Introductiom
Serous cystadenoma (SCA) is fundamentally a
multilobular cystic tumor that consists of a thin capsule and small
cysts only millimeters in diameter. SCA constitutes approximately
only 1 to 2% of all pancreatic tumors (1), but the number of patients with SCA has
been growing due to the frequent use of radiography and recent
improvements of imaging modalities. SCA is currently categorized
into four subtypes: microcystic, macrocystic, mixed, and solid
types. The most common subtype is microcystic type, and the cystic
structure can be easily recognized in the former three subtypes. On
the other hand, the solid type accounts for only 3% of all SCAs in
a Japanese multicenter study (1),
and it is described as noncystic, meaning that a cystic structure
is too tiny to be detected macroscopically. Solid SCA is usually
misdiagnosed preoperatively as neuroendocrine tumor (NET) (2). In several case reports, the authors
reported that magnetic resonance cholangiopancreatography (MRCP)
might be useful for preoperative diagnosis of SCA (3–5).
However, it is generally considered difficult to preoperatively
differentiate SCA from other solid pancreatic tumors by imaging
modalities.
The present report describes a rare case of solid
SCA in which the magnetic resonance imaging (MRI) findings were
very informative in the diagnosis and decision to perform middle
segment pancreatectomy as an organ-preserving surgical
strategy.
Case report
A 50-year-old woman was referred to the Department
of Surgery in Osaka University Hospital for investigation of a
pancreatic body mass detected during a health examination. She had
no chief complaint. A benign thyroid tumor had been found, and she
was followed up by annual ultrasonography. Physical examination
revealed normal abdominal findings. Laboratory examination revealed
no anemia, jaundice, or hyperglycemia. The serum level of
carcinoembryonic antigen and carbohydrate antigen 19-9 were within
the reference ranges, and no excess of pancreatic endocrine
hormones, including insulin, glucagon, and gastrin, was
observed.
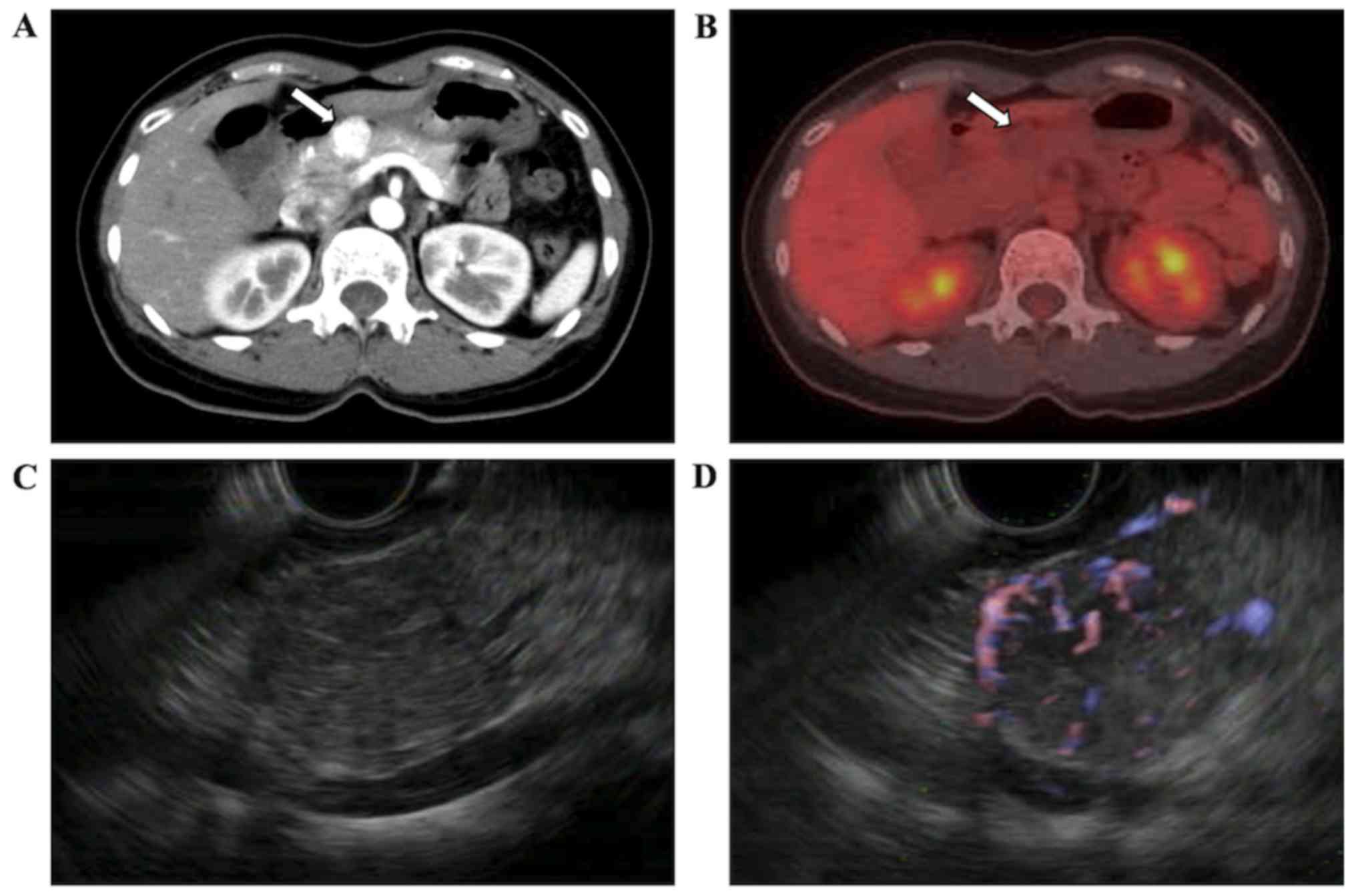
Contrast-enhanced computed tomography (CT)
demonstrated a 2-cm solid mass in the body of the pancreas, which
was strongly enhanced in the early phase (Fig. 1A). Fluorine-18 fluorodeoxyglucose
positron emission tomography-CT showed no abnormal accumulation of
the tracer in the tumor (Fig. 1B).
Endoscopic ultrasonography (EUS) revealed a hypoechoic,
heterogeneous, and hypervascular solid mass without posterior echo
enhancement in the body of the pancreas, and no cystic component
could be recognized (Fig. 1C and D).
We performed EUS-guided fine needle aspiration (FNA) three times,
but could not obtain adequate specimens for diagnosis because of
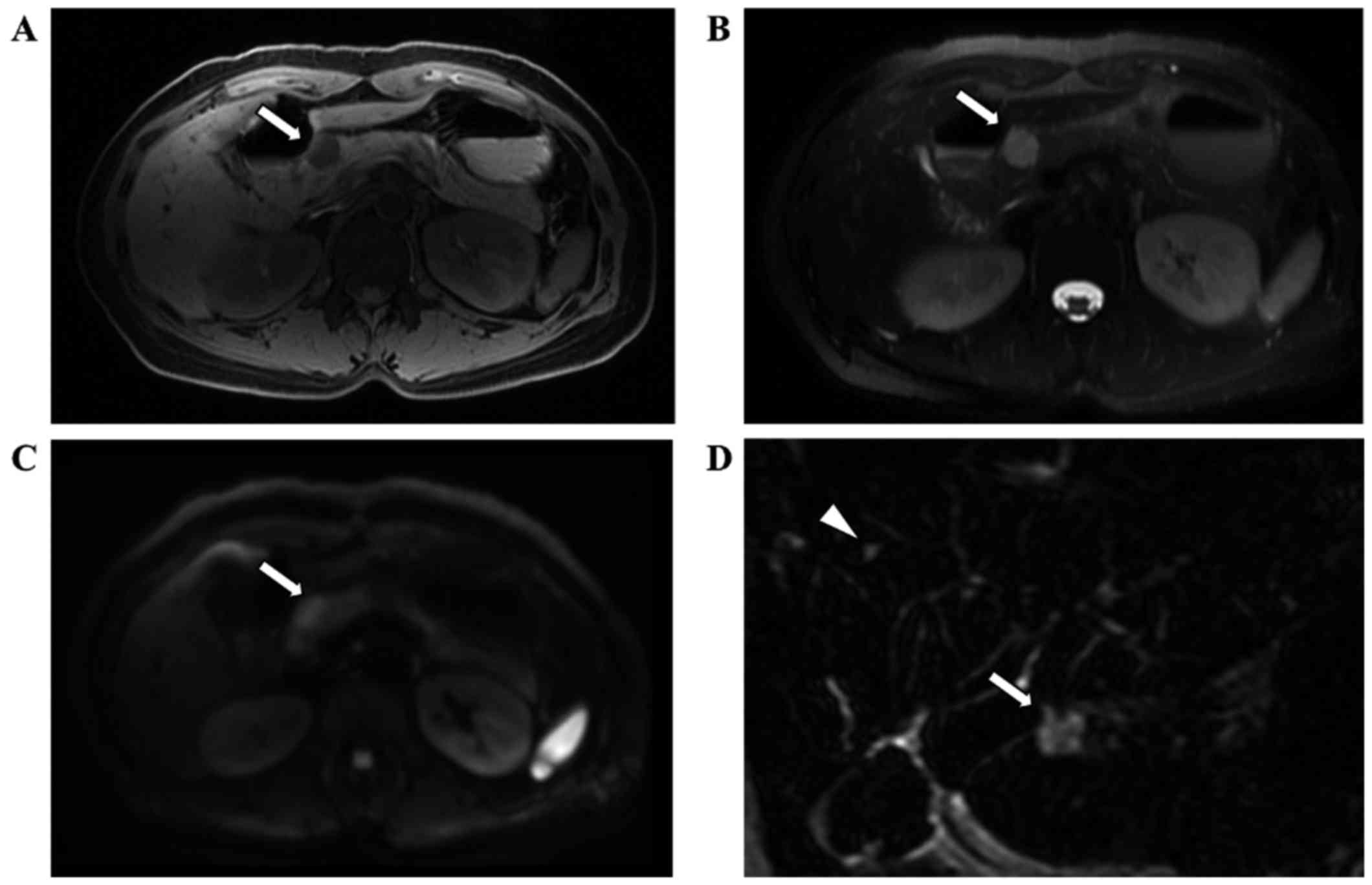
contamination of blood. MRI clearly showed a round mass with low
intensity on T1-weighted images (Fig.
2A) and high intensity on both T2-weighted images (Fig.2B) and diffusion-weighted images
(Fig. 2C). MRCP showed high
intensity, and the tumor signal intensity was similar to that of an
incidentally detected hepatic cyst (Fig.
2D). We strongly suspected the tumor to be a solid SCA based on
the radiological findings, including MRCP, but histological
confirmation could not be gained. We finally performed middle
segment pancreatectomy as a function-preserving surgery. The
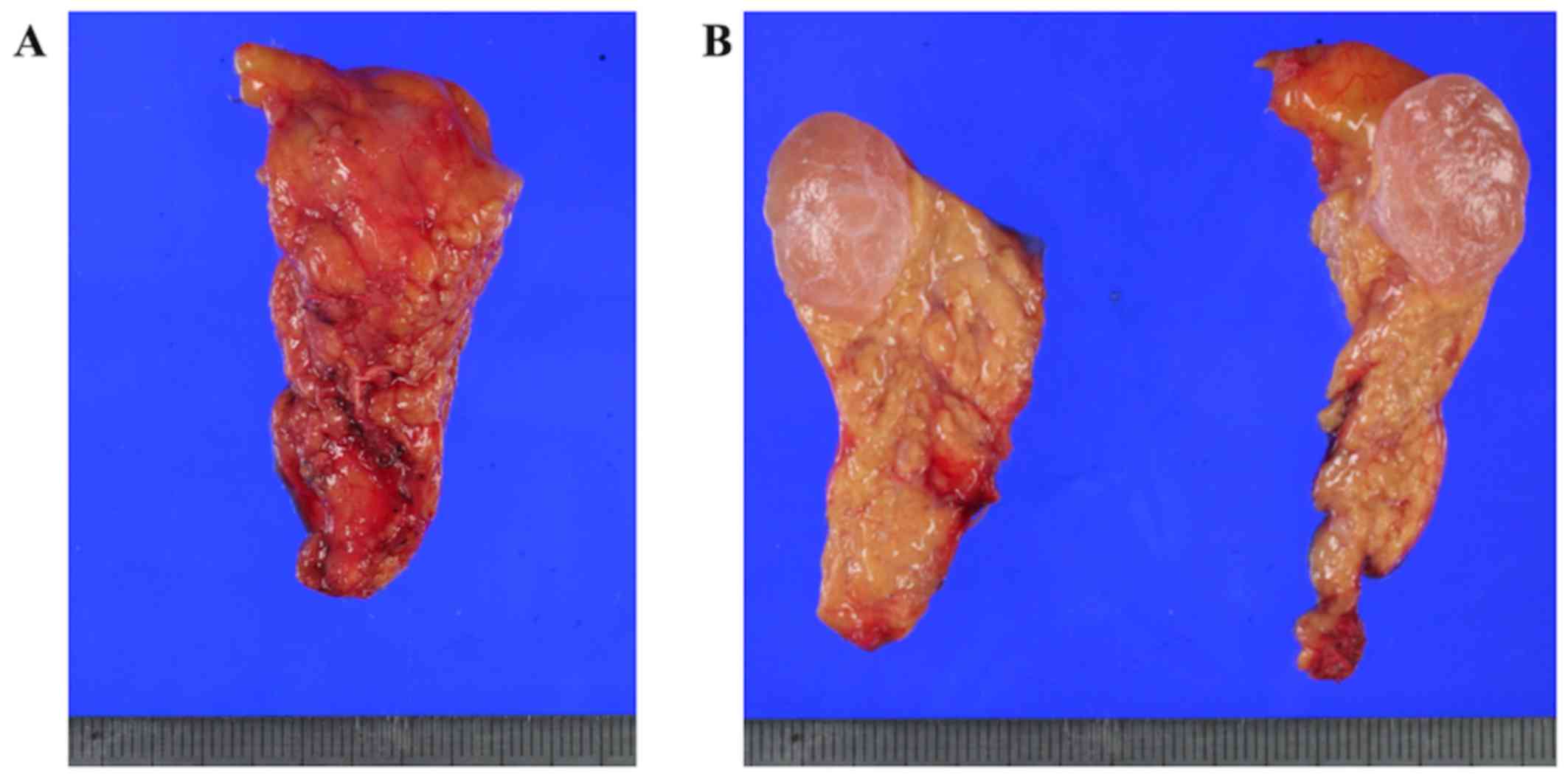
resected specimen was 2.6 cm in width, and surgical margin was
ascertained by intraoperative ultrasonography (Fig. 3A). The cranial stump was cut off
using a triple-row linear stapler and caudal stump was
reconstructed by pancreaticogastrostomy using mattress sutures
(6). The patient's recovery was
complicated by pulmonary embolism (Grade 3 by Common Terminology
Criteria for Adverse Events: CTCAE) and pancreatic fistula (Grade B
by International Study Group of Pancreatic Fistula classification:
ISGPF). The patient recovered with conservative treatment for
pancreatic fistula and anti-coagulation therapy with warfarin for
pulmonary embolism, and she was discharged on the 49 postoperative
day.
The pancreatic tumor was clearly circumscribed
within the resected specimen, and the cut surface of the tumor was
solid, glossy, and reddish with a central fibrous scar in a
stellate pattern (Fig. 3B). It was
2.2 cm in size, and no honeycomb structure characteristic of small
cysts was detected macroscopically. Formalin-fixed
paraffin-embedded tissue sections were prepared, and
immunohistochemical staining was conducted using following
antibodies; anti-mucin 6 antibody (Leica Biosystems, Nussloch,
Germany), anti-synaptophysin antibody, anti-chromogranin antibody
(both from Dako, Glostrup, Denmark) and anti-Ki-67 antibody (Roche
Diagnostics, Basel, Switzerland). Briefly, the sections were
incubated at room temperature with anti-mucin 6 for 32 min,
anti-synaptophysin for 16 min, anti-chromogranin A for 16 min and
anti-Ki-67 for 16 min, respectively. Microscopic examination
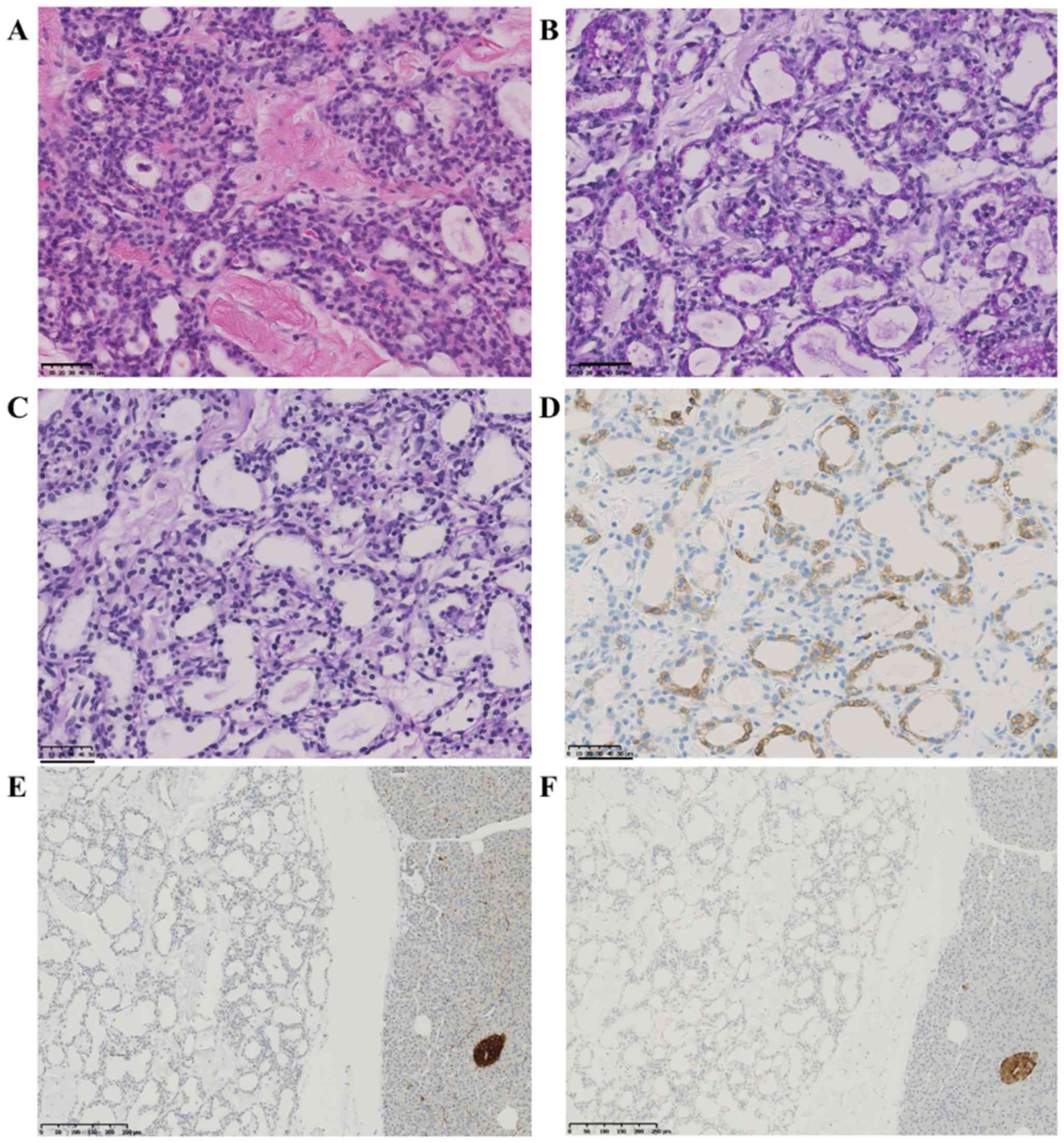
revealed numerous microcysts separated by hypocellular and dense
collagen fibers. The inner surface of the cysts was lined by a
single layer of cuboidal epithelium with clear cytoplasm (Fig. 4A). The cytoplasm was strongly stained
by periodic acid-Schiff and digested by diastase because of the
presence of abundant intracytoplasmic glycogen (Fig. 4B and C). The tumor cells were
positive for mucin 6 (Fig. 4D) and
negative for neuroendocrine differentiation labeling (synaptophysin
(Fig. 4E) or chromogranin A
(Fig. 4F). The Ki-67 labeling index
was 1 to 2%, and there was no evidence of malignancy or lymph node
metastasis. The final diagnosis was a solid SCA. She had been in
follow-up by every three months laboratory check and every six
months radiological check by CT. At the time of this writing (1
year postoperatively), the patient was clinically well with no
evidence of recurrence. She had no diarrhea and weight loss without
digestive enzymes. In addition, she also maintained favorable
glucose tolerance without oral hypoglycemic agents or insulin. The
preoperative and postoperative hemoglobin A1c level was not
worsened (5.8 and 6.0%, respectively). This clinical research was
approved by the Research Ethics Board of the Osaka University
Research Committee and conducted according to Institutional Review
Board guidelines. Written informed consent was obtained from the
patient prior to publication of the present case report.
Discussion
In 1978, Compagno and Oertel (7) first proposed the concept of serous
cystic neoplasm of the pancreas. SCA is morphologically classified
into four subtypes: microcystic, macrocystic, mixed, and solid
types. The solid variant of SCA was first described by
Perez-Ordonez et al (8) in
1996, who reported that the cells were arranged in small acini with
no or minute central lumina, resembling a solid tumor. The
characteristic radiological findings of SCA, such as a honeycomb
appearance, polycystic pattern, lobularity, sunburst appearance
(central calcification), and hemorrhage, are quite rare in solid
SCA. Solid SCA is so rare that the incidence is only 3.0% of all
SCAs, compared with microcystic type (58%) and macrocystic type
(20%) (1). Whether solid SCA is a
variant of SCA or a separate disease entity was historically
controversial (5,8,9), but it
is now considered a variant of SCA because the cytological and
immunohistological features of this tumor are very similar to those
of SCA. Compared with the microcystic type, which is composed of
numerous tiny cysts (usually ~2–10 mm), the solid type is formed by
far smaller cysts that cannot be recognized by the naked eye and
shows a homogeneous and glossy appearance.
We systematically reviewed the English literature by
using PubMed and Google Scholar from 1995 to 2017. The keywords of
‘solid serous cystadenoma’, ‘solid-type serous cystadenoma’,
‘serous cystic neoplasm’ or ‘solid serous adenoma’ were used. We
excluded the nonsurgical cases. To our knowledge, only 19 cases
including our case have been published with a pathological
confirmation (Table I) (2,3,5,8–19).
 | Table I.Literature review of the
clinicopathological findings of patients with solid-type serous
cystadenomas. |
Table I.
Literature review of the
clinicopathological findings of patients with solid-type serous
cystadenomas.
| No. | Authors | Year | Age (years) | Sex | Symptoms | Location | Size (cm) | Enhancement | MRI
(T1/T2/MRCP) | Clinical
diagnosis | Operation | (Refs.) |
|---|
| 1 | Perez-Ordonez et
al | 1996 | 70 | F | Abdominal Pain | Pt | 4.0 | − | − | NET | DP | (8) |
| 2 | Kosmahl et
al | 2004 | 50 | M | − | Ph | 2.5 | − | − | − | PPPD | (10) |
| 3 | Yamamoto et
al | 2004 | 60 | M | Epigastric
distention | Ph | 2.0 | Yes | Low/high/high | NET | PPPD | (5) |
| 4 | Gabata et
al | 2005 | 59 | F | Abdominal Pain | Pb | 2.0 | Yes | Low/high/high | Solid SCA | DP | (3) |
| 5 | Yamaguchi | 2006 | 58 | F | None | Pb | 2.0 | Yes | − | NET | DP | (9) |
| 6 | Reese et
al | 2006 | 66 | M | None | Ph | 4.0 | Yes | − | NET | PPPD | (11) |
| 7 | Sanaka et
al | 2007 | 74 | M | None | Pb | 1.6 | Yes | − | NET | Enucleation | (12) |
| 8 | Stern et
al | 2007 | 62 | M | Abdominal Pain | Pbh | 4.2 | − | − | NET,
othersa | DP | (13) |
| 9 | Casadei et
al | 2008 | 59 | F | Abdominal Pain | Pt | 4.0 | Yes | − | Solid SCA | DP | (14) |
| 10 | Yasuda et
al | 2009 | 72 | F | None | Ph | 1.7 | Yes | −/high/− | NET | PPPD | (15) |
| 11 | Hayashi et
al | 2012 | 74 | F | − | Pb | 4.2 | Yes | − | − | − | (16) |
| 12 | Hayashi et
al | 2012 | 57 | F | − | Ph | 2.1 | Yes | − | − | − | (16) |
| 13 | Hayashi et
al | 2012 | 58 | F | − | Pb | 3.2 | Yes | − | − | − | (16) |
| 14 | Lee et
al | 2013 | 56 | M | None | Pt | 2.5 | Yes | − | NET | Laparoscopic
DP | (17) |
| 15 | Kishida et
al | 2013 | 58 | M | None | Pb | 2.8 | Yes | Low/high/high | NET | DP | (18) |
| 16 | Ishigami et
al | 2014 | 43 | F | − | Ph | − | Yes | Low/−/Not
detected | − | − | (19) |
| 17 | Ishigami et
al | 2014 | 65 | F | − | Ptb | − | Yes | −/−/Not
detected | − | − | (19) |
| 18 | Katsourakis et
al | 2016 | 72 | F | Abdominal Pain | Pt | 3.0 | Yes | −/− | NET | DP | (2) |
| 19 | Present case |
| 50 | F | None | Pb | 2.2 | Yes | Low/high/high | Solid SCA | MP |
Some reports have described the radiological
findings of solid SCA. A honeycomb structure is generally a typical
finding of SCA; however, this finding cannot be detected in solid
SCA even by EUS because the internal microlevel structure is
difficult to capture. As a result, solid SCA is difficult to
distinguish from other solid pancreatic tumors such as NET, acinar
cell carcinoma, solid pseudopapillary tumor, and metastatic
carcinoma. In most cases, NET is an especially important
differential diagnosis because it is clinically the most common
hypervascular solid tumor of the pancreas. Several case reports
have shown that MRCP is a useful tool in distinguishing solid SCA
from other solid pancreatic tumors (3–5). Solid
SCA is reported to show T1 low intensity and T2 high intensity
almost same as the other types of SCA (1). In general, MRCP involves heavily
T2-weighted sequences, and the echo time is prolonged to about 10
times longer than regular T2-weighted imaging. On MRCP images, only
a pure water cyst can gain hyperintensity. Both solid SCA and NET
generally show hyperintensity on regular T2-weighted images.
However, only solid SCA maintains this high intensity because of
its cystic nature on MRCP. The high intensity on MRCP is quite a
useful clue for clearly differentiating solid SCA from NET. MRI is
becoming as common as CT for diagnostic modality. It can provide
large amounts of valuable anatomical information preoperatively
(20–22). If a tumor reveals high intensity on
MRCP, this indicates that the tumor is composed mainly of not a
solid substance but rather a watery fluid. In the present case, the
signal intensity of the tumor was similar to that of the
incidentally detected hepatic cyst. This finding could also be
valuable for reaching the accurate preoperative diagnosis.
With respect to treatment, expectant management has
been proposed when SCA is small and definitely diagnosed because
this neoplasm grows slowly and there is a minimal risk of malignant
transformation (23). When
nonoperative management is selected, histological examination by
EUS-FNA is recommended to provide essential information in advance,
but an adequate specimen can be rarely obtained by EUS-FNA.
The role of EUS-FNA is to obtain preoperative
cytological confirmation of pancreatic malignant tumors (24), and tumor markers and DNA analysis of
cyst aspirates are reportedly helpful in improving diagnostic yield
(25). EUS-FNA is recommended for
NET as an informative tool in differentiating from other pancreatic
tumors because of its high diagnostic sensitivity, which is
reported as high as 87–90% (26–28).
However, the reported diagnostic accuracy of EUS-guided biopsy for
cystic tumors of the pancreas ranges from 17–21% (18,29–31), and
the specimen often lacks the epithelial tissue necessary for
diagnosis because of its cystic nature. Consequently, EUS-FNA alone
is unlikely to provide the high level of diagnostic evidence
necessary to support observational management. The indication for
EUS about solid SCA is not mentioned because of its rarity in the
guidelines about pancreatic cysts (25,32–34). In
the present case, the tumor was initially deemed a NET because of
its hypervascularity and solid appearance. However, the MRCP
finding strongly indicated the possibility of solid SCA. We tried
to gain histological confirmation by EUS-FNA, but ended up as a
failure, and the possibility of NET could not be excluded. We
finally selected surgical intervention because solid SCA is rare
and the final diagnosis ought to be based on histological
confirmation of a surgical specimen unless the diagnosis is
preoperatively assured by EUS-FNA.
We performed middle segment pancreatectomy as a
function-preserving surgery because the tumor was as small as 2.2
cm. An organ-preserving surgical procedure is generally recommended
for SCA because lymph node metastasis of SCA is quite rare and
lymphadenectomy is not necessary (35). In previous reports of SCA with clear
mention about operative procedure, all of the 13 patients underwent
pancreaticoduodenectomy, distal pancreatectomy, or enucleation
(Table I). Previous five cases
lacked in operation method, as those reports placed value in the
imaging pitfalls of solid SCA. Therefore, Our case is the first in
which middle segment pancreatectomy was performed for a solid SCA
as an organ-preserving surgery, and this procedure might lead to
better preservation of endocrine function (36).
Solid SCA of the pancreas is definitively a rare
disease, but oncologic surgeons should be aware of the
characteristics of this neoplasm to allow for a correct
preoperative diagnosis. Further investigation to accumulate more
evidence regarding this rare disease is expected.
We experienced a case of solid SCA of the pancreas
for which MRCP imaging was very helpful for accurate diagnosis and
decision-making regarding the surgical method.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used during the current study are
available from the corresponding author on reasonable request.
Authors' contributions
YO, TN, HE, MS, TT and YD conceived and designed
this study. YO, YI, DY, TA, KK, KG and EM acquired the data. YO,
SK, KU, YH, YT, MT and MM drafted the manuscript. All authors read
and approved the final manuscript.
Ethics approval and consent to
participate
This clinical research was approved by the Research
Ethics Board of the Osaka University Research Committee and
conducted according to Institutional Review Board guidelines.
Written informed consent was obtained from the patient prior to
publication of the present case report.
Consent for publication
Written informed consent was obtained from the
patient involved in this publication and accompanying images. A
copy of this written consent is available for review by the
Editor-in-Chief of this journal.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
CT
|
computed tomography
|
|
EUS
|
endoscopic ultrasonography
|
|
FNA
|
fine needle aspiration
|
|
MRCP
|
magnetic resonance
cholangiopancreatography
|
|
MRI
|
magnetic resonance imaging
|
|
NET
|
neuroendocrine tumor
|
|
SCA
|
serous cystadenoma
|
References
|
1
|
Kimura W, Moriya T, Hirai I, Hanada K, Abe
H, Yanagisawa A, Fukushima N, Ohike N, Shimizu M, Hatori T, et al:
Multicenter study of serous cystic neoplasm of the Japan pancreas
society. Pancreas. 41:380–387. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Katsourakis A, Dimitriou I, Noussios G,
Chatzis I and Chatzitheoclitos E: Solid Serous Adenoma of the
Pancreas: A Case Report and Review of the Literature. Case Rep
Surg. 2016:37302492016.PubMed/NCBI
|
|
3
|
Gabata T, Terayama N, Yamashiro M,
Takamatsu S, Yoshida K, Matsui O, Usukura M, Takeshita M and Minato
H: Solid serous cystadenoma of the pancreas: MR imaging with
pathologic correlation. Abdom Imaging. 30:605–609. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Machado MC and Machado MA: Solid serous
adenoma of the pancreas: an uncommon but important entity. Europ J
Surg Oncol. 34:730–733. 2008. View Article : Google Scholar
|
|
5
|
Yamamoto T, Takahashi N, Yamaguchi T and
Imamura Y: A case of solid variant type of pancreatic serous
cystadenoma mimicking islet cell tumor. Clin Imaging. 28:49–51.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Ohigashi H, Ishikawa O, Eguchi H, Sasaki
Y, Yamada T, Kishi K, Noura S, Takachi K, Miyashiro I, Oue M, et
al: A simple and safe anastomosis in pancreaticogastrostomy using
mattress sutures. Am J Surg. 196:130–134. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Compagno J and Oertel JE: Microcystic
adenomas of the pancreas (glycogen-rich cystadenomas): A
clinicopathologic study of 34 cases. Am J Clin Pathol. 69:289–298.
1978. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Perez-Ordonez B, Naseem A, Lieberman PH
and Klimstra DS: Solid serous adenoma of the pancreas. The solid
variant of serous cystadenoma? Am J Surg Pathol. 20:1401–1405.
1996. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Yamaguchi M: Solid serous adenoma of the
pancreas: A solid variant of serous cystadenoma or a separate
disease entity? J Gastroenterol. 41:178–179. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Kosmahl M, Wagner J, Peters K, Sipos B and
Klöppel G: Serous cystic neoplasms of the pancreas: An
immunohistochemical analysis revealing alpha-inhibin,
neuron-specific enolase, and MUC6 as new markers. Am J Surg Pathol.
28:339–346. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Reese SA, Traverso LW, Jacobs TW and
Longnecker DS: Solid serous adenoma of the pancreas: A rare variant
within the family of pancreatic serous cystic neoplasms. Pancreas.
33:96–99. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Sanaka MR, Kowalski TE, Brotz C, Yeo CJ,
McCue P and Palazzo J: Solid serous adenoma of the pancreas: A rare
form of serous cystadenoma. Dig Dis Sci. 52:3154–3156. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Stern JR, Frankel WL, Ellison EC and
Bloomston M: Solid serous microcystic adenoma of the pancreas.
World J Surg Oncol. 5:262007. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Casadei R, D'Ambra M, Pezzilli R, Ricci C,
Calculli L, Lega S, Antonacci N, Monari F and Minni F: Solid serous
microcystic tumor of the pancreas. JOP. 9:538–540. 2008.PubMed/NCBI
|
|
15
|
Yasuda A, Sawai H, Ochi N, Matsuo Y, Okada
Y and Takeyama H: Solid variant of serous cystadenoma of the
pancreas. Arch Med Sci. 7:353–355. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Hayashi K, Fujimitsu R, Ida M, Sakamoto K,
Higashihara H, Hamada Y and Yoshimitsu K: CT differentiation of
solid serous cystadenoma vs endocrine tumor of the pancreas. Eur J
Radiol. 81:e203–e208. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Lee SD, Han SS and Hong EK: Solid serous
cystic neoplasm of the pancreas with invasive growth. J
Hepatobiliary Pancreat Sci. 20:454–456. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Kishida Y, Matsubayashi H, Okamura Y,
Uesaka K, Sasaki K, Sawai H, Imai K and Ono H: A case of solid-type
serous cystadenoma mimicking neuroendocrine tumor of the pancreas.
J Dig Dis. 15:211–215. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Ishigami K, Nishie A, Asayama Y, Ushijima
Y, Takayama Y, Fujita N, Takahata S, Ohtsuka T, Ito T, Igarashi H,
et al: Imaging pitfalls of pancreatic serous cystic neoplasm and
its potential mimickers. World J Radiol. 6:36–47. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Shimizu Y, Otani T, Matsumoto J, Takanishi
K, Minami T, Tsunoda H and Miyazaki M: Cystic duct with no visible
signal on magnetic resonance cholangiography is associated with
laparoscopic difficulties: An analysis of 695 cases. Surg Today.
44:1490–1495. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Sueda T, Ohue M, Noura S, Shingai T,
Nakanishi K and Yano M: Prognostic significance of a preoperative
magnetic resonance imaging assessment of the distance of mesorectal
extension in clinical T3 lower rectal cancer. Surg Today.
46:1249–1257. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Kolodziejczyk E, Jurkiewicz E, Pertkiewicz
J, Wejnarska K, Dadalski M, Kierkus J, Woynarowski M, Ryzko J and
Oracz G: MRCP Versus ERCP in the Evaluation of Chronic Pancreatitis
in Children: Which Is the Better Choice? Pancreas. 45:1115–1119.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Tseng JF, Warshaw AL, Sahani DV, Lauwers
GY, Rattner DW and Castillo CF-d: Serous Cystadenoma of the
Pancreas. Transactions of the . Meeting of the American Surgical
Association. 123:111–118. 2005. View Article : Google Scholar
|
|
24
|
Ohtsuka T, Tamura K, Ideno N, Aso T,
Nagayoshi Y, Kono H, Ueda J, Takahata S, Aso A, Igarashi H, et al:
Role of ERCP in the era of EUS-FNA for preoperative cytological
confirmation of resectable pancreatic ductal adenocarcinoma. Surg
Today. 44:1887–1892. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Chiang AL and Lee LS: Clinical approach to
incidental pancreatic cysts. World J Gastroenterol. 22:1236–1245.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Figueiredo FA, Giovannini M, Monges G,
Bories E, Pesenti C, Caillol F and Delpero JR: EUS-FNA predicts
5-year survival in pancreatic endocrine tumors. Gastrointest
Endosc. 70:907–914. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Pais SA, Al-Haddad M, Mohamadnejad M,
Leblanc JK, Sherman S, McHenry L and DeWitt JM: EUS for pancreatic
neuroendocrine tumors: A single-center, 11-year experience.
Gastrointest Endosc. 71:1185–1193. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Gornals J, Varas M, Catala I, Maisterra S,
Pons C, Bargallo D, Serrano T and Fabregat J: Definitive diagnosis
of neuroendocrine tumors using fine-needle aspiration-puncture
guided by endoscopic ultrasonography. Rev Esp Enferm Dig.
103:123–128. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Brugge WR: Role of endoscopic ultrasound
in the diagnosis of cystic lesions of the pancreas. Pancreatology.
1:637–640. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Belsley NA, Pitman MB, Lauwers GY, Brugge
WR and Deshpande V: Serous cystadenoma of the pancreas: Limitations
and pitfalls of endoscopic ultrasound-guided fine-needle aspiration
biopsy. Cancer. 114:102–110. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Huang P, Staerkel G, Sneige N and Gong Y:
Fine-needle aspiration of pancreatic serous cystadenoma: Cytologic
features and diagnostic pitfalls. Cancer. 108:239–249. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Vege SS, Ziring B, Jain R and Moayyedi P:
Clinical Guidelines C and American Gastroenterology A: American
gastroenterological association institute guideline on the
diagnosis and management of asymptomatic neoplastic pancreatic
cysts. Gastroenterology. 148:819–822; quize812–813. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Tanaka M, Chari S, Adsay V, Fernandez-del
Castillo C, Falconi M, Shimizu M, Yamaguchi K, Yamao K and Matsuno
S: International Association of Pancreatology: International
consensus guidelines for management of intraductal papillary
mucinous neoplasms and mucinous cystic neoplasms of the pancreas.
Pancreatology. 6:17–32. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Tanaka M, Chari S, Adsay V, Fernandez-del
Castillo C, Falconi M, Shimizu M, Yamaguchi K, Yamao K and Matsuno
S: International Association of Pancreatology: International
consensus guidelines for management of intraductal papillary
mucinous neoplasms and mucinous cystic neoplasms of the pancreas.
Pancreatology. 6:17–32. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Galanis C, Zamani A, Cameron JL, Campbell
KA, Lillemoe KD, Caparrelli D, Chang D, Hruban RH and Yeo CJ:
Resected serous cystic neoplasms of the pancreas: A review of 158
patients with recommendations for treatment. J Gastrointest Surg.
11:820–826. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Colvin H, Mizushima T, Eguchi H, Takiguchi
S, Doki Y and Mori M: Gastroenterological surgery in Japan: The
past, the present and the future. Ann Gastroenterol Surg. 1:5–10.
2017. View Article : Google Scholar
|