Introduction
Esophageal squamous cell carcinoma (ESCC) is the
most common pathological type of esophageal cancer in Asia, and the
high rate of lymph node metastasis (LNM) has been demonstrated to
be closely associated with local tumor recurrence (1). Besides adjuvant chemotherapy,
postoperative irradiation has been a major treatment used to
decrease the local recurrence rate in patients with ESCC (2,3).
According to the nomination of lymph node (LN) stations by the
Japanese Society for Esophageal Disease (JSED) (4), it is accepted that 101, 104, 105 and
106 LN stations should be included in the treatment planning of
postoperative irradiation in upper thoracic ESCC, as well as 106,
107, 108, 110, 1, 2, 3 and 7 LN stations for middle thoracic ESCC
while 107, 108, 110, 112, 1, 2, 3 and 7 LN stations for lower
thoracic ESCC, respectively, which were based on the pattern of LNM
in ESCC reported by various researchers (4–6).
As well as the depth of tumor invasion and lesion
length, the pathological differentiation of the tumor is also
considered as a very important risk factor for LNM. It has been
widely recognized that poorly-differentiated (pd)ESCC has a higher
tendency of early lymphatic metastasis and skip metastasis in
distant LN stations (7). However,
the pattern of LNM in pdESCC has been rarely reported, and it is
not clear whether there are significant differences between pdESCC
and ESCC en bloc on the pattern of LNM. If a difference is
confirmed, LN stations included in the treatment planning for
postoperative irradiation should be adjusted according to this
pattern in the patient with pdESCC. To investigate this problem,
the present study was designed to limit the inclusion criterion
only to thoracic pdESCC in order to exclude potential
confounders.
Patients and methods
Patient population
The medical records of 690 patients consisting of
508 males and 182 females admitted to Linyi People's Hospital
(Linyi, China) who underwent radical surgery for esophageal
carcinoma between December 2012 and July 2016 were retrospectively
collected. The age range of patients was 39–85 years with mean age
of 60.597 years. The exclusion criteria were as follows: No
conformity with pdESCC histologically; preoperative chemotherapy
and/or radiotherapy received; <15 LNs resected; and co-current
malignant disease. The present study was approved by the Ethics
Review Board of Linyi People's Hospital and written informed
consent was collected from each patient.
In order to identify the pattern difference of LNM
between pdESCC and ESCC en bloc, the results of previous research
conducted by the authors of the present study were cited as a
comparison, which were collected from the medical records of 1,893
ESCC cases coming from Shandong Cancer Hospital (Jinan, China)
between February 2003 and September 2011 (1474 males and 419
females; mean age, 60.511 years) (4). The metastasis rates in the neck, upper
mediastinum, middle mediastinum, lower mediastinum and abdominal
cavity were compared. Further investigation on the difference of
metastasis rate in every LN station was performed.
Surgical procedures and
histopathological assessment
Patients received two-field or three-field
lymphadenectomy during esophagectomy. Three-field lymphadenectomy
was performed when cervical LN metastases were considered by
ultrasound examination or computed tomography scan prior to
surgery. Tumor-node-metastasis staging system (7th edition) was
used to evaluate tumor T and N stages (6).
The tumor tissue and lymph nodes were resected and
labeled corresponding to their sites by the surgeon at the end of
the surgical procedure. LNs were labeled under the instruction of
LN nomination principles of the Japanese Society for Esophageal
Diseases (8). The specimens were
sent to the Department of Pathology in Linyi People's Hospital
(Linyi, China) and fixed in 10% formalin at room temperature for 24
h, embedded with the thickness of 2 mm tissue in paraffin and
stained at room temperature for 0.5 h by haematoxylin-eosin. Two
pathologists evaluated the differentiation grade of tumor tissue
based on the morphological characteristics of tumor cells and LNM
under an optical microscope. The differentiation grades were
classified into three grades: Well-, moderately- and
poorly-differentiated, depending on the difficulty and extent of
identifing adenocarcinoma cell or squamous cancer cell under the
optical microscope. Patients with poorly-differentiated squamous
cell carcinoma was defined as pdESCC.
Statistical analysis
Results were presented as a number and percentage
for categorical variables. The Cochran-Mantel-Haenszel test was
used to assess the difference of LNM pattern between pdESCC and
ESCC in the neck, upper mediastinum, middle mediastinum, lower
mediastinum and abdominal cavity. The clinicopathological factors
associated with LNM were analyzed using Chi-squared tests, and
Fisher's exact test was used to assess the difference of LNM rates
between the neck and abdominal cavity. Risk factors associated with
LNM were identified by forward step-wise logistic-regression
analysis, and this method was also used to evaluate the risk
factors associated with LN stations that were not included in
conventional treatment planning of postoperative irradiation. All
analyses were performed using Stata IC 10.1 (Stata software v.
11.0; StataCorp LP., College Station, TX, USA). P<0.05 was
considered to indicate a statistically significant difference.
Results
Clinicopathological characteristics of
patients
The risk factors associated with LNM using
univariate analysis are demonstrated in Table I. Tumor location (P=0.250), depth of
tumor invasion (P<0.001), age of patients (P=0.450) and length
of tumor (P=0.001) were evaluated using univariate analysis and
further assessed in a multivariate analysis model. For multivariate
analysis, tumor location (P=0.042), depth of tumor invasion
(P<0.001) and length of tumor (P<0.001) were identified as
significant risk factors that were closely associated with LNM
(Table II). The above results of
pdESCC were similar to our previous report in ESCC (4,5). In the
present study, LNM was identified in 211 of 508 males and 70 of 182
females. LNM was confirmed in 17 cases with upper thoracic ESCC (41
cases totally), 160 cases with middle thoracic ESCC (524 cases
totally) and 44 cases with the lower thoracic ESCC (125 cases
totally). In total, 11,360 LNs were collected after surgery and
metastases were identified in 1,366 LNs.
 | Table I.Univariate analysis of
clinicopathological factors associated with LNM in
poorly-differentiated esophageal squamous cell carcinoma. |
Table I.
Univariate analysis of
clinicopathological factors associated with LNM in
poorly-differentiated esophageal squamous cell carcinoma.
| Characteristics | n | Cases with LNM
(n) |
X2 | P-value |
|---|
| Sex |
|
| 0.22 | 0.630 |
| Male | 508 | 211 |
|
|
|
Female | 182 | 70 |
|
|
| Tumor location |
|
| 2.73 | 0.250 |
| Upper
thoracic esophagus | 41 | 17 |
|
|
| Middle
thoracic esophagus | 524 | 190 |
|
|
| Lower
thoracic esophagus | 125 | 74 |
|
|
| Depth of tumor
invasion |
|
| 24.7 | <0.001 |
| T1 | 94 | 13 |
|
|
| T2 | 185 | 62 |
|
|
| T3 | 366 | 175 |
|
|
| T4 | 45 | 31 |
|
|
| Age, years |
|
| 1.57 | 0.450 |
| ≤40 | 4 | 1 |
|
|
|
41–59 | 295 | 132 |
|
|
| ≥60 | 391 | 148 |
|
|
| Length of tumor,
cm |
|
| 17.8 | 0.001 |
| ≤2.0 | 55 | 8 |
|
|
|
2.1–4.0 | 276 | 99 |
|
|
|
4.1–6.0 | 271 | 118 |
|
|
|
6.1–8.0 | 59 | 37 |
|
|
|
>8.0 | 29 | 19 |
|
|
 | Table II.Multivariate analysis of risk factors
associated with lymph node metastasis in poorly-differentiated
esophageal squamous cell carcinoma. |
Table II.
Multivariate analysis of risk factors
associated with lymph node metastasis in poorly-differentiated
esophageal squamous cell carcinoma.
| Parameters | Odds ratio | Standard error | Z | P-value | 95% confidence
interval |
|---|
| Location of
tumor | 1.430663 | 0.251992 | 2.03 | 0.042 |
1.013001–2.020529 |
| Length of tumor | 1.5345 | 0.152081 | 4.32 | <0.001 | 1.26359–1.863493 |
| Depth of tumor | 1.925458 | 0.224064 | 5.63 | <0.001 |
1.532782–2.418732 |
Difference in LNM pattern between
pdESCC and ESCC en bloc
For the tumor metastasis happened in the site of
neck, upper mediastinum, middle mediastinum, lower mediastinum and
abdominal cavity, the rates of LNM in patients with upper thoracic
pdESCC were significantly different than that of patients with
upper thoracic ESCC (P<0.001). Compared with the LNM rate in
upper mediastinum, the extra-thoracic LNM rate was relatively
higher in patients with upper thosracic pdESCC than that of
patients with upper thoracic ESCC. Similar results could be
identified in the patients with middle and lower thoracic pdESCC,
who had different rate of LNM than patients with middle and lower
thoracic ESCC in the site of neck, upper mediastinum, middle
mediastinum, lower mediastinum and abdominal cavity (P<0.001 and
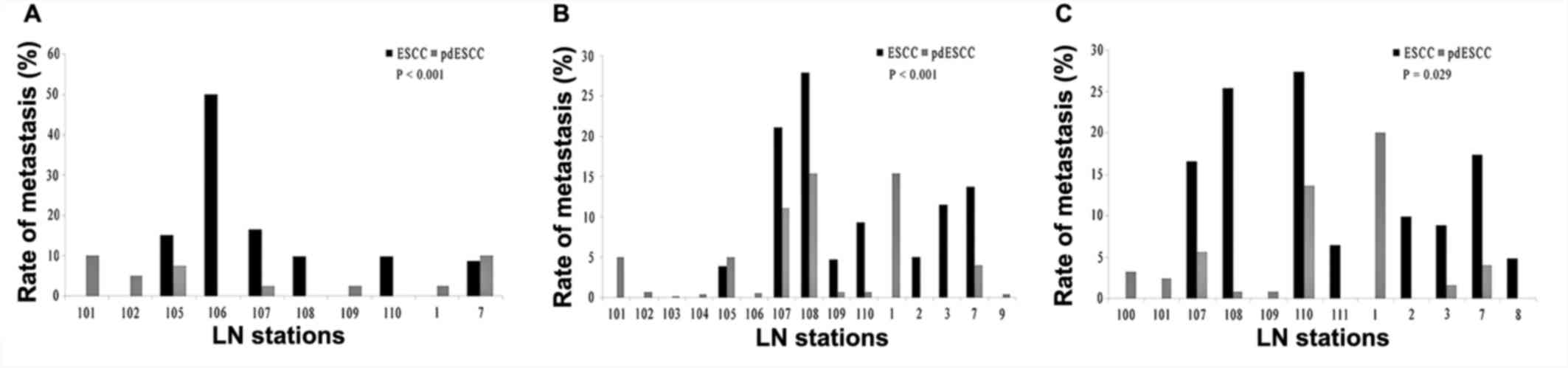
P=0.026, respectively; Fig. 1).
Significant differences of LNM distribution in LN stations between
patients with pdESCC and ESCC were demonstrated in this study, and
further investigation revealed that the upper, middle and lower
thoracic pdESCC cases had higher remote LNM rates than those of the
upper, middle and lower thoracic ESCC cases (P<0.001, P<0.001
and P=0.029, respectively; Fig.
2).
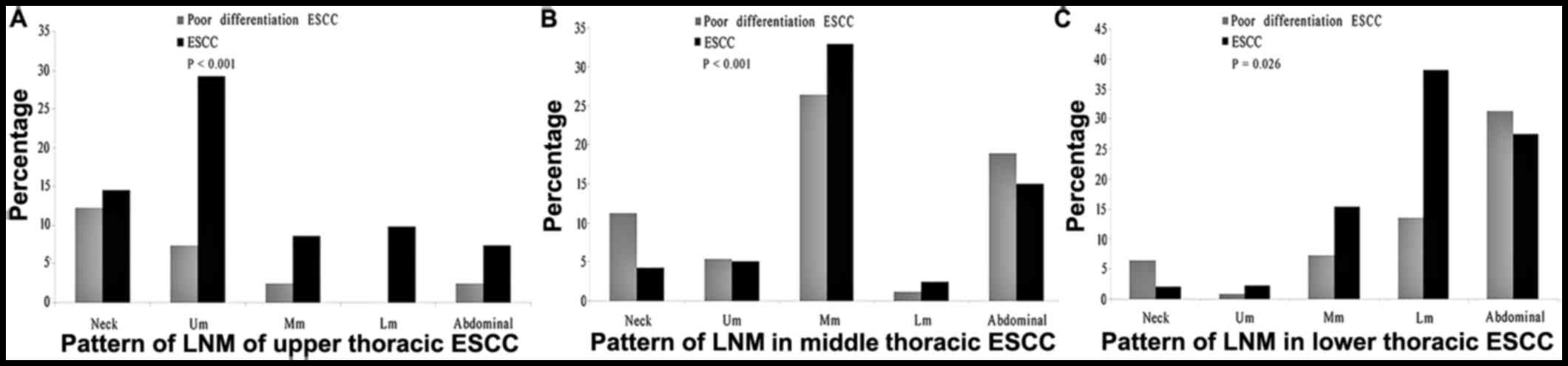 | Figure 1.Comparison of LNM distribution between
ESCC and pdESCC in the neck, Um, Mm, Lm and abdominal cavity. The
difference of LNM distribution in the site of neck, Um, Mm, Lm and
abdominal cavity between patients with thoracic ESCC and pdESCC
were significant. (A) upper thoracic ESCC vs. upper thoracic
pdESCC, P<0.001. (B) Middle thoracic ESCC vs. middle thoracic
pdESCC, P<0.001. (C) Lower thoracic ESCC vs. lower thoracic
pdESCC, P=0.026). LNM, lymph node metastasis; ESCC, esophageal
squamous cell carcinoma; pd, poorly-differentiated; Um, upper
mediastinum; Mm, middle mediastinum; Lm, lower mediastinum; EC,
Esophageal cancer. |
As a comparison of every LN station between pdESCC
and ESCC, the LNM rates of station 102 and 7 in the upper thoracic
pdESCC were higher than those of in upper thoracic pdESCC.
Similarly, the LNM rates of station 101 and 105 in pdESCC were
higher than those of ESCC in the middle thoracic pdESCC, while the
LNM rate of 100 station in pdESCC was higher than that of ESCC in
lower thoracic pdESCC. All of these LN stations were not delineated
in conventional treatment planning of postoperative irradiation,
which was based on the pattern of LNM in ESCC.
Differences in extra-thoracic
metastases between upper, middle and lower thoracic pdESCC
In the neck, significant differences in LNM were not
identified among the upper, middle and lower thoracic pdESCC
(P>0.05). However, in the abdominal cavity, significant
differences of LNM were confirmed among the upper, middle and lower
thoracic pdESCC (P<0.05). Further analysis indicated that
significant differences of bi-directional lymphatic metastasis (up
to neck and down to abdominal cavity, simultaneously) were
demonstrated in the middle (P<0.01) and lower thoracic pdESCC
(P<0.01), and not indicated in upper thoracic pdESCC (P=0.27;
Table III). Furthermore, higher
number of metastases was identified in the abdominal cavity
compared with the neck for middle and lower thoracic pdESCC (98
cases vs. 59 cases; 39 cases vs. 8 cases, respectively; Table III).
 | Table III.Pattern of LNM in the neck and
abdominal cavity from poorly-differentiated esophageal squamous
cell carcinoma. |
Table III.
Pattern of LNM in the neck and
abdominal cavity from poorly-differentiated esophageal squamous
cell carcinoma.
|
| Neck | Abdominal |
|---|
|
|
|
|
|---|
| Area | Total cases | Cases of LNM
(n) | P-value | Total cases | Cases of LNM | P-value |
|---|
| Ut EC | 41 | 5 | >0.05 | 41 | 2a | <0.05 |
| Mt EC | 524 | 59 |
| 524 | 98b |
|
| Lt EC | 125 | 8 |
| 125 | 39c |
Risk factors associated with
metastases of 102, 7, 101, 105 and 100 LN stations
Tumor location and upper mediastinum metastasis were
demonstrated to be the LNM risk factors of station 102 (P=0.01 and
P=0.001, respectively, Table IV),
while neck and middle mediastinum metastasis were identified as the
LNM risk factors of station 7 (P=0.03 and P=0.04, respectively,
Table IV). Despite this, station
102 and 7 were not included in treatment planning of postoperative
irradiation in upper thoracic pdESCC Station 101 and 105 were not
included in the treatment planning in middle thoracic pdESCC
Accordingly, tumor location, middle mediastinum metastasis, T stage
of tumor and abdominal metastasis (P=0.04) were the risk factors
for metastasis of station 101, while tumor location (P=0.04), upper
and lower mediastinum metastasis (P=0.01) were the risk factors for
metastasis of station 105 in middle thoracic pdESCC (Table IV). The T stage of tumor was
excluded from Table IV because of
the low impact of it on tumor metastasis. In lower thoracic pdESCC,
station 100 was excluded from the treatment planning of
postoperative irradiation conventionally in ESCC.
 | Table IV.Risk factors of metastasis for lymph
node stations in pdESCC. |
Table IV.
Risk factors of metastasis for lymph
node stations in pdESCC.
| Risk factors | Odds ratio | Standard error | Z | P-value | 95% confidence
interval |
|---|
| 102 station in
UtpdESCC |
|
|
|
|
|
| Tumor
location | 0.10 | 0.10 | −2.46 | 0.01 | 0.02–0.63 |
| Um
metastasis | 15.20 | 13.46 | 3.07 | 0.001 | 2.68–86.23 |
| 7 station in
UtpdESCC |
|
|
|
|
|
| Neck
metastasis | 3.03 | 1.52 | 2.21 | 0.03 | 1.13–8.11 |
| Mm
metastasis | 2.47 | 1.06 | 2.11 | 0.04 | 1.07–5.73 |
| 101 station in Mt
pdESCC |
|
|
|
|
|
|
Abdominal metastasis | 3.31 | 1.93 | 2.05 | 0.04 | 1.05–10.38 |
| 105 station in Mt
pdESCC |
|
|
|
|
|
| Tumor
location | 0.16 | 0.14 | −2.11 | 0.04 | 0.03–0.88 |
| Lm
metastasis | 35.17 | 46.64 | 2.69 | 0.01 | 2.62–472.97 |
Discussion
ESCC has been demonstrated to be characterized with
high LNM rate, which was closely related to poor progression-free
survival and overall survival (9).
Postoperative irradiation on LN stations with high metastasis risk
may be very important to decrease the local recurrence rate in
postoperative treatment (10).
However, bi-directional metastasis (downward and upward metastasis
at the same time) and skip metastasis have been observed to occur
in the early stage of ESCC (11,12).
From our previous study, using a large sample size, it was observed
that pathological differentiation was an important predictor for
LNM, which was consistent with other research (13).
To the best of our knowledge, the distribution
pattern of LNM in pdESCC has not previously been reported and the
difference of LNM pattern between pdESCC and ESCC was unclear.
Based on the present study, there was a significant difference in
the LNM pattern between pdESCC and ESCC, which existed not only in
the neck, upper mediastinum, middle mediastinum, lower mediastinum
and abdominal cavity, but also in every LN station.
The present study further demonstrated that there
were differences in the extra-thoracic metastasis ability among the
upper, middle and lower thoracic pdESCC. The abdominal cavity was
the major LNM area in middle and lower thoracic pdESCC, while not
in upper thoracic pdESCC. For the upper thoracic pdESCC, the
frequency of LNM in the abdominal cavity was not significantly
lower than in the neck. For the middle and lower thoracic pdESCC,
the frequency of LNM in the abdominal cavity was significantly
higher than that in the neck. This finding may indicate that the
anatomy structure in the upper mediastinum is different to that of
the middle and lower mediastinum; the upper mediastinum is rich in
lymphatic vessels, nerves and blood vessels making it possible for
metastasis to spread along them (14). It would be beneficial to understand
the mechanism of LNM in pdESCC.
Similar to the results of previously published
papers, tumor location, depth of tumor invasion and length of tumor
were demonstrated to be the risk factors associated with LNM in
univariate and multivariate analysis in the present study (15–17). Age
was not identified to be a significant risk factor for LNM, which
was concluded in our previously study of ESCC. The possible reason
for this may be that the influence of age on LNM was weaker than
that of differentiation in the multivariate analysis model in
pdESCC. This result implied that tumor differentiation was a more
powerful prognostic factor compared to age in pdESCC.
In ESCC, LN stations with metastasis rates higher
than 15% have been identified as high-risk areas that should be
included inside the clinical target volume (CTV) in the treatment
planning of postoperative irradiation (18–20).
However, in pdESCC, the threshold of LNM rate that should be
considered as the high-risk area may change, because the trend
oflymphatic skip metastasis in pdESCC was stronger than in ESCC
(21). It has been demonstrated that
the average rate of LNM in all LN stations may be a reasonable
threshold for metastasis risk in ESCC (22). Based on the result of the present
study, the rates of LNM of stations 102 and 7 were higher than the
average LNM rate in all LN stations in upper thoracic pdESCC (4.9
and 9.8 vs. 3.9%); the rates of LNM in station 101 and 105 were
higher than that in middle thoracic pdESCC (5.0 and 5.0 vs. 3.9%);
and the rate of LNM in station 100 was close to that in lower
thoracic pdESCC (3.2 vs. 4.3%). Coincidentally, station 102 and 7
were not included in the conventional treatment planning of
postoperative irradiation in upper thoracic ESCC. Similarly, it was
widely accepted that station 101 and 105 could not be included in
the conventional treatment planning of postoperative irradiation in
middle thoracic ESCC and station 100 could not be included in lower
thoracic ESCC. This result suggested that the average LNM rate in
all LN stations may be a reasonable cutoff value to determine
high-risk LN stations for metastasis in pdESCC.
However, more LN stations receiving irradiation
would mean that a larger CTV would be delivered to patients in the
treatment planning of radiotherapy, and a larger CTV may lead to
heavier toxicity of normal tissue. To solve this problem, it may be
effective to only consider the above LN stations when the risk
factors of tumor metastasis have been identified. Due to the
different LNM pattern between pdESCC and ESCC, treatment planning
of postoperative irradiation should be designed individually
depending on risk factors for the particular LN station in
pdESCC.
Based on the results of the present study, it can be
recommended that in the upper thoracic pdESCC, station 102 should
receive irradiation when upper mediastinum metastasis has been
confirmed, while station 7 is recommended to be delineated inside
CTV when neck and middle mediastinum metastases are identified. In
middle thoracic pdESCC, station 101 should be drawn inside the CTV
when middle mediastinum metastasis, T3-4 stage tumor and abdominal
metastasis are identified, while station 105 should be included
inside CTV iflower mediastinum metastasis is confirmed. The above
results suggested that the adjacent LN stations with metastases
were usually risk factors for metastasis in the next LN station,
which was similar to a report by Juloori et al (23). A much more interesting result found
in the present study was that neck metastasis was a significant
risk factor for LNM of station 7 in the upper thoracic pdESCC and
metastasis in the abdominal cavity was a risk factor for LNM of
station 101 in middle thoracic pdESCC, which were consistent with
the characteristic of ESCC that bi-directional and skip metastases
appeared frequently, particularly in pdESCC (24).
Based on the results of the present study, it is
strongly recommended that the distant LN stations should be
included in irradiation in postoperative radiotherapy individually
when risk factors have been confirmed. This outcome may also be
used as an implication to explore the distant LNM in
lymphadenectomy for patients with pdESCC.
The present study had some limitations. Firstly,
although the comparison of LNM pattern between pdESCC and ESCC was
performed, more results may be identified if the comparison between
pdESCC and moderate- or well-differentiated ESCC was conducted.
Secondly, the impact on prognosis of LNM pattern between pdESCC and
ESCC was not investigated, which may provide more important
recommendation for treatment planning design of postoperative
radiotherapy in pdESCC.
In summary, higher metastases were identified in
regional LN in pdESCC, and the LNM pattern was different compared
with that of ESCC. Distant LN stations should be individually
considered in postoperative radiotherapy when risk factors have
been identified in patients with pdESCC.
Acknowledgements
Not applicable.
Funding
The present study was supported by Shandong
Provincial Medical and Health Development Plan (grant no.
2013WSA13018, Dr Jinling Zhang), the Natural Science Foundation of
Shandong Province (grant no. ZR2014HL062, Dr Jinling Zhang) and the
National Nature Science Foundation of China (grant no. 81402538, Dr
Wei Huang).
Availability of data and materials
The datasets used and/or analysed during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
JZ, XH and BL designed the study. YL and FC
collected the information of patients and analyzed the data. JZ and
YL wrote the paper. XH and BL reviewed and edited the manuscript.
All authors read and approved the manuscript.
Ethics approval and consent to
participate
All procedures used in this study involving human
participants were in accordance with the ethical standards of the
institutional research committee, and with the 1964 Helsinki
declaration and its later amendment or comparable ethical
standards. The present study was approved by the Ethics Review
Board of Linyi People's Hospital and written informed consent was
collected from each patient.
Consent for publication
Informed consent was obtained from all participants
included in the study.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Esophageal Disease Society Research:
Preface, general principles, part IPatrick Barron J: Guidelines for
clinical and pathologic studies on carcinoma of the esophagus. 9.
Tokyo: Kanehara: pp. 72–73. 2004
|
|
2
|
Liu J, Liu Q, Wang Y, Xia Z and Zhao G:
Nodal skip metastasis is associated with a relatively poor
prognosis in thoracic esophageal squamous cell carcinoma. Eur J
Surg Oncol. 42:1202–1205. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Du D, Song T, Liang X, Fang M and Wu S:
Concurrent chemoradiotherapy with elective lymph node irradiation
for esophageal cancer: A systemic review and pooled analysis of the
literature. Dis Esophagus. 23:1–9. 2016.
|
|
4
|
Japan Esophageal Society, . Japanese
classification of esophageal cancer, 11th edition. Esophagus.
14:37–65. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Kim KH, Chang JS, Cha JH, Lee IJ, Kim DJ,
Cho BC, Park KR and Lee CG: Optimal adjuvant treatment for
curatively resected thoracic esophageal squamous cell carcinoma: A
radiotherapy perspective. Cancer Res Treat. 49:168–177. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Cheng J, Kong L, Huang W, Li B, Li H, Wang
Z, Zhang J, Zhou T and Sun H: Explore the radiotherapeutic clinical
target volume delineation for thoracic esophageal squamous cell
carcinoma from the pattern of lymphatic metastases. J Thorac Oncol.
8:359–365. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Li J, Wang L, Wang X, Zhao Y, Liu D, Chen
C, Zhang HP and Pan J: Preliminary study of the internal margin of
the gross tumor volume in thoracic esophageal cancer. Cancer
Radiother. 16:595–600. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Yamasaki M, Miyata H, Miyazaki Y,
Takahashi T, Kurokawa Y, Nakajima K, Takiguchi S, Mori M and Doki
Y: Evaluation of the nodal status in the 7th edition of the
UICC-TNM classification for esophageal squamous cell carcinoma:
Proposed modifications for improved survival stratification: Impact
of lymph node metastases on overall survival after esophagectomy.
Ann Surg Oncol. 21:2850–2856. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Li B, Chen H, Xiang J and Zhang Y, Li C,
Hu H and Zhang Y: Pattern of lymphatic spread in thoracic
esophageal squamous cell carcinoma: A single-institution
experience. J Thorac Cardiovasc Surg. 144:778–786. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Tanaka K, Yano M, Motoori M, Doki Y, Kishi
K, Miyashiro I, Shingai T, Gotoh K, Noura S, Takahashi H, et al:
The significance of abdominal para-aortic lymph node metastasis in
patients with lower thoracic esophageal cancer. Dis Esophagus.
25:146–152. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Chen H, Wu Z, Chen J, Lin X, Zheng C, Fan
Y, Zhang Z, Yao X, Wu J, Xu L, et al: Postoperative adjuvant
therapy for resectable thoracic esophageal squamous cell carcinoma:
A retrospective analysis of 426 cases. Med Oncol. 32:4172015.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Li M, Zhang X, Liao Z, Meng X, Kong L,
Zhang X, Shi F, Zhang Y, Wei G, Man H, et al: Failure patterns
after definitive chemoradiation therapy with involved-field
irradiation for locally advanced esophageal squamous cell
carcinoma. Int J Radiat Oncol Biol Phys. 90:12. 2014. View Article : Google Scholar
|
|
13
|
Wang S, Wang Z, Yang Z, Liu Y, Liu X,
Shang B and Jiang WP: Postoperative radiotherapy improves survival
in stage pT2N0M0 esophageal squamous cell carcinoma with high risk
of poor prognosis. Ann Surg Oncol. 23:265–272. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Huang W, Li B, Gong H, Yu J, Sun H, Zhou
T, Zhang Z and Liu X: Pattern of lymph node metastases and its
implication in radiotherapeutic clinical target volume in patients
with thoracic esophageal squamous cell carcinoma: A report of 1077
cases. Radiother Oncol. 95:229–233. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Deslauriers J: Preface. Thoracic anatomy:
Pleura and pleural spaces, mediastinum, diaphragm, and esophagus.
Thorac Surg Clin. 21:xiii–xiv. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Shinoda M, Ando N, Kato K, Ishikura S,
Kato H, Tsubosa Y, Minashi K, Okabe H, Kimura Y, Kawano T, et al:
Randomized study of low-dose versus standard-dose chemoradiotherapy
for unresectable esophageal squamous cell carcinoma (JCOG0303).
Cancer Sci. 106:407–412. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Chen J, Liu S, Pan J, Zheng X, Zhu K, Zhu
J, Xiao J and Ying M: The pattern and prevalence of lymphatic
spread in thoracic oesophageal squamous cell carcinoma. Eur J Surg
Oncol. 36:480–486. 2009.
|
|
18
|
Matsuda S, Tsubosa Y, Niihara M, Sato H,
Takebayashi K, Kawamorita K, Mori K, Tsushima T, Yasui H, Takeuchi
H, et al: Distribution of lymph node metastasis and clinical
validity of gastric tube reconstruction in lower thoracic
esophageal squamous cell carcinoma with gastric invasion. Ann Surg
Oncol. 22:617–623. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Ren X, Zhao Z, Huang W, Liu H, Dong C and
Li Y: Analysis of the characteristics and factors influencing lymph
node metastasis in thoracic esophageal carcinoma and cancer of the
gastric cardia. Hepatogastroenterology. 62:73–76. 2015.PubMed/NCBI
|
|
20
|
Van der Schaaf M, Johar A, Wijnhoven B,
Lagergren P and Lagergren J: Extent of lymph node removal during
esophageal cancer surgery and survival. J Natl Cancer Inst.
107:pii: djv043. 2015.PubMed/NCBI
|
|
21
|
Yokota T, Igaki H, Kato K, Tsubosa Y,
Mizusawa J, Katayama H, Nakamura K, Fukuda H and Kitagawa Y:
Accuracy of preoperative diagnosis of lymph node metastasis for
thoracic esophageal cancer patients from JCOG9907 trial. Int J Clin
Oncol. 21:283–288. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Jain S and Dhingra S: Pathology of
esophageal cancer and Barrett's esophagus. Ann Cardiothorac Surg.
6:99–109. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Juloori A, Tucker SL, Komaki R, Liao Z,
Correa AM, Swisher SG, Hofstetter WL and Lin SH: Influence of
preoperative radiation field on postoperative leak rates in
esophageal cancer patients after trimodality therapy. J Thorac
Oncol. 9:534–540. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Cavallin F, Alfieri R, Scarpa M, Cagol M,
Ruol A, Fassan M, Rugge M, Ancona E and Castoro C: Nodal skip
metastasis in thoracic esophageal squamous cell carcinoma: A cohort
study. BMC Surg. 17:492017. View Article : Google Scholar : PubMed/NCBI
|