Introduction
Metastatic colorectal cancer (mCRC) is one of the
leading types of cancer in developed countries. Despite treatment
and diagnostic advances, the mortality rates of mCRC remain high,
with a patient survival of 24–30 months. In Latvia, ~1,000 patients
with colon and rectum cancer are diagnosed annually, 25% of whom
are metastatic at presentation (1).
Cytotoxic agents, including a fluoropyrimidine,
irinotecan and oxaliplatin, and antibodies, such as bevacizumab (an
anti-vascular endothelial growth factor monoclonal antibody) and
cetuximab and panitumumab (anti-epidermal growth factor receptor
monoclonal antibodies) may significantly improve the survival of
patients with unresectable mCRC. However, chemotherapy-refractory
mCRC patients represent a major challenge for medical oncologists
in terms of selection of further treatment.
Trifluridine/tipiracil (FTD/TPI), also referred to
as TAS-102, is a combination of an antineoplastic thymidine-based
nucleoside analog, trifluridine, and the thymidine phosphorylase
inhibitor tipiracil hydrochloride at a molar ratio of 1:0.5. The
efficacy and safety of FTD/TPI in patients with mCRC who are
refractory to standard therapies were evaluated in the RECOURSE
trial (2).
Patients and methods
Study group
A total of 14 mCRC patients who received FTD/TPI
chemotherapy in two institutions in Latvia (Clinic of Oncology of
Pauls Stradins Clinical University Hospital and Oncology Centre of
Riga East University Hospital) were analyzed. The study was
performed with the Lonsurf Compassionate Use program (Expanded
Access program) and written informed consent was obtained from each
patient who participated in the study. The inclusion criteria were
as follows: Metastatic cancer of the colorectum, neutrophil count
>1,500/mm3, platelet count >75.000/mm3,
hemoglobin level >9.0 g/dl, at least 2 previous chemotherapy
lines, refractory to or intolerant of fluoropyrimidines,
oxaliplatin and irinotecan. The patient inclusion period was only 1
month (April 2016), and a maximum of 15 patients were allowed to
participate in this program in Latvia. Data on clinical follow-up
were obtained until June 2017. The patients underwent 1–13 months
of follow-up.
Treatment
FTD/TPI (with each dose consisting of 35
mg/m2) was administered orally twice daily, for 5 days a
week, with 2 days of rest, for 2 weeks, followed by a 14-day rest
period, thus completing one cycle. The regimen was repeated every 4
weeks. The dose was recommended as standard in all sites
participating in the Lonsurf Compassionate Use program (2). Treatment was discontinued upon
clinically or radiologically confirmed disease progression.
Statistical analysis
Progression-free survival (PFS) was calculated from
the start of FTD/TPI until clinical or radiological progression,
and overall survival (OS) was calculated from the start of FTD/TPI
until death from any cause or censoring at the last follow-up. The
median OS and PFS (mOS and mPFS, respectively) were estimated using
the Kaplan-Meier method. The log-rank test was used to calculate
any significant differences between the subgroups (patients with
vs. without grade 3–4 neutropenia, and duration of previous
treatment ≤ vs. >18 months) by univariate analysis. Significance
levels were set at P<0.05. All statistical analyses were
performed by MedCalc software, version 16.4.8 (MedCalc Software,
Ostend, Belgium).
Results
Patients
Of the 15 patients, 14 received FTD/TPI treatment in
the Lonsurf Compassionate Use program in Latvia between April 2016
and January 2017 in the two participating institutions (1 patient
was excluded from the program due to rapid cancer progression). The
clinical characteristics of the participants are listed in Table I. A total of 8 patients (57.2%) had
primary metastatic cancer (stage IV), and 6 patients developed
metastases after treatment of the primary cancer. All the patients
had received 2–4 previous lines of chemotherapy and progressed.
 | Table I.Patient clinical characteristics
(n=14). |
Table I.
Patient clinical characteristics
(n=14).
| Characteristics | No (%) |
|---|
| Age, years |
|
| Median
(range) | 65 (52–76) |
| Sex |
|
| Male | 6 (42.8) |
|
Female | 8 (57.2) |
| ECOG PS |
|
| 0 | 5 (35.7) |
| 1 | 9 (64.3) |
| Primary site |
|
|
Colon | 9 (64.3) |
|
Rectum | 5 (35.7) |
| KRAS status |
|
|
Wild-type | 1 (7.1) |
|
Mutant | 4 (28.6) |
|
Unknown | 9 (64.3) |
| Metastases |
|
|
Synchronous - stage IV at
diagnosis | 8 (57.2) |
|
Metachronous | 6 (42.8) |
| Median time to
metastases, months (range) | 22 (8–36) |
| Stage II at
diagnosis | 1 |
| Stage III at
diagnosis | 5 |
| Median time from
start of first-line chemotherapy, month (range) | 32.2 (8–90) |
|
>18 | 9 (64.3) |
| ≤18 | 5 (35.7) |
| Median number of
prior lines (range) | 2.7 (2–4) |
| Prior
chemotherapeutic agents |
|
| 5-FU | 14 (100.0) |
|
Ftorafur | 3 (21.4) |
|
Capecitabine | 3 (21.4) |
|
Oxaliplatin | 13 (92.8) |
|
Irinotecan | 14 (100.0) |
|
Bevacizumab | 6 (42.8) |
|
Aflibercept | 1 (7.1) |
|
Cetuximab | 1 (7.1) |
Treatment and adverse events
The staring dose of FTD/TPI was 35 mg/m2,
and the duration of treatment was 1–9 cycles (median, 5.8
cycles).
One patient had a delay of 1 week due to grade 3
neutropenia; none of the patients required dose reduction, and all
patients eventually discontinued FTD/TPI due to disease
progression. All treatment-related adverse events are listed in
Table II.
 | Table II.Adverse events (n=14). |
Table II.
Adverse events (n=14).
| Adverse event | No (%) |
|---|
| Neutropenia, | 4 (28.5) |
| Diarrhea, any
grade | 1 (7.1) |
| Nausea, any
grade | 5 (35.7) |
Survival data
At the median follow-up time of 7.1 months (range,
1–14 months), the mPFS was 5 months [95% confidence interval (CI):
4.09–5.90], and the mOS was 7 months (95% CI: 5.95–8.04). The
6-month PFS was 35.7% and the 6-month OS was 57.1%. All 14 patients
progressed on FTD/TPI treatment and 9 deaths were reported.
Increased PFS and OS were reported in patients with
grade 3–4 neutropenia: The mPFS was 7 months in patients with
neutropenia vs. 5 months in those without neutropenic events
[hazard ratio (HR)=0.24, 95% CI: 0.07–0.89; P=0.033]; the mOS was 7
months in patients without neutropenia, whereas in the neutropenic
group mOS was not met (HR=0.25, 95% CI: 0.06–1.14, P=0.075;
Fig. 1).
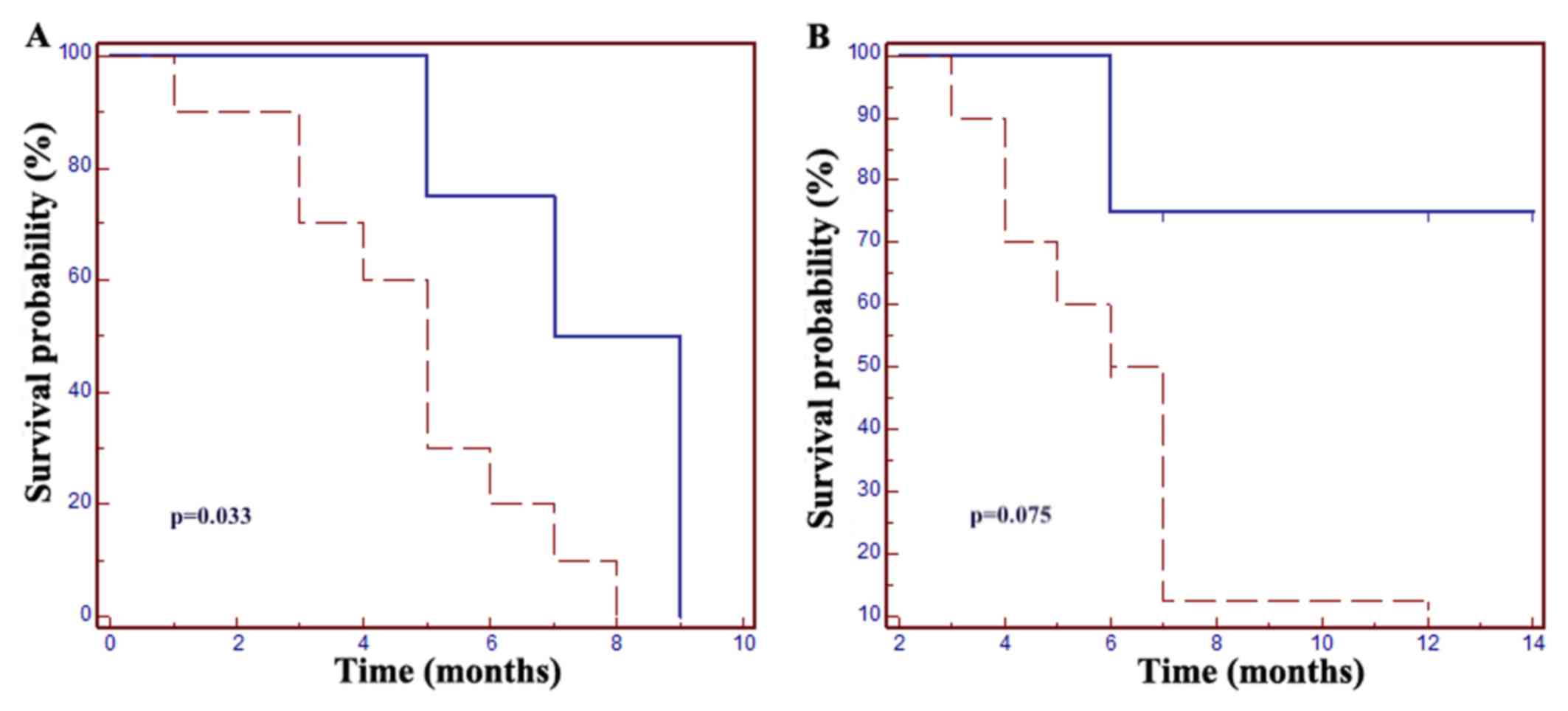 | Figure 1.Grade 3–4 neutropenia is a prognostic
factor in patients with metastatic colorectal cancer. (A) The mPFS
was 5 months in patients without neutropenic events vs. 7 months in
the neutropenia group (HR=0.24, 95% CI: 0.07–0.89; P=0.033. (B) The
mOS was 7 months in patients without neutropenia, but in the
neutropenic group mOS was not met (HR=0.25, 95% CI: 0.06–1.14,
P=0.075). Dotted line, grade ≤2 neutropenia; solid line, grade ≥3
neutropenia. mPFS, median progression-free survival; mOS, median
overall survival; HR, hazard ratio; CI, confidence interval. |
Furthermore, increased PFS and OS were observed in
patients with a time of >18 months from the start of
chemotherapy for mCRC, with a mPFS of 7 months vs. 5 months in
patients with a shorter (≤18 months) previous treatment duration
(HR=0.15, 95% CI: 0.03–0.83, P=0.029); the mOS was not met in
patients with a time of >18 months, whereas it was 6 months in
the ≤18 months patient group (HR=0.23, 95% CI: 0.05–1.12, P=0.069;
Fig. 2).
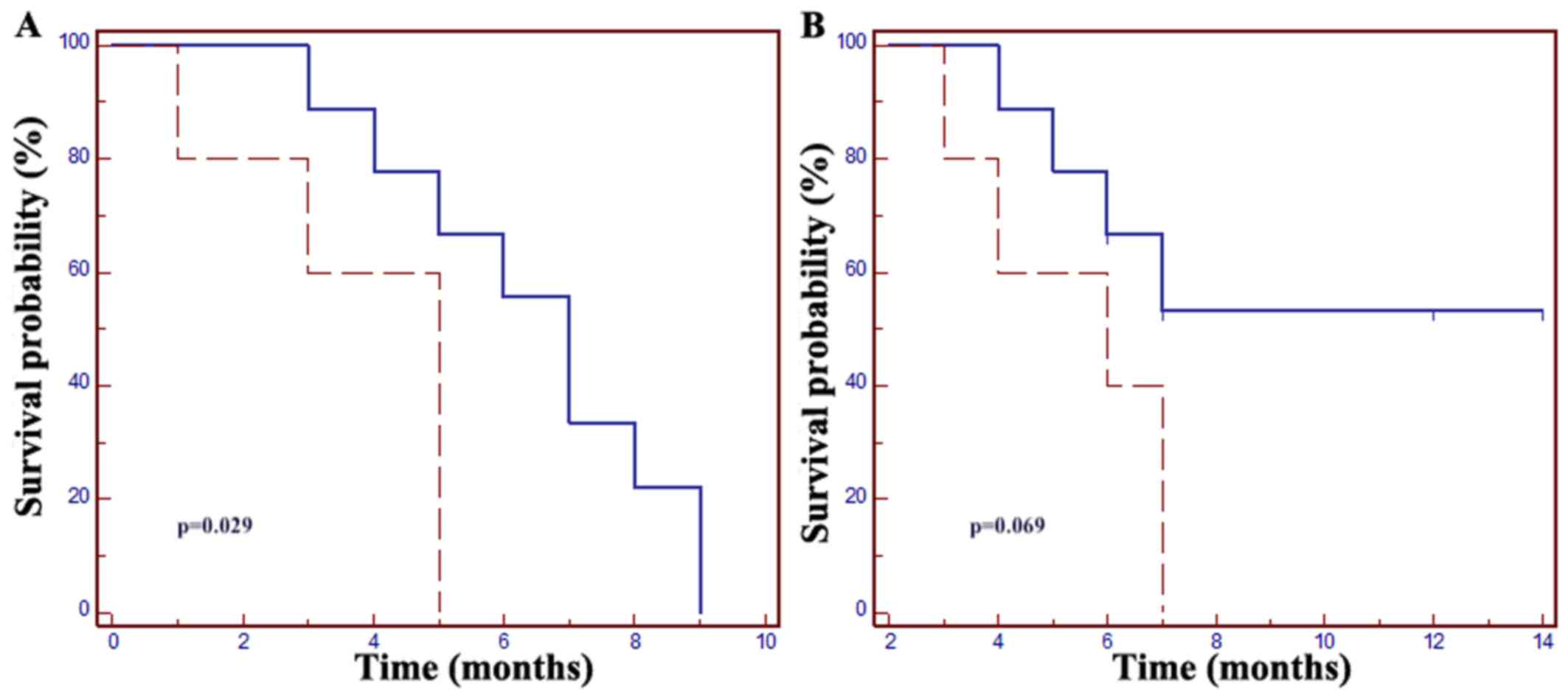 | Figure 2.(A) The mPFS in patients with a time
of >18 months from the start of first-line mCRC treatment was 7
months vs. 5 months in those with a time of ≤18 months (HR=0.15,
95% CI: 0.03–0.83; P=0.029). (B) The mOS in patients with a time of
≤18 months from the start of first-line mCRC treatment was 6
months, but the mOS was not met in patients with a time of >18
months (HR=0.23, 95% CI: 0.05–1.12, P=0.069). Dotted line, time ≤18
months from the start of first-line mCRC treatment; solid line,
time >18 months from the start of first-line mCRC treatment.
mCRC, metastatic colorectal cancer; mPFS, median progression-free
survival; mOS, median overall survival; HR, hazard ratio; CI,
confidence interval. |
Discussion
The aim of the present study was to compare survival
and clinical data from the participants in the Lonsurf
Compassionate Use program and to report possible prognostic factors
for better treatment response. In total, 2,093 patients
participated in the Lonsurf Compassionate Use program in 20
countries (3). Previously published
data from a phase III trial confirmed the efficacy of FTD/TPI in
patients with pretreated mCRC, as treatment with FTD/TPI was
associated with a significant improvement in mOS (7.1 vs. 5.3
months; P<0.0001) and mPFS (2.0 vs. 1.7 months; P<0.0001) vs.
placebo, with a disease control rate of 44% (2). Similar survival data were observed in
the present retrospective study, with a mPFS of 5 months and a mOS
of 7 months. The reports from other counties that participated in
this Expanded Access program (4–6),
revealed a similar trend in safety and efficacy.
In the present study, grade 3–4 neutropenia was the
most frequently observed clinically meaningful adverse event,
occurring in 28% of the patients. This adverse event was associated
with better treatment outcomes (mPFS of 7 months and increased
mOS). Previously reported data demonstrated that, in patients with
advanced colorectal cancer, FTD/TPI-induced severe neutropenia was
associated with superior survival (2,6–8). The reported frequency of grade 3–4
neutropenia in FTD/TPI users is 31–50% (2,7–9). The global incidence of grade 3–4
neutropenia is quite low compared to the RECOURSE study, whereas
the mPFS is relatively high.
Despite the small number of included patients, it
was observed that the time from the start of first-line
chemotherapy to the start of FTD/TPI treatment may be a prognostic
factor, as the mPFS in patients with a time of >18 months from
the start of mCRC treatment was 7 months (HR=0.15), and the HR for
cancer-specific mortality was 0.23 (mOS was not met). It may be
suggested that patients with a shorter duration of previous
treatment lines and rapid progression under previous chemotherapy
may benefit less from FTD/TPI treatment. This observation may lead
to the hypothesis that more aggressive and chemotherapy-resistant
tumors possibly harbor mutations leading to FTD/TPI resistance.
Therefore, a variety of previous chemotherapy lines, patient
performance status, primary tumor and metastases location may
affect the duration of PFS and OS.
There were certain limitations to the present study.
Due to the limited number of participants in the Lonsurf
Compassionate Use program, the research was conducted on a small
size of the mCRC population (n=14). Therefore, to generalize the
results for a larger population, the study would require more
participants.
In conclusion, severe neutropenia may be considered
as a surrogate marker for predicting FTD/TPI treatment outcomes. In
addition, patients with a time of <18 months from the start of
first-line mCRC treatment to the first study drug administration
have a poor prognosis. However, further studies are required to
confirm these findings.
Acknowledgements
Not applicable.
Funding
The article processing charges were funded by the
Latvian Association of Medical Oncologists.
Availability of data and materials
The analysed data sets generated during the study
are available from the corresponding author on reasonable request.
All authors had full access to all the data in this study and take
complete responsibility for the integrity of the data and accuracy
of the data analysis.
Authors' contributions
All named authors meet the International Committee
of Medical Journal Editors (ICMJE) criteria for authorship for this
manuscript, take responsibility for the integrity of the work as a
whole, and have given final approval for the version to be
published. ES participated in Lonsurf Compassionate Use program,
managed the database, computed results and wrote the paper. All
other authors participated in the Lonsurf Compassionate Use
program, interpreted the results, commented on the manuscript and
approved the final version.
Ethics approval and consent to
participate
This article does not contain any new studies with
human or animal subjects performed by any of the authors. The study
was performed with the Lonsurf Compassionate Use program (Expanded
Access program) approved by the State Agency of Medicines of
Latvia. Written informed consent was obtained from each patient who
participated in the study.
Consent for publication
Written informed consent was obtained from each
patient who participated in the study.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Latvian cancer registry. https://www.spkc.gov.lv/upload/Veselibas%20aprupes%20statistika/Gadagramata/2015/3_sabiedribas_veseliba_2015_1.pdfMarch
20–2018
|
|
2
|
Mayer RJ, Van Cutsem E, Falcone A, Yoshino
T, Garcia-Carbonero R, Mizunuma N, Yamazaki K, Shimada Y, Tabernero
J, Komatsu Y, et al: RECOURSE Study Group: Randomized trial of
TAS-102 for refractory metastatic colorectal cancer. N Engl J Med.
372:1909–1919. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Salvatore L, Niger M, Bellu L, Tamburini
E, Garcia-Alfonso P, Amellal N, Delmas AS, Wahba M and Prager G:
Compassionate use program for trifluridine/tipiracil (TAS-102) in
metastatic colorectal cancer: A real-life overview. Ann Oncol. 27
suppl_6:512P2016. View Article : Google Scholar
|
|
4
|
Fernández CC, Roces LV, López EL, Ferreras
LR, de Segura Iriarte AG and María CR: DI-064 Evaluationof tas-102
as an expanded access in refractory colorectal cancer. Eur J Hosp
Pharm Sci Pract. 24:A141–A142. 2017.
|
|
5
|
O'Brien C, Callaghan S, Papaxoinis G,
Bennett J, Lee CS, Evans RM, Iveson T, Adams R and Mullamitha S:
TAS 102 in refractory metastatic colorectal cancer: UK Expanded
Access Programme experience. J Clin Oncol. 35:e150432017.
|
|
6
|
Kotani D, Pashtoon K, Cecchini M, Shitara
K, Ohtsu A, Ramanathan R, Hochster H, Grothey A and Yoshino T:
PD-010 Association between chemotherapy-induced neutropenia at
1-month and overall survival in patients receiving TAS-102 for
metastatic colorectal cancer. Ann Oncol. 27 suppl 2:ii1052016.
View Article : Google Scholar
|
|
7
|
Hamauchi S, Yamazaki K, Masuishi T, Kito
Y, Komori A, Tsushima T, Narita Y, Todaka A, Ishihara M, Yokota T,
et al: Neutropenia as a predictive factor in metastatic colorectal
cancer treated with TAS-102. Clin Colorectal Cancer. 16:51–57.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Kimura M, Usami E, Iwai M, Teramachi H and
Yoshimura T: Severe neutropenia: A prognosticator in patients with
advanced/recurrent colorectal cancerunderoral
trifluridine-tipiracil (TAS-102) chemotherapy. Pharmazie. 72:49–52.
2017.PubMed/NCBI
|
|
9
|
Yoshino T, Mizunuma N, Yamazaki K, Nishina
T, Komatsu Y, Baba H, Tsuji A, Yamaguchi K, Muro K, Sugimoto N, et
al: TAS-102 monotherapy for pretreated metastatic colorectal
cancer: A double-blind, randomised, placebo-controlled phase 2
trial. Lancet Oncol. 13:993–1001. 2012. View Article : Google Scholar : PubMed/NCBI
|
















