT, Stott SL, Wittner BS,
Ozsolak F, Paul S, Ciciliano JC, Smas ME, Winokur D, Gilman AJ, et
al: RNA sequencing of pancreatic circulating tumour cells
implicates WNT signalling in metastasis. Nature. 487:510–513. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Xu X, Su B, Xie C, Wei S, Zhou Y, Liu H,
Dai W, Cheng P, Wang F, Xu X, et al: Sonic hedgehog-Gli1 signaling
pathway regulates the epithelial mesenchymal transition (EMT) by
mediating a new target gene, S100A4, in pancreatic cancer cells.
PLoS One. 9:e964412014. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Bao B, Wang Z, Ali S, Kong D, Li Y, Ahmad
A, Banerjee S, Azmi AS, Miele L and Sarkar FH: Notch-1 induces
epithelial-mesenchymal transition consistent with cancer stem cell
phenotype in pancreatic cancer cells. Cancer Lett. 307:26–36. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Kaushik N, Kim MJ, Kim RK, Kumar Kaushik
N, Seong KM, Nam SY and Lee SJ: Low-dose radiation decreases tumor
progression via the inhibition of the JAK1/STAT3 signaling axis in
breast cancer cell lines. Sci Rep. 7:433612017. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Mobley RJ, Raghu D, Duke LD, Abell-Hart K,
Zawistowski JS, Lutz K, Gomez SM, Roy S, Homayouni R, Johnson GL,
et al: MAP3K4 Controls the Chromatin Modifier HDAC6 during
Trophoblast Stem Cell Epithelial-to-Mesenchymal Transition. Cell
Reports. 18:2387–2400. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Qiu DZ, Zhang ZJ, Wu WZ and Yang YK:
Bufalin, a component in Chansu, inhibits proliferation and invasion
of hepatocellular carcinoma cells. BMC Complement Altern Med.
13:1852013. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Li C, Hashimi SM, Cao S, Mellick AS, Duan
W, Good D and Wei MQ: The mechanisms of chansu in inducing
efficient apoptosis in colon cancer cells. Evid Based Complement
Alternat Med. 2013:8490542013.PubMed/NCBI
|
|
18
|
Zou Z, Luo X, Nie P, Wu B, Zhang T, Wei Y,
Wang W, Geng G, Jiang J and Mi Y: Inhibition of SRC-3 enhances
sensitivity of human cancer cells to histone deacetylase
inhibitors. Biochem Biophys Res Commun. 478:227–233. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Zhang J, Sha J, Zhou Y, Han K, Wang Y, Su
Y, Yin X, Hu H and Yao Y: Bufalin Inhibits Proliferation and
Induces Apoptosis in Osteosarcoma Cells by Downregulating
MicroRNA-221. Evid Based Complement Alternat Med. 2016:73194642016.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Wang J, Chen C, Wang S, Zhang Y, Yin P,
Gao Z, Xu J, Feng D, Zuo Q, Zhao R, et al: Bufalin Inhibits HCT116
Colon Cancer Cells and Its Orthotopic Xenograft Tumor in Mice Model
through Genes Related to Apoptotic and PTEN/AKT Pathways.
Gastroenterol Res Pract. 2015:4571932015. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Sun P, Feng LX, Zhang DM, Liu M, Liu W, Mi
T, Wu WY, Jiang BH, Yang M, Hu LH, et al: Bufalin derivative BF211
inhibits proteasome activity in human lung cancer cells in vitro by
inhibiting β1 subunit expression and disrupting proteasome
assembly. Acta Pharmacol Sin. 37:908–918. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Wang H, Ning Z, Li Y, Zhu X and Meng Z:
Bufalin suppresses cancer stem-like cells in gemcitabine-resistant
pancreatic cancer cells via Hedgehog signaling. Mol Med Rep.
14:1907–1914. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Hong SH, Kim GY, Chang YC, Moon SK, Kim WJ
and Choi YH: Bufalin prevents the migration and invasion of T24
bladder carcinoma cells through the inactivation of matrix
metalloproteinases and modulation of tight junctions. Int J Oncol.
42:277–286. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Wang H, Zhang C, Xu L, Zang K, Ning Z,
Jiang F, Chi H, Zhu X and Meng Z: Bufalin suppresses hepatocellular
carcinoma invasion and metastasis by targeting HIF-1α via the
PI3K/AKT/mTOR pathway. Oncotarget. 7:20193–20208. 2016.PubMed/NCBI
|
|
25
|
Liu M, Feng LX, Sun P, Liu W, Wu WY, Jiang
BH, Yang M, Hu LH, Guo DA and Liu X: A Novel Bufalin Derivative
Exhibited Stronger Apoptosis-Inducing Effect than Bufalin in A549
Lung Cancer Cells and Lower Acute Toxicity in Mice. PLoS One.
11:e01597892016. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Shen S, Zhang Y, Wang Z, Liu R and Gong X:
Bufalin induces the interplay between apoptosis and autophagy in
glioma cells through endoplasmic reticulum stress. Int J Biol Sci.
10:212–224. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Liu X, Xiao XY, Shou QY, Yan JF, Chen L,
Fu HY and Wang JC: Bufalin inhibits pancreatic cancer by inducing
cell cycle arrest via the c-Myc/NF-κB pathway. J Ethnopharmacol.
193:538–545. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Zhai X, Lu J, Wang Y, Fang F, Li B and Gu
W: Reversal effect of bufalin on multidrug resistance in K562/VCR
vincristine-resistant leukemia cell line. J Tradit Chin Med.
34:678–683. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Heldin CH, Vanlandewijck M and Moustakas
A: Regulation of EMT by TGFβ in cancer. FEBS Lett. 586:1959–1970.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Gaarenstroom T and Hill CS: TGF-β
signaling to chromatin: How Smads regulate transcription during
self-renewal and differentiation. Semin Cell Dev Biol. 32:107–118.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Lombaerts M, van Wezel T, Philippo K,
Dierssen JW, Zimmerman RM, Oosting J, van Eijk R, Eilers PH, van de
Water B, Cornelisse CJ, et al: E-cadherin transcriptional
downregulation by promoter methylation but not mutation is related
to epithelial-to-mesenchymal transition in breast cancer cell
lines. Br J Cancer. 94:661–671. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Kahata K, Dadras MS and Moustakas A: TGF-β
Family Signaling in Epithelial Differentiation and
Epithelial-Mesenchymal Transition. Cold Spring Harb Perspect Biol.
10:0221942018. View Article : Google Scholar
|
|
33
|
Ikushima H and Miyazono K: Cellular
context-dependent ‘colors’ of transforming growth factor-beta
signaling. Cancer Sci. 101:306–312. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Zhao L, Liu S, Che X, Hou K, Ma Y, Li C,
Wen T, Fan Y, Hu X, Liu Y, et al: Bufalin inhibits TGF-β-induced
epithelial-to-mesenchymal transition and migration in human lung
cancer A549 cells by downregulating TGF-β receptors. Int J Mol Med.
36:645–652. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Zhao R, Yu H, Shi X, Qiu Y, Qian Y and Yin
P: The influence of Bufalin on TGF-β1-induced epithelial
mesenchymal transition in HCT-116 cells. Surg Res N Tech.
4:217–222. 2015.
|
|
36
|
Wang S, Lin J, Xing L and Chen T:
Mechanism of Bufalin inhibiting invasion of human colon cancer
HCT116 cells. China J Cancer Prev Treat. 23:52016.
|
|
37
|
Wang SQ, Wang C, Chang LM, Zhou KR, Wang
JW, Ke Y, Yang DX, Shi HG, Wang R, Shi XL, et al: Geridonin and
paclitaxel act synergistically to inhibit the proliferation of
gastric cancer cells through ROS-mediated regulation of the
PTEN/PI3K/Akt pathway. Oncotarget. 7:72990–73002. 2016.PubMed/NCBI
|
|
38
|
Wang R, Song Y, Liu X, Wang Q, Wang Y, Li
L, Kang C and Zhang Q: UBE2C induces EMT through Wnt/β catenin and
PI3K/Akt signaling pathways by regulating phosphorylation levels of
Aurora-A. Int J Oncol. 50:1116–1126. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Yoo YA, Kang MH, Lee HJ, Kim BH, Park JK,
Kim HK, Kim JS and Oh SC: Sonic hedgehog pathway promotes
metastasis and lymphangiogenesis via activation of Akt, EMT, and
MMP-9 pathway in gastric cancer. Cancer Res. 71:7061–7070. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Hong KO, Kim JH, Hong JS, Yoon HJ, Lee JI,
Hong SP and Hong SD: Inhibition of Akt activity induces the
mesenchymal-to-epithelial reverting transition with restoring
E-cadherin expression in KB and KOSCC-25B oral squamous cell
carcinoma cells. J Exp Clin Cancer Res. 28:282009. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Zhang L, Huang G, Li X, Zhang Y, Jiang Y,
Shen J, Liu J, Wang Q, Zhu J, Feng X, et al: Hypoxia induces
epithelial-mesenchymal transition via activation of SNAI1 by
hypoxia-inducible factor-1α in hepatocellular carcinoma. BMC
Cancer. 13:1–9. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Chen YY, Lu HF, Hsu SC, Kuo CL, Chang SJ,
Lin JJ, Wu PP, Liu JY, Lee CH, Chung JG, et al: Bufalin inhibits
migration and invasion in human hepatocellular carcinoma SK-Hep1
cells through the inhibitions of NF-kB and matrix
metalloproteinase-2/-9-signaling pathways. Environ Toxicol.
30:74–82. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Liu F, Tong D, Li H, Liu M, Li J, Wang Z
and Cheng X: Bufalin enhances antitumor effect of paclitaxel on
cervical tumorigenesis via inhibiting the integrin α2/β5/FAK
signaling pathway. Oncotarget. 7:8896–8907. 2016.PubMed/NCBI
|
|
44
|
Nusse R and Varmus HE: Wnt genes. Cell.
69:1073–1087. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Serman L, Martic Nikuseva T, Serman A and
Vranic S: Epigenetic alterations of the Wnt signaling pathway in
cancer: A mini review. Bosn J Basic Med Sci. 14:191–194. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Ma Y, Zhu B, Liu X, Yu H, Yong L, Liu X,
Shao J and Liu Z: Inhibition of oleandrin on the proliferation show
and invasion of osteosarcoma cells in vitro by suppressing
Wnt/β-catenin signaling pathway. J Exp Clin Cancer Res. 34:1152015.
View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Kim K, Lu Z and Hay ED: Direct evidence
for a role of β-catenin/LEF-1 signaling pathway in induction of
EMT. Cell Biol Int. 26:463–476. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Gai JQ, Sheng X, Qin JM, Sun K, Zhao W and
Ni L: The effect and mechanism of bufalin on regulating
hepatocellular carcinoma cell invasion and metastasis via
Wnt/β-catenin signaling pathway. Int J Oncol. 48:338–348. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Nüsslein-Volhard C and Wieschaus E:
Mutations affecting segment number and polarity in Drosophila.
Nature. 287:795–801. 1980. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
McMillan R and Matsui W: Molecular
pathways: The hedgehog signaling pathway in cancer. Clin Cancer
Res. 18:4883–4888. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Chun HW and Hong R: Significance of the
hedgehog pathway-associated proteins Gli-1 and Gli-2 and the
epithelial-mesenchymal transition-associated proteins Twist and
E-cadherin in hepatocellular carcinoma. Oncol Lett. 12:1753–1762.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Hui CC and Angers S: Gli proteins in
development and disease. Annu Rev Cell Dev Biol. 27:513–537. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Chong Y, Tang D, Gao J, Jiang X, Xu C,
Xiong Q, Huang Y, Wang J, Zhou H, Shi Y, et al: Galectin-1 induces
invasion and the epithelial-mesenchymal transition in human gastric
cancer cells via non-canonical activation of the hedgehog signaling
pathway. Oncotarget. 7:83611–83626. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Sheng X, Sun X, Sun K, Sui H, Qin J and Li
Q: Inhibitory effect of bufalin combined with Hedgehog signaling
pathway inhibitors on proliferation and invasion and metastasis of
liver cancer cells. Int J Oncol. 49:1513–1524. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Sen R and Baltimore D: Multiple nuclear
factors interact with the immunoglobulin enhancer sequences. Cell
1986. 46: 705–716. J Immunol. 177:7485–7496. 2006.PubMed/NCBI
|
|
56
|
Karin M: NF-kappaB and cancer: Mechanisms
and targets. Mol Carcinog. 45:355–361. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Torii S, Yamamoto T, Tsuchiya Y and
Nishida E: ERK MAP kinase in G cell cycle progression and cancer.
Cancer Sci. 97:697–702. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Kim EK and Choi EJ: Pathological roles of
MAPK signaling pathways in human diseases. Biochim Biophys Acta.
1802:396–405. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Huang C, Jacobson K and Schaller MD: MAP
kinases and cell migration. J Cell Sci. 117:4619–4628. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Cong Q, Jia H, Li P, Qiu S, Yeh J, Wang Y,
Zhang ZL, Ao J, Li B and Liu H: p38α MAPK regulates proliferation
and differentiation of osteoclast progenitors and bone remodeling
in an aging-dependent manner. Sci Rep. 7:459642017. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Chueh FS, Chen YY, Huang AC, Ho HC, Liao
CL, Yang JS, Kuo CL and Chung JG: Bufalin-inhibited migration and
invasion in human osteosarcoma U-2 OS cells is carried out by
suppression of the matrix metalloproteinase-2, ERK, and JNK
signaling pathways. Environ Toxicol. 29:21–29. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Krol J, Loedige I and Filipowicz W: The
widespread regulation of microRNA biogenesis, function and decay.
Nat Rev Genet. 11:597–610. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Othman N, In LL, Harikrishna JA and Hasima
N: Bcl-xL silencing induces alterations in hsa-miR-608 expression
and subsequent cell death in A549 and SK-LU1 human lung
adenocarcinoma cells. PLoS One. 8:e817352013. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Ho CS, Yap SH, Phuah NH, In LL and Hasima
N: MicroRNAs associated with tumour migration, invasion and
angiogenic properties in A549 and SK-Lu1 human lung adenocarcinoma
cells. Lung Cancer. 83:154–162. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Li Y, Su J, Li F, Chen X and Zhang G:
MiR-150 regulates human keratinocyte proliferation in hypoxic
conditions through targeting HIF-1α and VEGFA: Implications for
psoriasis treatment. PLoS One. 12:e01754592017. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Mohammadi A, Mansoori B and Baradaran B:
Regulation of miRNAs by herbal medicine: An emerging field in
cancer therapies. Biomed Pharmacother. 86:262–270. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Qiu YY, Hu Q, Tang QF, Feng W, Hu SJ,
Liang B, Peng W and Yin PH: MicroRNA-497 and bufalin act
synergistically to inhibit colorectal cancer metastasis. Tumour
Biol. 35:2599–2606. 2014. View Article : Google Scholar : PubMed/NCBI
|