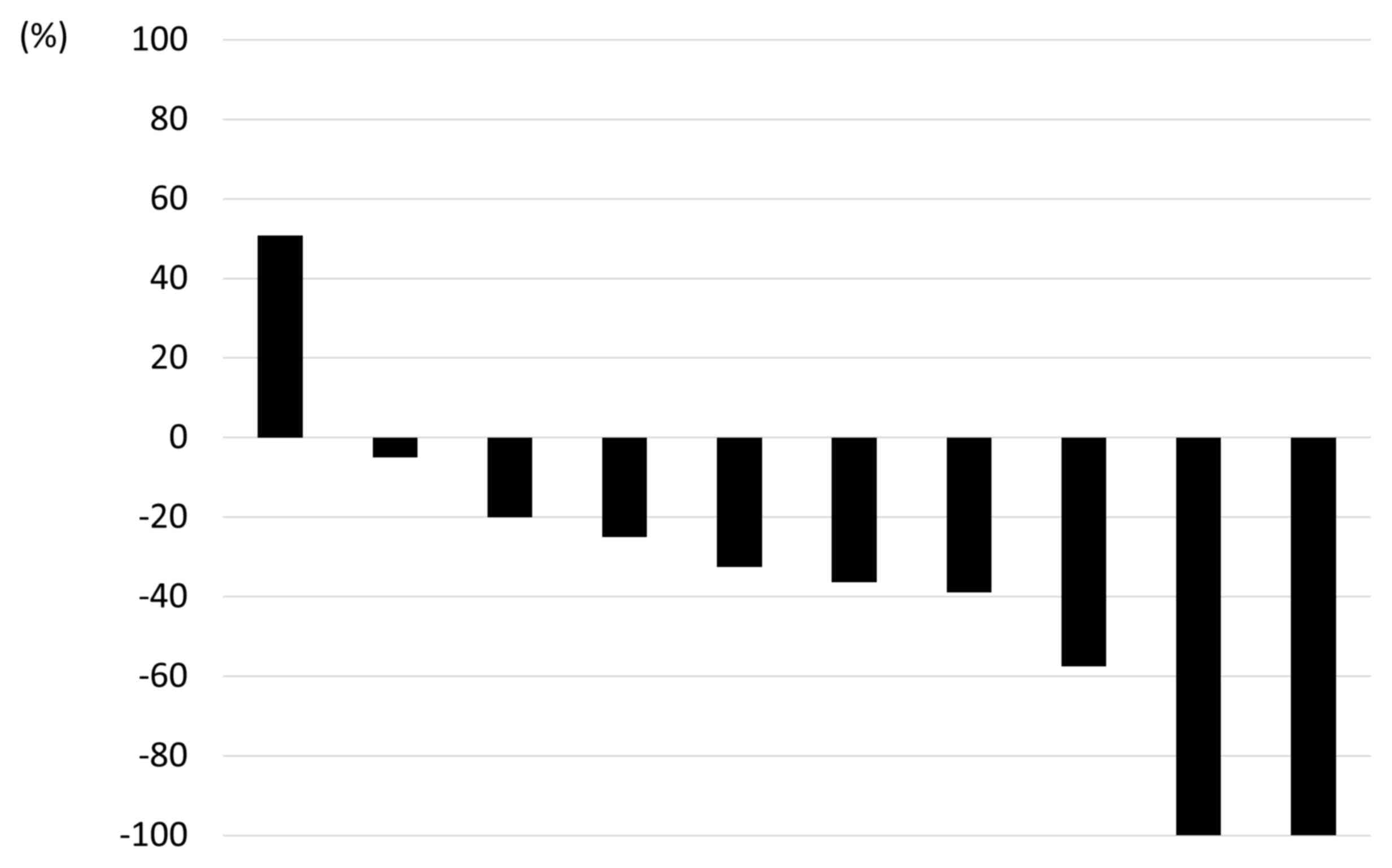Introduction
In 2017, six molecular targeted agents were approved
for the treatment of metastatic renal cell carcinoma (mRCC) in
Japan (1). Results of large-scale
clinical trials (2–7), as well as from our institution
(8), and other investigators in
Japan (9–12) have reported real-world clinical data
showing an improvement in the survival rate of patients with mRCC.
However, it is uncertain for which type of patient will this
treatment be effective. Differences in survival rates according to
the Memorial Sloan Kettering Cancer Center (MSKCC) criteria
(13) and the International
Metastatic Renal Cell Carcinoma Database Consortium (IMDC) model
(14) are being studied. Motzer
et al reported that Eastern Cooperative Oncology Group
performance status, serum hemoglobin level, time from diagnosis to
treatment, and corrected calcium, alkaline phosphatase, and lactate
dehydrogenase levels were significant independent predictors for
survival (15). It was also reported
that the prognostic factors used in the MSKCC classification are
robust and applicable in the contemporary era of targeted therapy.
Although these models correlated well with cancer survival, only a
few reports presented the survival rate as classified by metastatic
organ. In the cytokine therapy era, there was minimal variation in
the metastatic organ among Japanese patients (16,17).
Liver, bone, lymph node, and brain metastases were independent risk
factors for mRCC due to interferon-α administration. However, the
relationship between metastatic organ and mRCC treatment using
molecular targeted drugs (10,18,19) are
not well studied. Therefore, we aimed to investigate the survival
rate classified according to the metastatic lesion in the molecular
targeted agents era for mRCC in Japanese patients.
Patients and methods
We retrospectively analyzed 180 consecutively
treated patients who had received molecular targeted drugs for
mRCC. Regarding administration of first-line drugs, sunitinib 50 mg
was administered orally (PO) every day over 2 or 4 weeks, followed
by a 1- or 2-week washout period. Dose reductions, if needed, were
made in decrements of 12.5 mg. Sorafenib was administered
continuously at a full dose of 400 mg PO twice a day, with an
allowed dose reduction of 200 mg (2,3).
Temsirolimus was administered at a full dose of 25 mg div weekly
(7). For second-line drugs,
everolimus was administered continuously at a full dose of 10 mg PO
per day (4). Axitinib was
administered continuously at a full dose of 10 mg PO per day, with
allowed dose escalation of up to 20 mg, and dose reduction of up to
2 mg (20). According to the
therapeutic strategy in our institute, sorafenib is used as the
first-line therapy and sunitinib or everolimus as the second-line
therapy. From 2010 onward, we usually used sunitinib as the
first-line therapy, and from 2012 onward, we usually used axitinib
as the second-line therapy.
We calculated the overall survival (OS), OS
classified according to the MSKCC criteria (21), the number of metastatic organs, the
metastatic lesion, and presence or absence of nephrectomy. OS
period commenced from treatment with the initial targeted therapy.
OS was estimated using the Kaplan-Meier method, and the differences
were determined using the log-rank test. Cox proportional stepwise
multivariate analysis was used to evaluate the association between
the number of metastatic organ, metastatic site, MSKCC criteria,
presence or absence of nephrectomy and OS.
Response assessment was performed by using computed
tomography or magnetic resonance imaging scans every 10–12 weeks,
and evaluated according to the Response Evaluation Criteria in
Solid Tumors (RECIST) v.1.1, and the change in the pancreatic tumor
size was calculated by the fraction of decrease or increase in the
sum of the largest diameter of the target lesions (22). A P-value <0.05 was considered
statistically significant. All statistical analyses were performed
using Microsoft Excel® (Microsoft, Redmond, Washington,
USA). Permission to access the database for review of the medical
records of these patients was approved by the Local Research Ethics
Committee at Osaka City University (approval number 3441).
Results
The median age of the patients was 67 years (range:
35–84) Other patient characteristics and treatments are shown in
Table I. Patients belonging to the
intermediate risk class accounted for about 50% of all risk
classes. The number of metastatic organs was almost evenly
allocated to single or multiple. Lungs were the most common
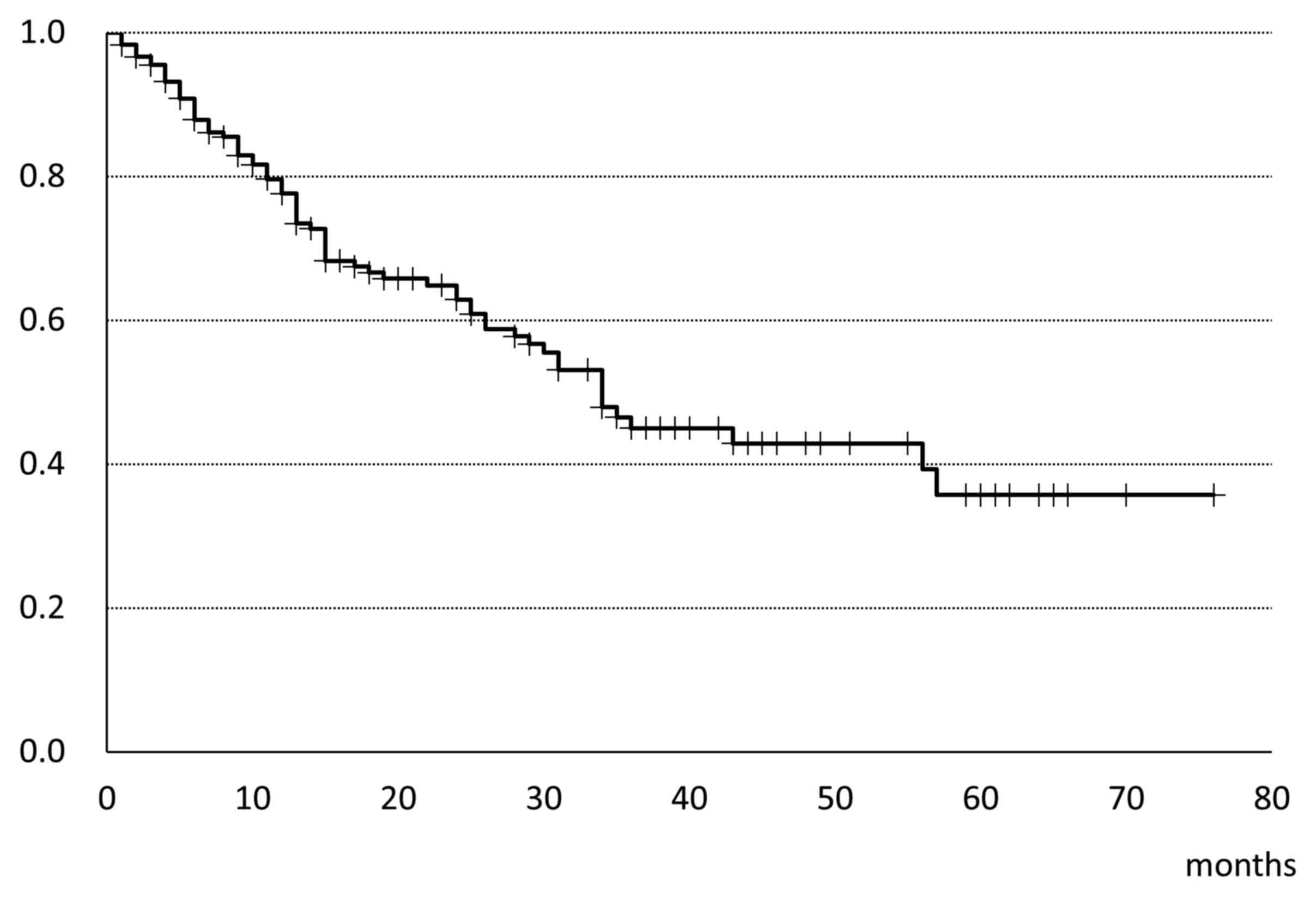
metastatic organ, followed by lymph nodes and bone. The median OS
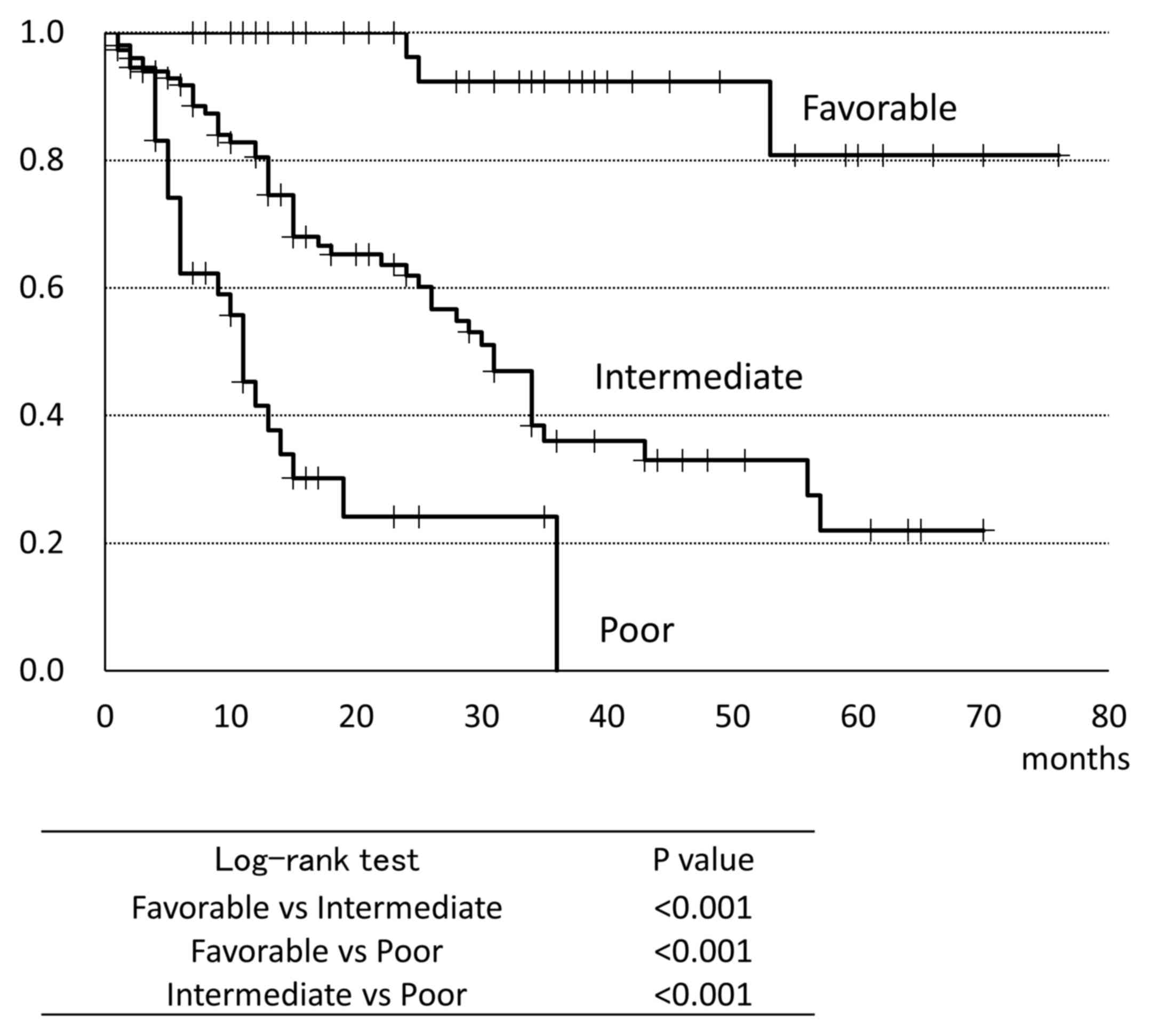
was 34 months (Fig. 1). Fig. 2 shows the OS classified by the MSKCC
criteria. Patients in the favorable group had a significantly
prolonged survival than those in the other groups; conversely, the
patients in the poor group had significantly shorter survival time
compared to those in the other groups (favorable: Not reached;
intermediate 31.0 months; poor: 11.0 months). In univariate
analysis, patients who performed nephrectomy or cytoreductive
nephrectomy had a significantly prolonged survival than those who
did not performed. Concerning the intermediate risk class (126
patients in total), this was subdivided into Intermediate group 1
(64 patients) and intermediate group 2 (62 patients). The median
age of intermediate group 1 was 67 years (range: 40–80); that of
intermediate group 2 was 69 years (range: 39–83). The other patient
characteristics and treatments are detailed in Table II.
 | Table I.Patients characteristics and
treatments (N=180). |
Table I.
Patients characteristics and
treatments (N=180).
| Characteristics | Nο. of patients | Percentage |
|---|
| Sex |
|
|
| Male | 140 |
|
|
Female | 40 |
|
| Age, years
(median) | 66 | range, 35–84 |
| MSKCC |
|
|
|
Favorable | 44 | 24.3% |
|
Intermediate | 99 | 55.2% |
| Poor | 37 | 20.5% |
| Nο. of metastatic
organ |
|
|
|
Single | 98 | 54.1% |
|
Multiple | 82 | 45.9% |
| Sites of
metastasis |
|
|
| Lung | 127 | 46.7% |
| Lymph
node | 54 | 20.0% |
| Bone | 51 | 18.8% |
|
Pancreas | 14 | 5.1% |
|
Liver | 13 | 4.8% |
|
Brain | 13 | 4.8% |
| Prior
nephrectomy |
|
|
| Yes | 165 | 91.6% |
| No | 15 | 8.4% |
| Molecular targeted
agents |
|
|
|
1st |
|
|
|
Sunitinib | 108 |
|
|
Sorafenib | 66 |
|
|
Temsilorimus | 6 |
|
|
2nd |
|
|
|
Everolimus | 30 |
|
|
Axitinib | 33 |
|
|
Sunitinib | 20 |
|
|
Temsilorimus | 14 |
|
|
Sorafenib | 2 |
|
|
3rd |
|
|
|
Everolimus | 21 |
|
|
Sunitinib | 7 |
|
|
Axitinib | 7 |
|
|
Sorafenib | 5 |
|
|
Temsirolimus | 5 |
|
|
Pazopanib | 3 |
|
|
4th |
|
|
|
Everolimus | 21 |
|
|
Sunitinib | 7 |
|
|
Axitinib | 7 |
|
|
Sorafenib | 5 |
|
|
Temsirolimus | 5 |
|
|
Pazopanib | 3 |
|
|
5th |
|
|
|
Axitinib | 3 |
|
|
Sorafenib | 2 |
|
 | Table II.Results of the Cox proportional
stepwise multivariate analysis for the association between the
clinicopathological variables and cause specific survival. |
Table II.
Results of the Cox proportional
stepwise multivariate analysis for the association between the
clinicopathological variables and cause specific survival.
|
|
| Unadjusted | Adjusted |
|---|
|
|
|
|
|
|---|
| Comparison | Overall survival
(months) (median) | HR (95% CI) | P-value | HR (95% CI) | P-value |
|---|
| Lung vs. other
organs | 31.0 vs. 34.0 | 1.03
(0.74–1.42) | 0.846 |
|
|
| Lung only vs. other
organs | 36.0 vs. 31.0 | 0.66
(0.40–1.07) | 0.097 |
|
|
| Pancreas vs. other
organs | not reached vs.
31.0 | 0.20
(0.04–0.80) | 0.024 | 0.22
(0.05–0.94) | 0.042 |
| Brain vs. other
organs | 15.0 vs. 34.0 | 1.66
(0.81–3.38) | 0.163 |
|
|
| Lymph node vs.
other organs | 28.0 vs. 34.0 | 1.15
(0.76–1.74) | 0.496 |
|
|
| Liver vs. other
organs | 15.0 vs. 34.0 | 1.88
0.83–4.26) | 0.129 |
|
|
| Bone vs. other
organs | 15.0 vs. 34.0 | 1.23
(0.80–1.89) | 0.334 |
|
|
| Single organ vs.
multiple organs | 44.4 vs. 32.3 |
0.53(0.33–0.84) | 0.007 | 0.51
(0.33–0.82) | 0.005 |
| MSKCC |
|
|
|
|
|
|
Favorable vs. others | not reached vs.
26.0 |
0.07(0.02–0.25) | <0.001 | 0.10
(0.03–0.33) | <0.001 |
| Poor
vs. others | 12.0 vs. 53.0 |
4.21(2.52–7.02) | <0.001 | 2.33
(1.39–3.93) | 0.001 |
| Nephrectomy |
|
|
|
|
|
| Yes vs.
no | 35 vs. 13 |
0.46(0.23–0.93) | 0.030 |
|
|
Patients who had a single metastatic organ lived
significantly longer than those with multiple metastatic organs
(Fig. 3). OS classified according to
metastatic lesions was analyzed using univariate logistic
regression (Table II). Patients
with pancreatic metastasis had a good response to molecular
targeted drugs. Other metastatic lesions did not have a significant
impact on the patients' survival. In multivariate analysis,
pancreatic metastasis, the number of metastatic organs, and MSKCC
criteria were independent risk factors for OS.
Next, we focused on pancreatic metastasis due to
RCC. The characteristics of patients with pancreatic metastasis and
other metastasis are shown in Table
III. Three out of 14 cases had metastases confined only to the
pancreas; however, all the 3 cases had multiple pancreatic
metastases in pancreatic head and there was no indication for
surgical resection. In patients with pancreatic metastasis, the
time from diagnosis to metastasis was significantly longer than in
cases with other metastasis (unpaired t test, Welch's test). The
maximum reduction from the baseline of the pancreatic tumors in the
10 evaluable patients is shown in Fig.
4. Partial remission was achieved in 6 cases.
 | Table III.Characteristics of patients with or
without pancreatic metastasis. |
Table III.
Characteristics of patients with or
without pancreatic metastasis.
|
| Pancreatic
metastasis (N=14) | Other metastasis
(N=166) | P-value |
|---|
| Other metastatic
organs |
|
|
|
|
Lung | 10 | 117 |
|
|
Liver | 1 | 12 |
|
|
Brain | 1 | 11 |
|
| Lymph
node | 4 | 50 |
|
|
Bone | 3 | 48 |
|
|
Others | 6 | 27 |
|
|
None | 3 | – |
|
| Time from diagnosis
to metastasis (months) | 81 (1.5–182.5) | 37.4 (0–232.4) | 0.004 |
Discussion
Treatment of mRCC has changed over the last few
years, and treatment by molecular targeted drugs has become common
(2–4,6,7,20).
Although a number of treatment outcomes have been reported in the
real-world setting (10,23), there are only few reports on the
treatment outcomes classified according to metastatic lesion
(19). We retrospectively
investigated the treatment outcome by metastatic lesion with
molecular targeted drugs, and consequently, identified that
pancreatic metastasis had better response compared to metastasis to
other organs in the molecular targeted therapy era.
In the cytokine therapy era, there was minimal
variation in the metastatic organ in Japanese patients (17). It was reported that lymph node, bone,
hepatic, and brain metastasis correlated with progression on
univariate analysis, but not on multivariate analysis. Shinohara
et al (16) reported that
liver or bone metastasis were independent risk factors. In the
targeted therapy era, McKay et al reported that the presence
of bone and liver metastasis had a negative impact on survival
(19), and another report indicated
that lymph node metastases was associated with poor prognosis in
mRCC patients treated with targeted therapy (24). In Japanese patients, liver metastasis
was an independent factor for OS (10). However, patients with pancreatic
metastasis were very few among the population, and the relationship
between survival and pancreatic metastasis was not studied in these
previous studies. Yuasa et al reported that the pancreatic
metastasis (N=20) occurs a long time (median 7.8 years) after
nephrectomy, and that the OS of these patients is long (median not
reached, 10 year survival rate; 80%) (25), although, among 20 patients, only 6
patients were treated by molecular targeted drugs. Only Grassi
et al (26) and Kalra et
al (27) reported that
pancreatic metastasis is an independent prognostic variable in the
targeted therapy era. We also identified that OS of patients with
pancreatic metastasis was longer than that of those with metastasis
to other organs (not reached vs. 31 months, respectively) in
Japanese patients. According to our findings, the reason that
pancreatic metastasis might carry good prognosis is that pancreatic
metastasis occurred late, showed good response to molecular
targeted drugs, and few patients were classified to have poor
prognosis according to the MSKCC criteria. In fact, our study
showed that time to pancreatic metastasis was longer than that to
other metastases (81 vs. 37.4 months, respectively), and 60% of
patients with pancreatic metastasis achieved partial response.
Moreover, no patients with pancreatic metastasis were classified as
poor risk in our study population. As opposed, Chrom et al
(28) recently reported that the
presence of pancreatic metastasis was not an independent prognostic
factor.
OS for patients with mRCC treated by molecular
targeted drugs was 34 months, this result is comparable to other
reports by Japanese investigators (10,12).
According to the MSKCC criteria (13), survival rates in each group were
clearly stratified. IMDC criteria, which is another risk
classification, is now often adapted to mRCC; however, some of our
study participants had no available neutrophil/lymphocyte ratio,
especially those who were diagnosed before 2010; hence, we did not
investigate the IMDC risk. Although MSKCC criteria was established
in the cytokine therapy era, our results showed that it can be
applied efficiently, even in the era of molecular targeted drugs
(21).
Next, we investigated the relationship between the
number of metastatic organ and OS. OS for patients who had one
metastatic organ was superior to those with multiple organs.
Gerlinger et al mentioned that intratumor heterogeneity can
lead to underestimation of the tumor genomics landscape portrayed
from single tumor-biopsy samples, and may present major challenges
to personalized-medicine and biomarker development (29). Therefore, it can be said that it is a
reasonable result that the prognosis is better if the metastatic
lesion is in a single organ. As might be expected, a single
metastasis in a single organ must be enucleated because complete
resection of RCC metastases may be associated with log-term
survival (30,31), and we also performed metastatectomy
in such cases. In this study, the patient recruited was inoperable
due to the presence of multiple metastases in a single organ.
Despite that the cases who underwent metastasis resection were not
included, the prognosis was better in the single organ metastasis
group than in the multiple organ group; it is said that early
detection and prompt treatment of metastasis are useful.
This study was a retrospective study and thus has
certain limitations. Treatment strategy for mRCC patients is
changing across time, so there is minimum variation due to the
class of molecular targeted drugs. Treatment with immune checkpoint
inhibitors has also increased, and future investigation is
necessary.
In conclusion, the presence of pancreatic metastasis
in patients with mRCC treated with molecular targeted therapy has a
positive impact on survival. The site of metastasis may possibly be
used for risk-stratification of patients with mRCC.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets during and/or analysed during the
current study available from the corresponding author on reasonable
request.
Authors' contributions
YS and ST conceived and designed the study and were
major contributors in writing the manuscript. TI, MK, NN, TN and TY
designed the study and revised the manuscript. SY analyzed and
interpreted the data.
Ethics approval and consent to
participate
Permission to access the database for review of the
medical records of these patients was approved by the Local
Research Ethics Committee at Osaka City University (approval nο.
3441).
Consent for publication
Not applicable.
Competing interests
Dr Satoshi Tamada received remuneration for a
lecture from Pfizer Japan (Tokyo, Japan), Bayer Japan (Tokyo,
Japan) and Novartis Pharma Japan (Tokyo, Japan). The other authors
have declared that they have no competing interests.
References
|
1
|
Shinohara N and Abe T: Prognostic factors
and risk classifications for patients with metastatic renal cell
carcinoma. Int J Urol. 22:888–897. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Escudier B, Eisen T, Stadler WM, Szczylik
C, Oudard S, Siebels M, Negrier S, Chevreau C, Solska E, Desai AA,
et al: Sorafenib in advanced clear-cell renal-cell carcinoma. N
Engl J Med. 356:125–134. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Motzer RJ, Hutson TE, Tomczak P,
Michaelson MD, Bukowski RM, Rixe O, Oudard S, Negrier S, Szczylik
C, Kim ST, et al: Sunitinib versus interferon alfa in metastatic
renal-cell carcinoma. N Engl J Med. 356:115–124. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Motzer RJ, Escudier B, Oudard S, Hutson
TE, Porta C, Bracarda S, Grünwald V, Thompson JA, Figlin RA,
Hollaender N, et al: Efficacy of everolimus in advanced renal cell
carcinoma: A double-blind, randomised, placebo-controlled phase III
trial. Lancet. 372:449–456. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Rini BI, Escudier B, Tomczak P, Kaprin A,
Szczylik C, Hutson TE, Michaelson MD, Gorbunova VA, Gore ME,
Rusakov IG, et al: Comparative effectiveness of axitinib versus
sorafenib in advanced renal cell carcinoma (AXIS): A randomised
phase 3 trial. Lancet. 378:1931–1939. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Motzer RJ, Hutson TE, Cella D, Reeves J,
Hawkins R, Guo J, Nathan P, Staehler M, de Souza P, Merchan JR, et
al: Pazopanib versus sunitinib in metastatic renal-cell carcinoma.
N Engl J Med. 369:722–731. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Hudes G, Carducci M, Tomczak P, Dutcher J,
Figlin R, Kapoor A, Staroslawska E, Sosman J, McDermott D, Bodrogi
I, et al: Temsirolimus, interferon alfa, or both for advanced
renal-cell carcinoma. N Engl J Med. 356:2271–2281. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Ninomiya N, Tamada S, Kato M, Yamasaki T,
Iguchi T and Nakatani T: Prolonging survival in metastatic renal
cell carcinoma patients treated with targeted anticancer agents: A
single-center experience of treatment strategy modifications. Can J
Urol. 22:7798–7804. 2015.PubMed/NCBI
|
|
9
|
Kondo T, Takagi T, Kobayashi H, Iizuka J,
Nozaki T, Hashimoto Y, Ikezawa E, Yoshida K, Omae K and Tanabe K:
Superior tolerability of altered dosing schedule of sunitinib with
2-weeks-on and 1-week-off in patients with metastatic renal cell
carcinoma-comparison to standard dosing schedule of 4-weeks-on and
2-weeks-off. Jpn J Clin Oncol. 44:270–277. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Miyake H, Miyazaki A, Harada K and
Fujisawa M: Assessment of efficacy, safety and quality of life of
110 patients treated with sunitinib as first-line therapy for
metastatic renal cell carcinoma: Experience in real-world clinical
practice in Japan. Med Oncol. 31:9782014. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Akaza H, Oya M, Iijima M, Hyodo I, Gemma
A, Itoh H, Adachi M, Okayama Y, Sunaya T and Inuyama L: A
large-scale prospective registration study of the safety and
efficacy of sorafenib tosylate in unresectable or metastatic renal
cell carcinoma in Japan: Results of over 3200 consecutive cases in
post-marketing all-patient surveillance. Jpn J Clin Oncol.
45:953–962. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Shinohara N, Obara W, Tatsugami K and
Naito S, Kamba T, Takahashi M, Murai S, Abe T, Oba K and Naito S:
Prognosis of Japanese patients with previously untreated metastatic
renal cell carcinoma in the era of molecular-targeted therapy.
Cancer Sci. 106:618–626. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Motzer RJ, Bacik J, Murphy BA, Russo P and
Mazumdar M: Interferon-alfa as a comparative treatment for clinical
trials of new therapies against advanced renal cell carcinoma. J
Clin Oncol. 20:289–296. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Ko JJ, Xie W, Kroeger N, Lee JL, Rini BI,
Knox JJ, Bjarnason GA, Srinivas S, Pal SK, Yuasa T, et al: The
International Metastatic Renal Cell Carcinoma Database Consortium
model as a prognostic tool in patients with metastatic renal cell
carcinoma previously treated with first-line targeted therapy: A
population-based study. Lancet Oncol. 16:293–300. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Motzer RJ, Hutson TE, Tomczak P,
Michaelson MD, Bukowski RM, Oudard S, Negrier S, Szczylik C, Pili
R, Bjarnason GA, et al: Overall survival and updated results for
sunitinib compared with interferon alfa in patients with metastatic
renal cell carcinoma. J Clin Oncol. 27:3584–3590. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Shinohara N, Nonomura K, Abe T, Maruyama
S, Kamai T, Takahashi M, Tatsugami K, Yokoi S, Deguchi T, Kanayama
H, et al: A new prognostic classification for overall survival in
Asian patients with previously untreated metastatic renal cell
carcinoma. Cancer Sci. 103:1695–1700. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Naito S, Yamamoto N, Takayama T, Muramoto
M, Shinohara N, Nishiyama K, Takahashi A, Maruyama R, Saika T,
Hoshi S, et al: Prognosis of Japanese metastatic renal cell
carcinoma patients in the cytokine era: A cooperative group report
of 1463 patients. Eur Urol. 57:317–325. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Patil S, Figlin RA, Hutson TE, Michaelson
MD, Négrier S, Kim ST, Huang X and Motzer RJ: Prognostic factors
for progression-free and overall survival with sunitinib targeted
therapy and with cytokine as first-line therapy in patients with
metastatic renal cell carcinoma. Ann Oncol. 22:295–300. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
McKay RR, Kroeger N, Xie W, Lee JL, Knox
JJ, Bjarnason GA, MacKenzie MJ, Wood L, Srinivas S, Vaishampayan
UN, et al: Impact of bone and liver metastases on patients with
renal cell carcinoma treated with targeted therapy. Eur Urol.
65:577–584. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Motzer RJ, Escudier B, Tomczak P, Hutson
TE, Michaelson MD, Negrier S, Oudard S, Gore ME, Tarazi J,
Hariharan S, et al: Axitinib versus sorafenib as second-line
treatment for advanced renal cell carcinoma: Overall survival
analysis and updated results from a randomised phase 3 trial.
Lancet Oncol. 14:552–562. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Motzer RJ, Bukowski RM, Figlin RA, Hutson
TE, Michaelson MD, Kim ST, Baum CM and Kattan MW: Prognostic
nomogram for sunitinib in patients with metastatic renal cell
carcinoma. Cancer. 113:1552–1558. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Eisenhauer EA, Therasse P, Bogaerts J,
Schwartz LH, Sargent D, Ford R, Dancey J, Arbuck S, Gwyther S,
Mooney M, et al: New response evaluation criteria in solid tumours:
Revised RECIST guideline (version 1.1). Eur J Cancer. 45:228–247.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Wahlgren T, Harmenberg U, Sandstrom P,
Sandström P, Lundstam S, Kowalski J, Jakobsson M, Sandin R and
Ljungberg B: Treatment and overall survival in renal cell
carcinoma: A Swedish population-based study (2000–2008). Br J
Cancer. 108:1541–1549. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Kroeger N, Pantuck AJ, Wells JC, Lawrence
N, Broom R, Kim JJ, Srinivas S, Yim J, Bjarnason GA, Templeton A,
et al: Characterizing the impact of lymph node metastases on the
survival outcome for metastatic renal cell carcinoma patients
treated with targeted therapies. Eur Urol. 68:506–515. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Yuasa T, Inoshita N, Saiura A, Yamamoto S,
Urakami S, Masuda H, Fujii Y, Fukui I, Ishikawa Y and Yonese J:
Clinical outcome of patients with pancreatic metastases from renal
cell cancer. BMC Cancer. 15:462015. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Grassi P, Verzoni E, Mariani L, De Braud
F, Coppa J, Mazzaferro V and Procopio G: Prognostic role of
pancreatic metastases from renal cell carcinoma: Results from an
Italian center. Clin Genitourin Cancer. 11:484–488. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Kalra S, Atkinson BJ, Matrana MR, Matin
SF, Wood CG, Karam JA, Tamboli P, Sircar K, Rao P, Corn PG, et al:
Prognosis of patients with metastatic renal cell carcinoma and
pancreatic metastases. BJU Int. 117:761–765. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Chrom P, Stec R, Bodnar L and Szczylik C:
Prognostic significance of pancreatic metastases from renal cell
carcinoma in patients treated with tyrosine kinase inhibitors.
Anticancer Res. 38:359–365. 2018.PubMed/NCBI
|
|
29
|
Gerlinger M, Rowan AJ, Horswell S, Math M,
Larkin J, Endesfelder D, Gronroos E, Martinez P, Matthews N,
Stewart A, et al: Intratumor heterogeneity and branched evolution
revealed by multiregion sequencing. N Engl J Med. 366:883–892.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Alt AL, Boorjian SA, Lohse CM, Costello
BA, Leibovich BC and Blute ML: Survival after complete surgical
resection of multiple metastases from renal cell carcinoma. Cancer.
117:2873–2882. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Naito S, Kinoshita H, Kondo T, Shinohara
N, Kasahara T, Saito K, Takayama T, Masumori N, Takahashi W,
Takahashi M, et al: Prognostic factors of patients with metastatic
renal cell carcinoma with removed metastases: A multicenter study
of 556 patients. Urology. 82:846–851. 2013. View Article : Google Scholar : PubMed/NCBI
|