Introduction
Immature teratoma (IM) is a germ cell tumor that
develops in the ovary of the relatively young women (1). Chemotherapy involving cisplatin,
etoposide and bleomycin (BEP) has improved its prognosis, but
recurrence of IM is occasionally encountered (2–4). Thus,
it is important to assess for recurrence in the follow-up
examination. In addition to IM recurrence, gliomatosis peritonitis
(GP) and growing teratoma syndrome (GTS) can develop after
treatment for IM (5–7).
GP is characterized by mature glial tissue in the
peritoneum, and GTS is defined as an increase in tumor size, which
is composed of only a mature teratoma, after treatment for IM. Both
are histologically benign tumors. In some cases, a tumor becomes
large and requires resection. However, when symptoms are not
present, we do not have to resect the tumor does not require
resection (5–7). On the other hand, we start treatment as
soon as possible in cases involving the recurrence of IM.
Thus, it is very important to distinguish whether
the tumor represents IM recurrence or the development of GP and
GTS.
Imaging examination, such as computed tomography
(CT) and fluorodeoxyglucose positron emission tomography (FDG-PET),
is widely used clinically. Given that these techniques can be used
to visualize vascularization and glucose transport, which are
characteristics of malignant cells, they are useful (8–10).
Here, we report on a tumor that developed after
treatment for IM and exhibited both the contrast enhancement and
accumulation of FDG. Diagnostic laparoscopy was useful to obtain an
accurate diagnosis. The patient is now undergoing regular follow-up
without any evidence of IM recurrence.
Case report
A 30-year-old nulliparous woman presented with
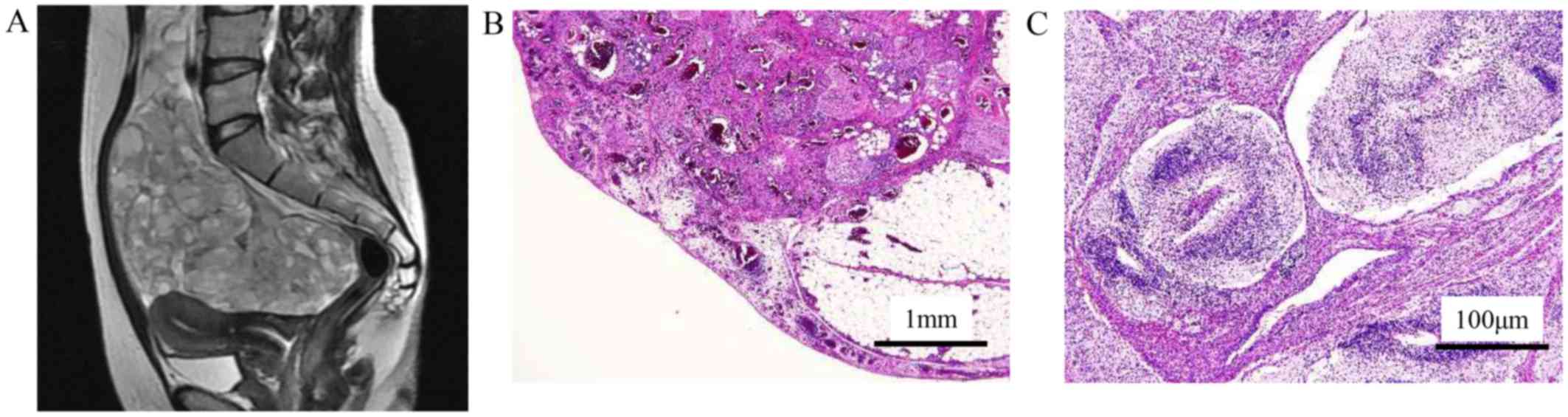
abdominal discomfort. MR image suggested malignant ovarian cancer
(Fig. 1A), and a total abdominal
hysterectomy, and bilateral salpingo-oophorectomy and partial
omentectomy were performed. She was diagnosed with a grade 2
immature teratoma (Fig. 1B and C),
and the FIGO stage was IIIB (pT3bNXM0). Four cycles of BEP
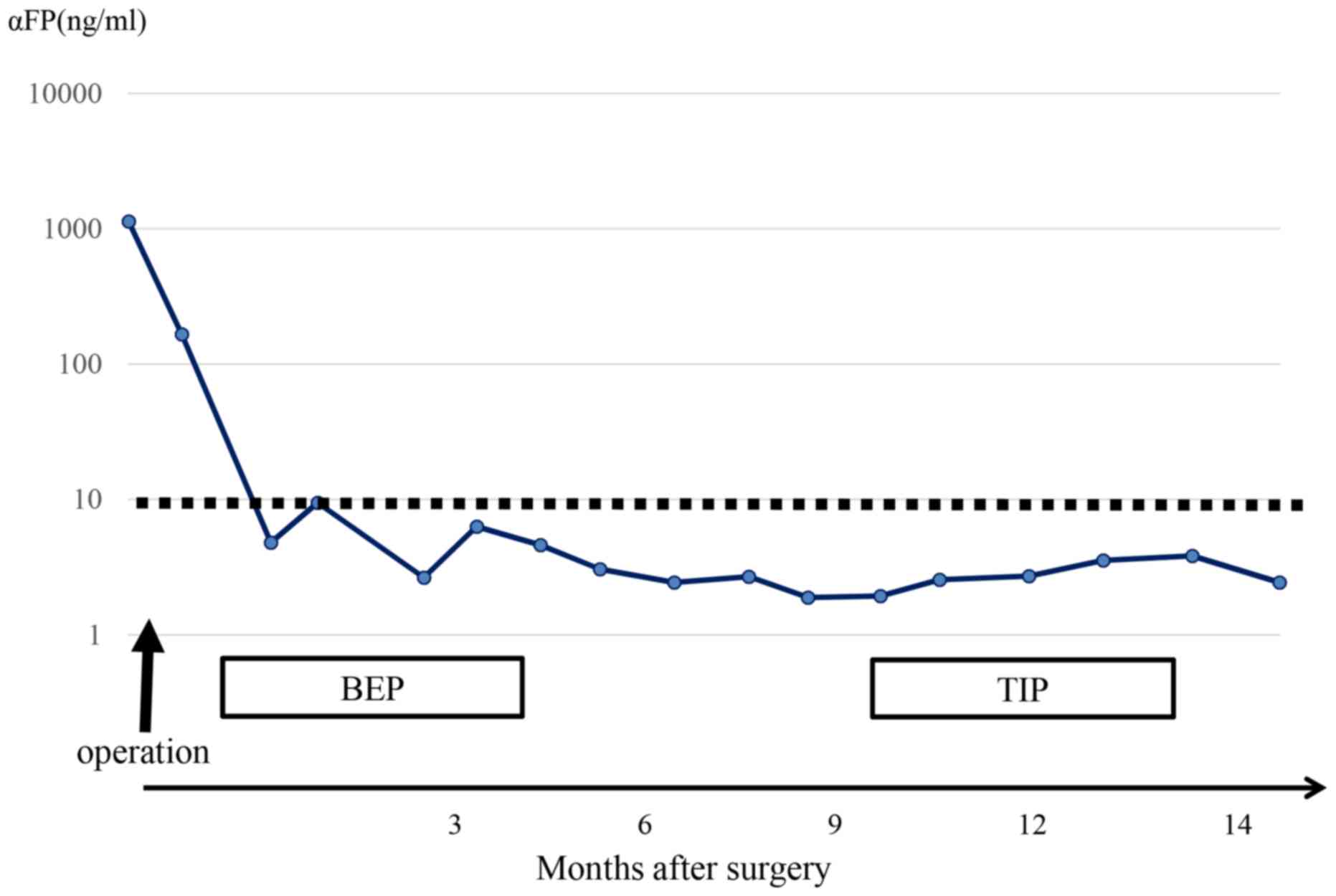
chemotherapy were administered after surgery. α-feto protein (AFP),
which was markedly elevated before treatment, decreased rapidly. At
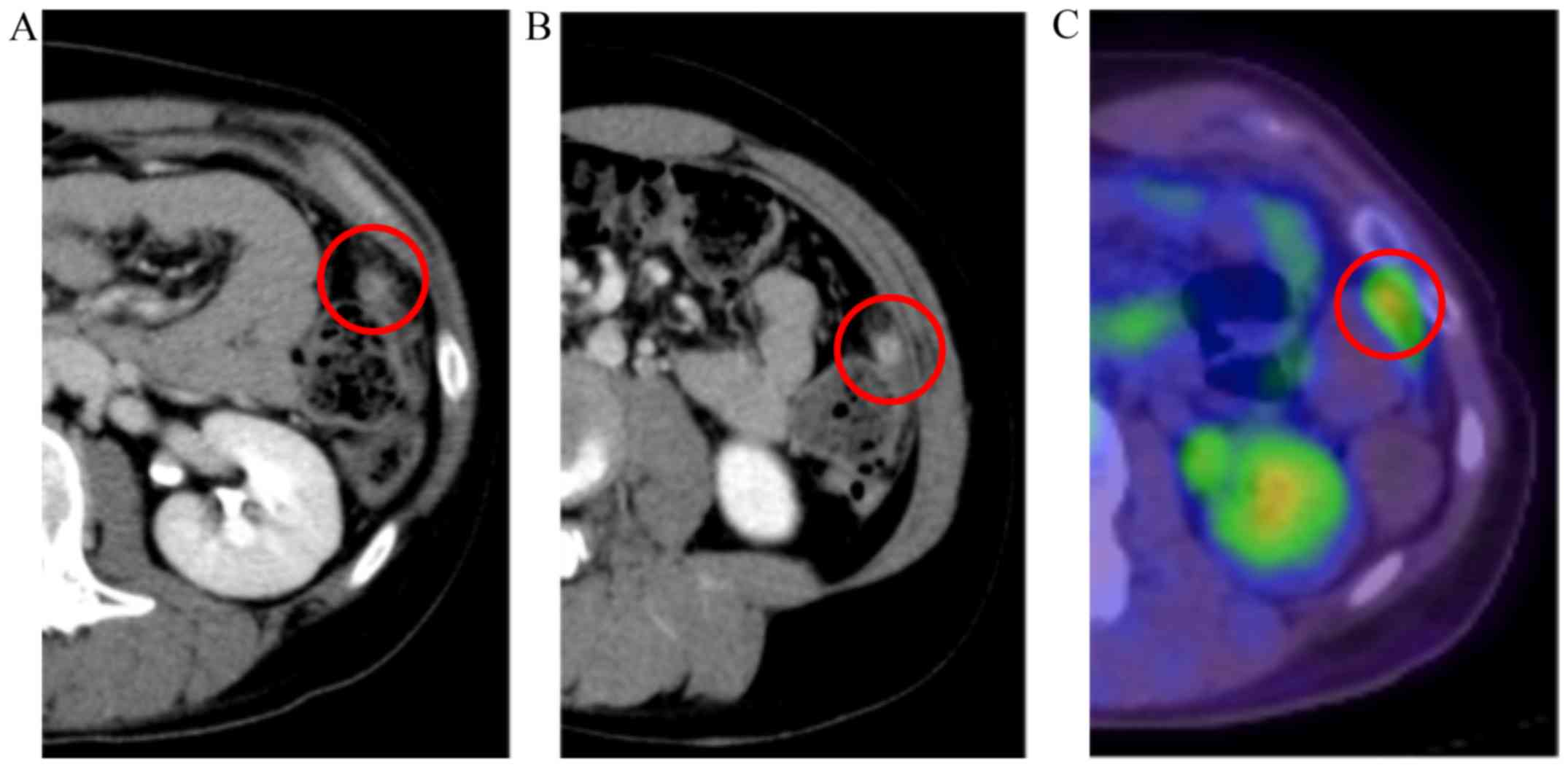
the end of treatment, a CT test was performed, and we did not find
any evidence of tumor recurrence (Fig.
2A).
Six months after initial treatment, follow-up CT was
performed. A tumor with contrast enhancement developed on the
splenic flexure (Fig. 2B). To
distinguish whether the tumor was a recurrence of IM or GP and GTS,
a PET/CT test was performed. As a result, accumulation of FDG was
noted in the same place (SUVmax, 3.43) (Fig. 2C). AFP levels remained normal
(Fig. 3).
At that time, no reports were available that
referred to GP and GTS with FDG accumulation. Although low levels
of AFP are atypical of recurrence of IM, we thought that the tumor
represented recurrent IM. Upon patient consent, four cycles of
paclitaxel, ifomide and cisplatin (TIP) chemotherapy were
administered.
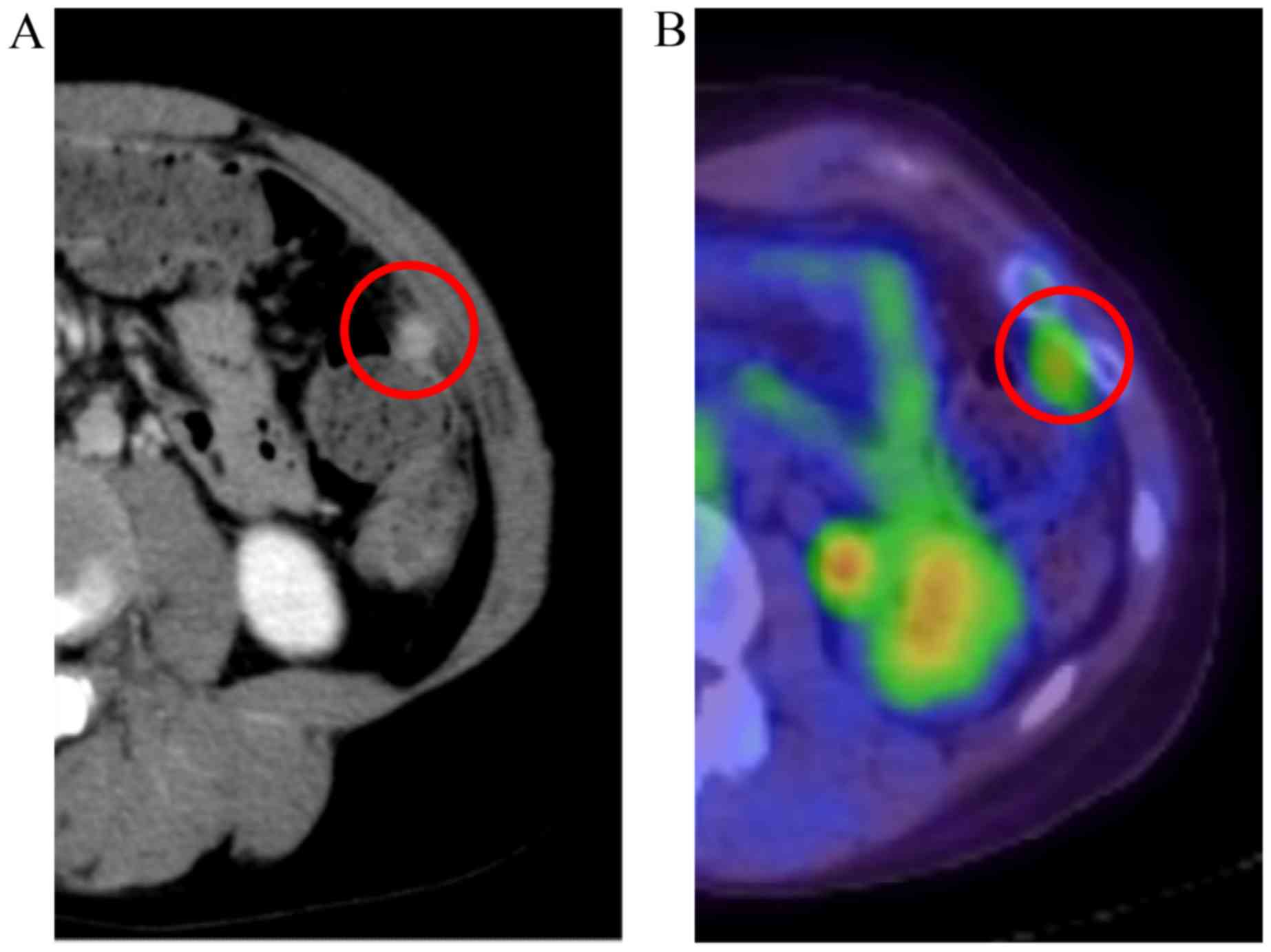
However, contrast enhancement of the tumor remained
after TIP treatment (Fig. 4A). FDG
accumulation also remained (SUVmax, 3.15) (Fig. 4B). AFP levels remained normal
(Fig. 3).
Surgery can be indicated for local and
chemorefractory recurrent IM. Although there was no report that
referred to GP and GTS with FDG accumulation at that time, we
proposed the possibility of GP and GTS with FDG accumulation. Upon
obtaining patient consent, diagnostic laparoscopy was performed to
make an accurate diagnosis.
With the help of digestive surgery doctors,
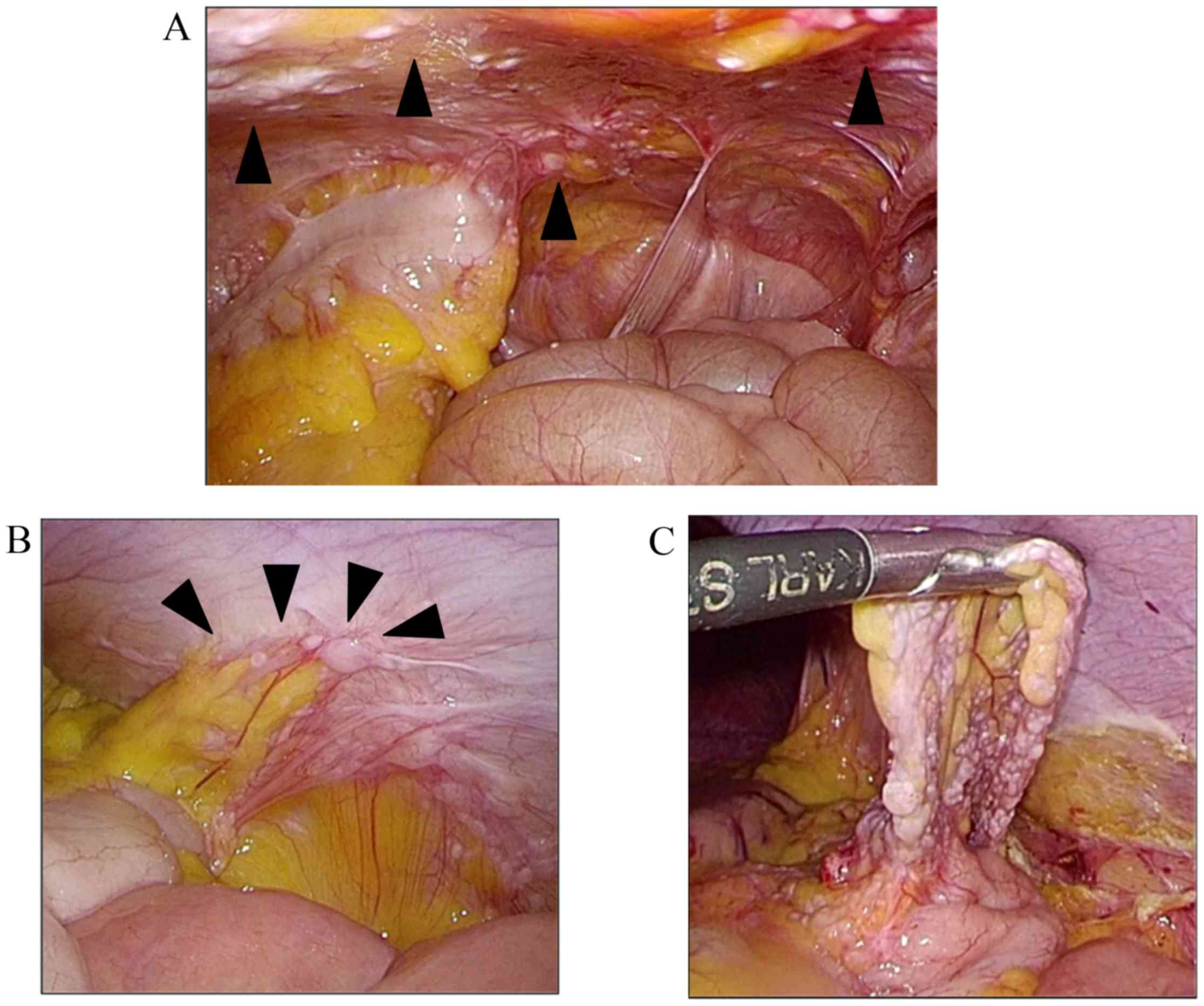
diagnostic laparoscopy was performed. Numerous white nodes were
located in the peritoneum (Fig. 5A).
A tumor on the splenic flexure adhered to the peritoneum wall
surrounded by omentum (Fig. 5B).
Under laparoscopy, the adhesion was removed
carefully, and the tumor was resected (Fig. 5C). We also resected several white
perinoneal nodes. Intraoperative rapid diagnosis revealed GP, and
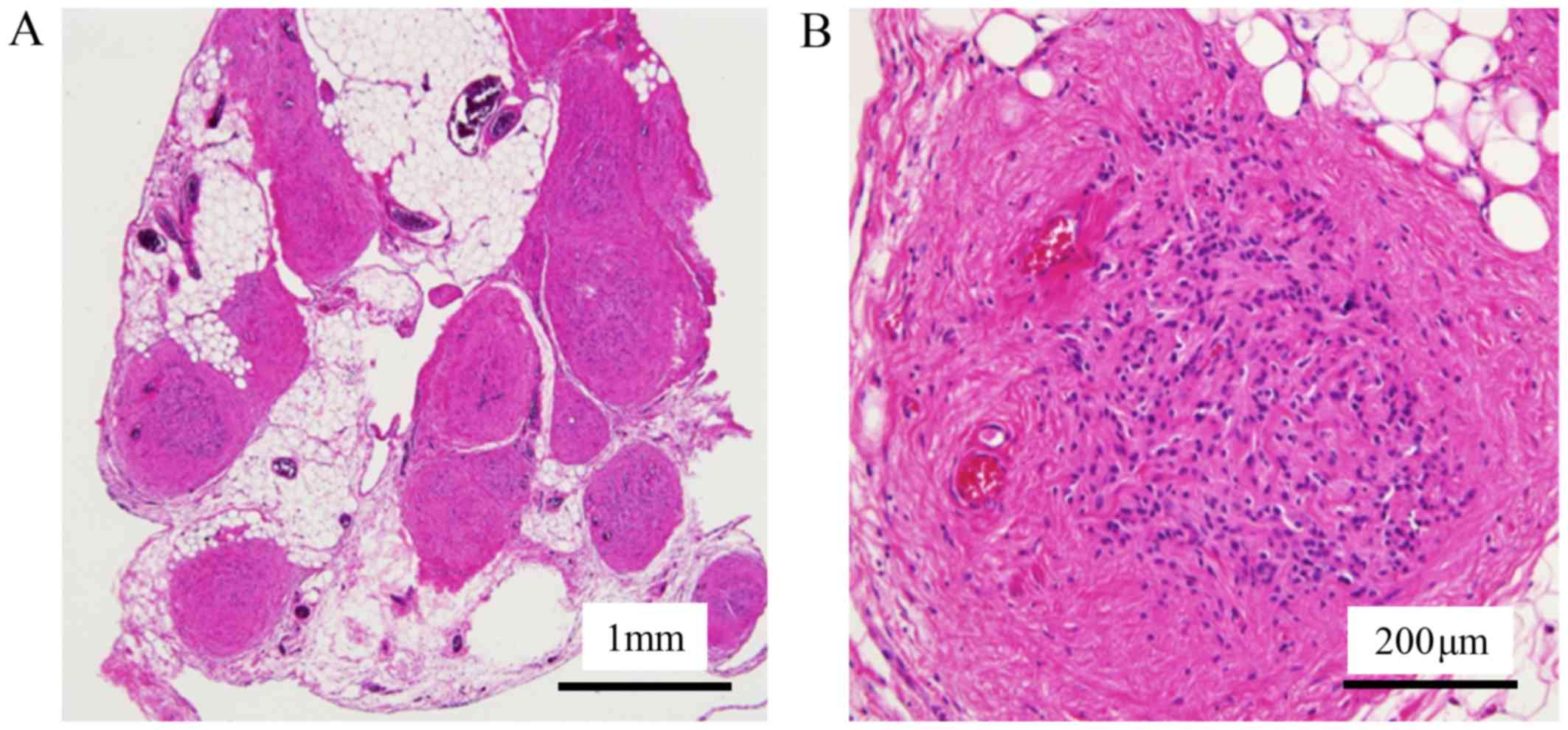
no evidence of IM was found. Detailed pathological examination
revealed mature glioma and fibrosis in the tumor (Fig. 6). The tumor was diagnosed as GP.
At the one-year follow-up examination after
diagnostic laparoscopy, we did not find any evidence of IM
recurrence. The patient is currently undergoing regular
follow-up.
Discussion
In this report, we describe a case of GP with both
contrast enhancement and FDG accumulation, which developed after
treatment for IM. As a result, the tumor was GP not recurrent IM.
To date, only one report describes GP with FDG accumulation
developed after treatment for IM (11). However, to our knowledge, this is the
first report that describes GP with both FDG accumulation and
contrast enhancement.
In the follow-up of malignancies, imaging tests play
an important role. Vascularization and facilitated glucose
transport are characteristics of malignant tumor. Given that
enhanced CT can evaluate tumor vascularization, FDG-PET can
evaluate glucose transport. These tests represent useful imaging
tests to evaluate tumor function.
In this case, the tumor harbored both contrast
enhancement and FDG accumulation. Thus, we initially assumed IM
recurrence and administered 2nd line chemotherapy. However, the 2nd
line chemotherapy TIP did not alter contrast enhancement, FDG
accumulation or tumor size.
Given that tumor status and levels of the tumor
marker AFP remained stable, we assumed GP and GTS, which are known
to develop in relation to IM.
Occasionally, GP or GTS become large and must be
resected if symptoms develop. However, these lesions are
pathologically benign tumors. We believe that glucose transport
should be reduced in GP compared with malignant tumors. However,
recently, a case of GP with high FDG uptake was reported. Although
a detailed mechanism must be elucidated, glia that consist of GP
may occasionally exhibit increased glucose transport regardless of
the degree of malignancies.
When we cannot make an accurate diagnosis based on
imaging tests, pathological examination is very important. In this
case, we could make an accurate diagnosis by diagnostic
laparoscopy. As laparoscopic surgery becomes popular, it is useful
in the field of gynecologic malignancies. Compared with laparotomy,
laparoscopic surgery reduces intraoperative blood loss,
post-operative pain, and the length of hospital stay. This
technique also offers a more speedy recovery. In addition, we
obtain a wide intra-abdominal view with a smaller incision. If an
accurate diagnosis is difficult to obtain, close observation and
biopsy of the lesion with laparoscopy can be considered.
In conclusion, we report a case of GP with both
contrast enhancement and high FDG uptake that developed after
treatment for IM. We could not distinguish GP or GTP from IM
recurrence by imaging tests. Biopsy and pathological examination of
the lesion by laparoscopic surgery were useful. In the follow-up
examination after treatment for IM, we should consider that GP,
which cannot be completely distinguished from IM recurrence by
imaging tests alone, can develop. In such a case, we should
consider diagnostic laparoscopy to obtain an accurate
diagnosis.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
All data has been presented in this published
study.
Authors' contributions
OT performed treatments and wrote the manuscript; YK
performed treatments and edited the manuscript; IY, OJ, SM, SH, HT,
YK and SK performed treatments.
Ethics approval and consent to
participate
Written informed consent was obtained from the
patient in the present study.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Zalel Y, Piura B, Elchalal U, Czernobilsky
B, Antebi S and Dgani R: Diagnosis and management of malignant germ
cell ovarian tumors in young females. Int J Gynaecol Obstet.
55:1–10. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Williams S, Blessing JA, Liao SY, Ball H
and Hanjani P: Adjuvant therapy of ovarian germ cell tumors with
cisplatin, etoposide and bleomycin: A trial of the Gynecologic
Oncology Group. J Clin Oncol. 12:701–706. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Norris HJ, Zirkin HJ and Benson WL:
Immature (malignant) teratoma of the ovary: A clinical and
pathologic study of 58 cases. Cancer. 37:2359–2372. 1976.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Mangili G, Scarfone G, Gadducci A,
Sigismondi C, Ferrandina G, Scibilia G, Vigano R, Tateo S, Villa A
and Lorusso D: Is adjuvant chemotherapy indicated in stage I pure
immature ovarian teratoma (IT)? Amulticentre Italian trial in
ovarian cancer (MITO-9). Gynecol Oncol. 119:48–52. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
André F, Fizazi K, Culine S, Droz J,
Taupin P, Lhommé C, Terrier-Lacombe M and Théodore C: The growing
teratoma syndrome: Results of therapy and long-term follow-up of 33
patients. Eur J Cancer. 36:1389–1394. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Fortt RW and Mathie IK: Gliomatosis
peritonei caused by ovarian teratoma. J Clin Pathol. 22:348–353.
1969. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Yoon NR, Lee JW, Kim BG, Bae DS, Sohn I,
Sung CO and Song SY: Gliomatosis pertonei is associated with
frequent recurrence, but does not affect overall survival in
patients with ovarian immature teratoma. Vichows Arch. 461:299–304.
2012. View Article : Google Scholar
|
|
8
|
Gu P, Pan LL, Wu SQ, Sun L and Huang G:
CA125, PET alone, PET-CT, CT and MRI in diagnosing reccurent
ovarian carcinoma: A systematic review and meta-analysis. Eur J
Radiol. 71:164–174. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Beyer T, Townsend DW, Brun T, Kinahan PE,
Charron M, Roddy R, Jerin J, Young J, Byars L and Nutt R: A
combined PET/CT scanner for clinical oncology. J Nucl Med.
41:1369–1379. 2000.PubMed/NCBI
|
|
10
|
Oldan JD and Patel PS: Positron emission
tomography/computed tomography for gynecologic malignancies. Obstet
Gynecol Surv. 71:545–556. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Lavoie JM, Lacroix-Poisson F, Hoang LN,
Wilson DC, Seckl MJ and Tinker AV: 18F FDG positron-emission
tomography findings of gliomatosis peritonei: A case report and
review of the literature. Gynecol Oncol Rep. 20:105–107. 2017.
View Article : Google Scholar : PubMed/NCBI
|