Introduction
Lung cancer is a common cause of mortality for both
men and women (1). Despite advances
in treatment, the five-year overall survival (OS) rate is only
16.3% (2). In the majority of cases,
>80% of lung cancer diagnoses are of the non-small cell lung
cancer (NSCLC) type (3). To date,
the disease prognosis is mainly based on the tumor-node-metastasis
(TNM) staging system, the histologic type and certain mutational
genetic analyses (3,4). Although these factors strongly affect
the treatment choice and outcomes of patients with NSCLC, the
majority of these factors cannot be determined without invasive
procedures, and the required mutational genetics analysis
procedures are costly and provide insufficient evidence for
validation (5–7). Therefore, it is necessary and
worthwhile to identify clinical biomarkers that could economically
and conveniently predict the prognosis of patients with NSCLC.
Hemoglobin is a biochemical biomarker assessed
during clinical examination. Several reports have indicated that
low hemoglobin levels are associated with poor survival in patients
with NSCLC (8–10); however, contradictory reports also
exist (11,12). Whether hemoglobin levels, especially
low pre-treatment hemoglobin (LPHb) levels, are an independent
predictor for poor prognosis in patients with NSCLC requires
further study for clarification.
In Henan, China, there reside ~1,000,000,000 people;
to the best of our knowledge there is no regional data regarding
the prognostic value of pre-treatment hemoglobin levels in patients
with NSCLC. The aim of the present study was to investigate the
prognostic value of pre-treatment hemoglobin levels for the
survival of patients with NSCLC.
Patients and methods
Patients
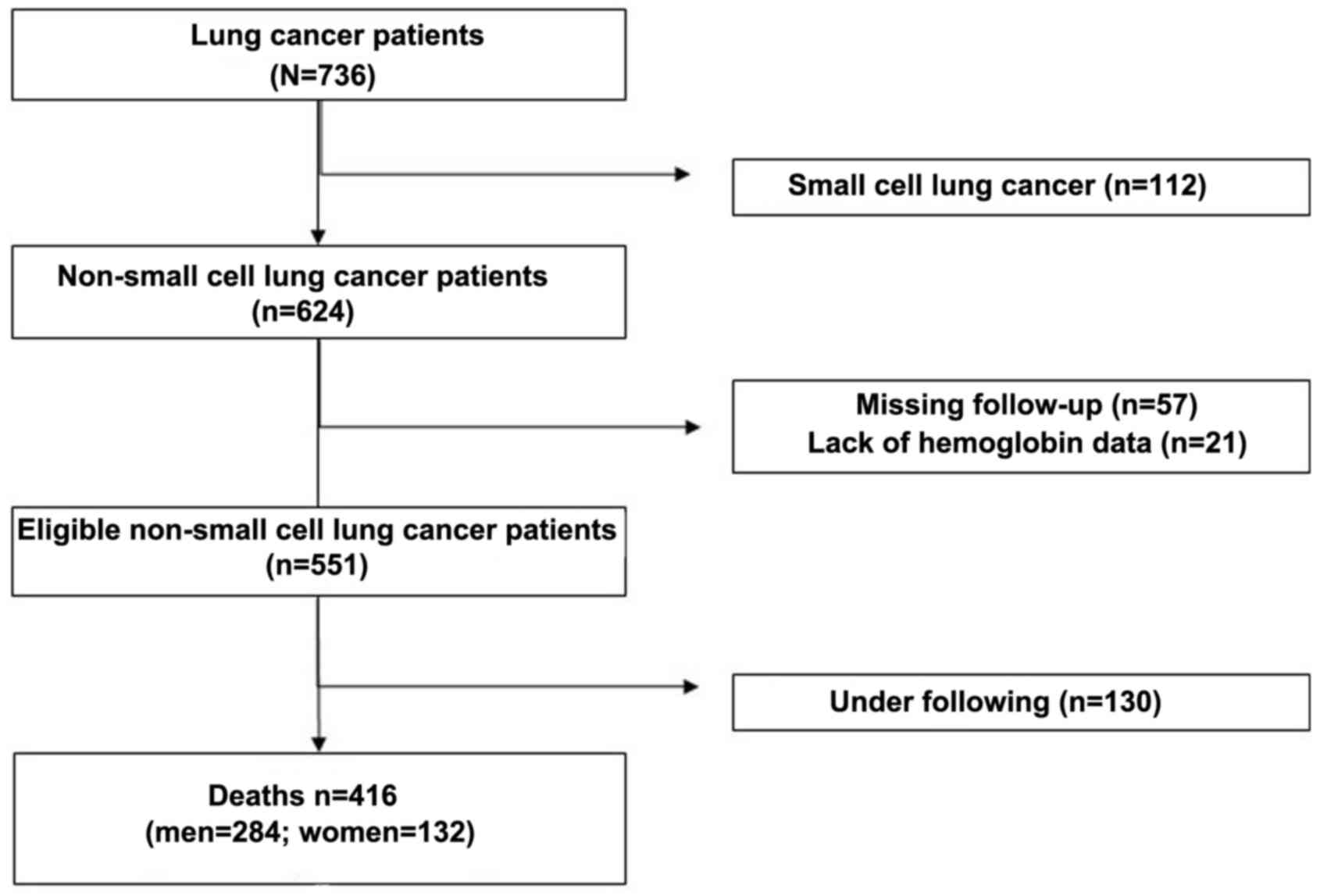
From May 2010 to June 2017, 736 patients with lung
cancer were diagnosed at the Henan University Huaihe Hospital
(Henan, China). The clinical data were retrospectively collected.
After excluding 320 ineligible subjects, a total of 416 patients
with NSCLC (284 men and 132 women) were selected as subjects for
the present study (Fig. 1). All
cases of NSCLC were pathologically confirmed. The survival period
for each subject was defined as the number of days from the date of
diagnosis to the date of mortality. Patients were included in the
present study if they had a verified diagnosis of NSCLC, regardless
of whether they had received prior lung lobectomy, chemotherapy or
radiotherapy treatments.
The clinical stage was assigned on the basis of the
8th Edition of the TNM Classification for Lung Cancer (13). Data regarding age, sex, histological
cancer type, TNM stage, Karnofsky performance status (KPS)
(14), lung lobectomy, chemotherapy,
radiotherapy, smoking status, alcohol consumption, family history,
diagnosis date, hemoglobin levels and date of mortality were
obtained retrospectively from the patients’ medical records, local
death registration departments and telephone follow-ups. The study
was approved by the Medical Ethics Committee of Henan University
Huaihe Hospital.
Methods
The pre-treatment hemoglobin levels of the patients
were obtained. The LPHb level was defined as <120 g/l of
hemoglobin in men, and as <110 g/l in women. All patients were
dichotomized into an LPHb group (n=104) and a normal pre-treatment
hemoglobin (NPHb) group (n=312). Comparisons of clinical
characteristics between the LPHb and NPHb groups were conducted
using the Chi-squared (χ2) test. For univariate Cox
proportional hazards regression, age, sex, TNM stages, KPS scores,
lung lobectomy status, chemotherapy, radiotherapy, smoking status,
alcohol consumption, family history, and hemoglobin levels were
dichotomized into a favorable group and an unfavorable group.
Hazard ratios (HR) and 95% confidence intervals (CI) were
calculated to estimate associations between the observed factors
and case fatality rate of patients with NSCLC. A subsequent
multivariate analysis using Cox proportional hazards model
estimated the prognostic influence of age, sex, TNM stage, KPS,
lung lobectomy, chemotherapy, radiotherapy and hemoglobin levels on
the case fatality rate of patients with NSCLC.
Survival curves were generated using the
Kaplan-Meier analysis method, and the log-rank test was used to
examine differences in survival between the various hemoglobin
groups. All statistical analyses were performed using the Stata
software version 13 (Stata Corporation, College Station, TX, USA).
P<0.05 was considered statistically significant for all
analyses.
Results
Patient characteristics
As presented in Table
I, of the 416 patients, 178 (42.8%) were non-smokers and 238
(57.2%) were smokers. Histological diagnoses included 232 (55.8%)
adenocarcinomas, 139 (33.4%) squamous cell carcinomas and 45
(10.8%) other NSCLC types. In total, 83 (20.0%) patients were at
TNM stage I–III, 206 (49.5%) at stage IV and 127 (30.5%) patients
had an unknown stage. Statistical analysis revealed that there were
significant differences in hemoglobin levels between patients ≥65
and <65 years of age (P<0.001), men and women (P=0.002),
histological types (P=0.004), KPS scores (P=0.005), treatment with
or without lung lobectomy (P=0.006), treatment with or without
chemotherapy (P=0.02), smokers and non-smokers (P=0.002), and
survival time (P<0.001).
 | Table I.Pre-treatment hemoglobin levels among
clinicopathological and lifestyle factors in NSCLC patients. |
Table I.
Pre-treatment hemoglobin levels among
clinicopathological and lifestyle factors in NSCLC patients.
| Factors | NPHb | LPHb | P-value |
|---|
| Age (years) |
|
| <0.001 |
| ≥65 | 140 | 64 |
|
|
<65 | 172 | 40 |
|
| Sex |
|
| 0.002 |
| Male | 200 | 84 |
|
|
Female | 112 | 20 |
|
| Histology |
|
| 0.004 |
|
Adenocarcinoma | 188 | 44 |
|
| SqCC | 91 | 48 |
|
|
Other | 33 | 12 |
|
| TNM Stage |
|
| 0.089 |
|
I–III | 70 | 13 |
|
| IV | 150 | 56 |
|
|
Others | 92 | 35 |
|
| KPS |
|
| 0.005 |
|
<80 | 131 | 60 |
|
|
≥80 | 181 | 44 |
|
| Lung lobectomy |
|
| 0.006 |
|
Yes | 79 | 13 |
|
| No | 233 | 91 |
|
| Chemotherapy |
|
| 0.020 |
|
Yes | 148 | 63 |
|
| No | 164 | 41 |
|
| Radiotherapy |
|
| 0.066 |
|
Yes | 57 | 11 |
|
| No | 255 | 93 |
|
| Cigarette
smoking |
|
| 0.002 |
|
Yes | 165 | 73 |
|
| No | 147 | 31 |
|
| Alcohol
drinking |
|
| 0.112 |
|
Yes | 61 | 28 |
|
| No | 251 | 76 |
|
| Family history of
cancer |
|
| 0.994 |
|
Yes | 18 | 6 |
|
| No | 294 | 98 |
|
| Survival year |
|
| <0.001 |
| <1
year | 125 | 74 |
|
| ≥1
year | 187 | 30 |
|
Univariate Cox proportional hazards regression
analysis demonstrated that patients who were at TNM stage IV (HR,
1.55; 95% CI, 1.28–1.89), had KPS scores <80 (HR, 1.50; 95% CI,
1.24–1.83), did not receive lung lobectomy (HR, 1.96; 95% CI,
1.55–2.48), did not receive chemotherapy (HR, 1.47; 95% CI;
1.21–1.78), did not receive radiotherapy (HR, 1.34; 95% CI,
1.03–1.74), or had LPHb levels (HR, 2.05; 95% CI, 1.63–2.57) had a
significantly increased case fatality rate (Table II). However, age, sex, smoking
status, alcohol consumption and family history did not have any
significant associations with the case fatality rate of patients
with NSCLC (Table II).
 | Table II.Univariate analysis of prognostic
factors in patients with NSCLC. |
Table II.
Univariate analysis of prognostic
factors in patients with NSCLC.
| Factors | Favorable | Unfavorable | Hazard ratio
(HR) | 95% CI | P-value |
|---|
| Age (years) | <65 | ≥65 | 1.19 | 0.98–1.45 | 0.078 |
| Sex | Female | Male | 0.90 | 0.73–1.11 | 0.313 |
| TNM Stage | I–III | IV | 1.55 | 1.28–1.89 | <0.001 |
| KPS scores | ≥80 | <80 | 1.50 | 1.24–1.83 | <0.001 |
| Lung lobectomy | Yes | No | 1.96 | 1.55–2.48 | <0.001 |
| Chemotherapy | Yes | No | 1.47 | 1.21–1.78 | <0.001 |
| Radiotherapy | Yes | No | 1.34 | 1.03–1.74 | 0.030 |
| Cigarette
smoking | No | Yes | 1.07 | 0.88–1.30 | 0.483 |
| Alcohol
consumption | No | Yes | 0.95 | 0.75–1.20 | 0.660 |
| Family history of
cancer | No | Yes | 0.93 | 0.62–1.39 | 0.720 |
| Hemoglobin | NPHb | LPHb | 2.05 | 1.63–2.57 | <0.001 |
Multivariate Cox proportional hazards regression
analysis demonstrated that LPHb levels were independently
associated with an increased case fatality rate (HR, 1.86; 95% CI,
1.47–2.36; Table III). In
addition, not receiving lung lobectomy (HR, 1.46; 95% CI,
1.10–1.93), not receiving chemotherapy (HR, 1.34; 95% CI,
1.07–1.67) and TNM stage IV were also independent and unfavorable
prognostic factors (Table
III).
 | Table III.Multivariate analysis of prognostic
factors in patients with NSCLC. |
Table III.
Multivariate analysis of prognostic
factors in patients with NSCLC.
| Factors | Favorable | Unfavorable | Hazard ratio
(HR) | 95% CI | P-value |
|---|
| Age (years) | <65 | ≥65 | 1.01 | 0.82–1.25 | 0.892 |
| Sex | Female | Male | 0.90 | 0.72–1.12 | 0.356 |
| TNM Stage | I–III | IV | 1.31 | 1.04–1.65 | 0.022 |
| KPS scores | <80 | ≥80 | 1.13 | 0.92–1.41 | 0.247 |
| Lung lobectomy | Yes | No | 1.46 | 1.10–1.93 | 0.008 |
| Chemotherapy | Yes | No | 1.34 | 1.07–1.67 | 0.011 |
| Radiotherapy | Yes | No | 1.09 | 0.82–1.45 | 0.558 |
| Hemoglobin | NPHb | LPHb | 1.86 | 1.47–2.36 | <0.001 |
Kaplan-Meier survival curve estimations revealed
that patients with LPHb had a poorer OS than did patients with NPHb
levels (log-rank test, χ2=39.50; P<0.001; Fig, 2A). When the patients were subdivided
by sex, the male LPHb patients had a poorer OS than the male NPHb
patients (log-rank test, χ2=38.38; P<0.001; Fig. 2B), a difference not observed between
the counterpart female groups (log-rank test, χ2=3.16; P=0.076;
Fig. 2B). When the patients were
subdivided according to lung lobectomy, the LPHb group had a poorer
OS than the NPHb group in both the no lung lobectomy group
(log-rank test, χ2=27.35; P<0.001; Fig. 2C) and the lung lobectomy group
(log-rank test, χ2=4.87; P=0.027; Fig. 2C). The subdivision of patients
according to chemotherapy treatment also demonstrated that the LPHb
group had a poorer OS than the NPHb group, in both the no
chemotherapy group (log-rank test, χ2=21.36; P<0.001;
Fig. 2D) and the chemotherapy group
(log-rank test, χ2=12.30; P<0.001; Fig. 2D).
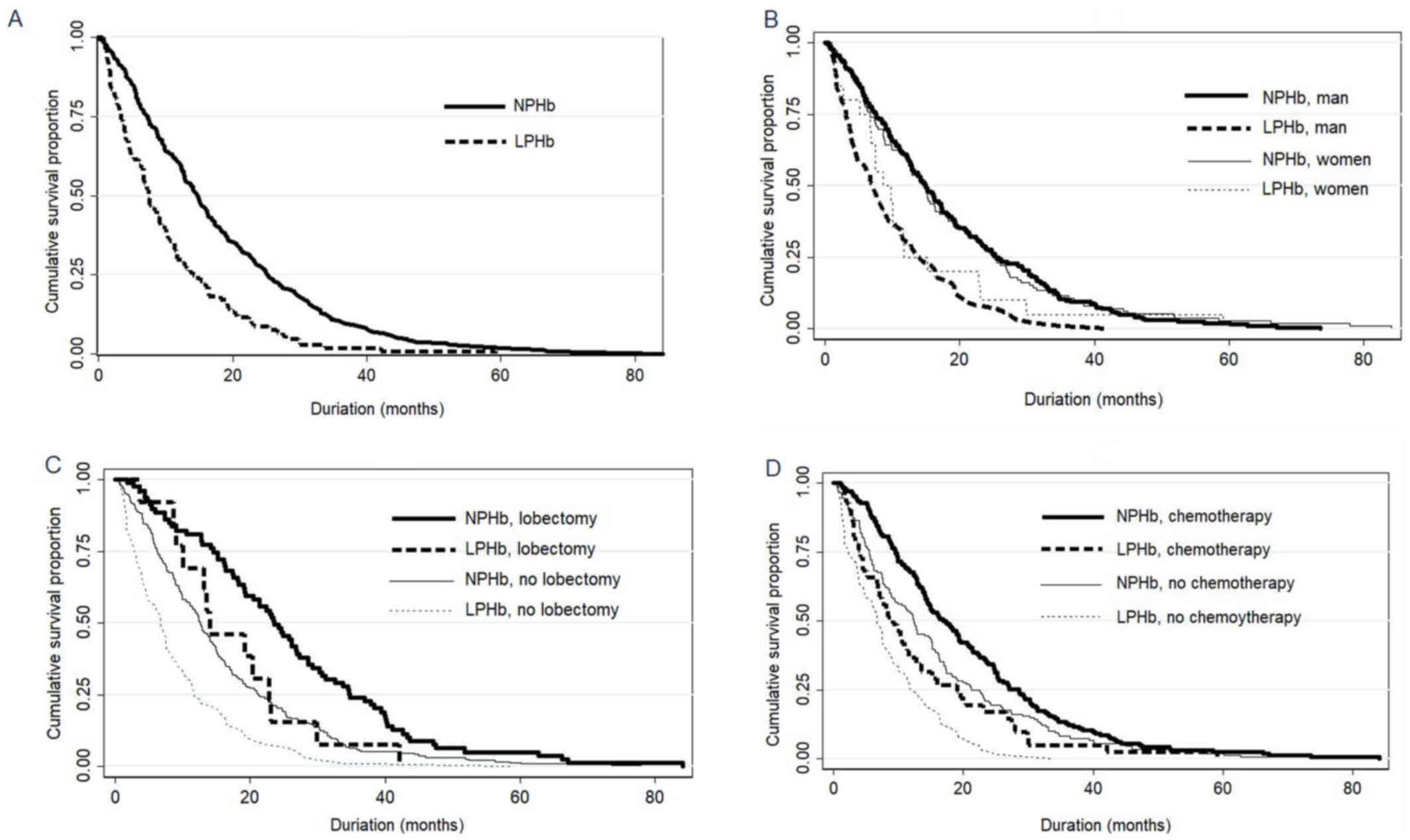 | Figure 2.Cumulative survival proportion of
patients with NSCLC according to their pre-treatment hemoglobin
levels. (A) Overall survival of NPHb and LPHb patients with NSCLC
(log-rank test, χ2=39.50; P<0.001). (B) LPHb patients
had a poorer overall survival than NPHb patients among men
(log-rank test, χ2=38.38; P<0.001), but not among
women (log-rank test, χ2=3.16; P=0.076). (C) LPHb
patients had a poorer overall survival than NPHb patients in the no
lung lobectomy group (log-rank test, χ2=27.35;
P<0.001) and the lung lobectomy group (log-rank test,
χ2=4.87; P=0.027). (D) LPHb group had a poorer overall
survival than NPHb patients in the no chemotherapy group (log-rank
test, χ2=21.36; P<0.001) and the chemotherapy group
(log-rank test, χ2=12.30; P<0.001). NSCLC, non-small
cell lung cancer; NPHb, normal pre-treatment hemoglobin (men,
120–160 g/l; women, 110–150 g/l); LPHb, low pre-treatment
hemoglobin (men, <120 g/l; women ≤110 g/l). |
Discussion
The present data suggested that pre-treatment
hemoglobin levels, measured at the time of diagnosis, may be an
independent predictor for the prognosis of patients with NSCLC.
These data are concordant with those of previous studies (8–10); to
the best of our knowledge, this is the first report of associations
between pre-treatment hemoglobin levels and the prognosis of
patients with NSCLC, independent of whether they had received
chemotherapy and/or lobectomy, in Henan, China. Compared with
previous studies (8–11,15), the
multivariate models performed in the current study included more
factors, rendering the present results less confounded.
Low hemoglobin is common in oncological diseases,
including in lung (16,17), breast (17), gastric (18) and ovarian cancer (19). There is evidence for a correlation
between hemoglobin levels and the prognosis of patients with NSCLC.
The precise underlying mechanisms are not fully understood. Tumor
cells secrete a number of soluble molecules, including
interleukin-6 (IL-6) and tumor necrosis factor-α (TNF-α). These
molecules could decrease hemoglobin by changing the hematopoietic
environment (20,21), suppressing erythropoiesis and
erythropoietin (EPO) (22), and
impairing the EPO response in erythroid progenitor cells (23). Furthermore, in patients with bone
metastasis, bone marrow involvement may lead to bone morrow
failure, which may then cause low hemoglobin levels (24) and subsequently lead to hypoxia, which
could induce genomic changes and enhance the development of
malignancy (25). Hypoxia may also
boost tumor angiogenesis and accelerate metastasis (26). In addition, hypoxia may enhance tumor
cell resistance to chemotherapy and radiotherapy through the
development of multi-drug resistance (27).
A major strength of the present study was the
inclusion of a large number of patients with NSCLC, all with a
complete set of clinical data, including the pre-treatment
hemoglobin levels, the complete survival period, records of
multiple treatments, the family history and lifestyle details,
including smoking status and alcohol consumption; this enabled us
to investigate the prognostic value of pre-treatment hemoglobin
levels with decreased sample bias and offset heterogeneity.
However, there are also limitations to the present study. First, it
was retrospective, and the information on post-treatment recurrence
was insufficient. Second, these data did not observe interaction of
post-treatment hemoglobin levels with survival rate.
Both lung lobectomy and chemotherapy treatments were
associated with the prognosis of patients with NSCLC. However,
neither significantly affected the prognostic value of the
pre-treatment hemoglobin levels in the present study. The TNM stage
was also independently associated with the NSCLC prognosis, which
is in line with previous studies (28,29).
In conclusion, the present study suggests that low
pre-treatment hemoglobin levels could be an independent biomarker
for poor prognosis in patients with NSCLC. In future clinical
studies, hemoglobin levels should be considered during the work-up
of patients with NSCLC in prospective trials, in order to confirm
its prognostic significance.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contirbutions
YHZ made substantial contributions to data
collection and was a major contributor in writing the manuscript.
YQL analyzed and interpretated the data, contributed to manuscript
preparation and revision and gave final approval for the version to
be published. HL was responsible for the acquisition of data and
the Institutional Review Board application, conducted data
interpretation, and gave final approval for the version to be
published. MWZ and YMZ agreed to be accountable for all aspects of
the work in ensuring that questions related to the accuracy or
integrity of any part of the work are appropriately investigated
and resolved. XLL made substantial contributions to conception and
design of the present study. PL and XYZ made substantial
contributions to the design of the present study and acquisition of
data. All authors read and approved the final manuscript.
Ethics approval and consent to
participate
The study was approved by the Medical Ethics
Committee of Henan University Huaihe Hospital.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no compteing
interests.
Glossary
Abbreviations
Abbreviations:
|
NSCLC
|
non-small cell lung cancer
|
|
TNM
|
tumor-node-metastasis
|
|
KPS
|
Karnofsky performance status
|
|
NPHb
|
normal pre-treatment hemoglobin
|
|
LPHb
|
low pre-treatment hemoglobin
|
|
HR
|
hazard ratio
|
|
CI
|
confidence interval
|
|
OS
|
overall survival
|
References
|
1
|
Siegel RL, Miller KD and Jemal A: Cancer
Statistics, 2017. CA Cancer J Clin. 67:7–30. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Alberg AJ, Brock MV, Ford JG, Samet JM and
Spivack SD: Epidemiology of lung cancer: Diagnosis and management
of lung cancer, 3rd ed: American college of chest physicians
evidence-based clinical practice guidelines. Chest. 143 5
Suppl:e1S–e29S. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Ettinger DS, Wood DE, Aisner DL, Akerley
W, Bauman J, Chirieac LR, D'Amico TA, DeCamp MM, Dilling TJ,
Dobelbower M, et al: Non-small cell lung cancer, version 5.2017,
NCCN clinical practice guidelines in oncology. J Natl Compr Canc
Netw. 15:504–535. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Clark GM: Prognostic factors versus
predictive factors: Examples from a clinical trial of erlotinib.
Mol Oncol. 1:406–412. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Brown T, Pilkington G, Bagust A, Boland A,
Oyee J, Tudur-Smith C, Blundell M, Lai M, Saborido Martin C,
Greenhalgh J, et al: Clinical effectiveness and cost-effectiveness
of first-line chemotherapy for adult patients with locally advanced
or metastatic non-small cell lung cancer: A systematic review and
economic evaluation. Health Technol Assess. 17:1–278. 2013.
View Article : Google Scholar
|
|
6
|
Greenhalgh J, Dwan K, Boland A, Bates V,
Vecchio F, Dundar Y, Jain P and Green JA: First-line treatment of
advanced epidermal growth factor receptor (EGFR) mutation positive
non-squamous non-small cell lung cancer. Cochrane Database Syst
Rev: CD010383. 2016. View Article : Google Scholar
|
|
7
|
Palma JF, Das P and Liesenfeld O: Lung
cancer screening: Utility of molecular applications in conjunction
with low-dose computed tomography guidelines. Expert Rev Mol Diagn.
16:435–447. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Tomita M, Shimizu T, Hara M, Ayabe T and
Onitsuka T: Impact of preoperative hemoglobin level on survival of
non-small cell lung cancer patients. Anticancer Res. 28:1947–1950.
2008.PubMed/NCBI
|
|
9
|
Gauthier I, Ding K, Winton T, Shepherd FA,
Livingston R, Johnson DH, Rigas JR, Whitehead M, Graham B and
Seymour L: Impact of hemoglobin levels on outcomes of adjuvant
chemotherapy in resected non-small cell lung cancer: The JBR.10
trial experience. Lung Cancer. 55:357–363. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Aoe K, Hiraki A, Maeda T, Katayama H,
Fujiwara K, Tabata M, Kiura K, Ueoka H and Tanimoto M: Serum
hemoglobin level determined at the first presentation is a poor
prognostic indicator in patients with lung cancer. Intern Med.
44:800–804. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Trufelli DC, Moraes TV, Lima AA and Giglio
AD: Epidemiological profile and prognostic factors in patients with
lung cancer. Rev Assoc Med Bras (1992). 62:428–433. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Watine J: Blood hemoglobin as an
independent prognostic factor in surgically resected stages I and
II non-small cell lung cancer patients. Ann Thorac Surg.
73:2034–2035, Author reply 2035. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Kay FU, Kandathil A, Batra K, Saboo SS,
Abbara S and Rajiah P: Revisions to the tumor, node, metastasis
staging of lung cancer (8th edition): Rationale, radiologic
findings and clinical implications. World J Radiol. 9:269–279.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Schag CC, Heinrich RL and Ganz PA:
Karnofsky performance status revisited: Reliability, validity, and
guidelines. J Clin Oncol. 2:187–193. 1984. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Langendijk H, de Jong J, Wanders R, Lambin
P and Slotman B: The importance of pre-treatment haemoglobin level
in inoperable non-small cell lung carcinoma treated with radical
radiotherapy. Radiother Oncol. 67:321–325. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Holgersson G, Sandelin M, Hoye E,
Bergström S, Henriksson R, Ekman S, Nyman J, Helsing M, Friesland
S, Holgersson M, et al: Swedish lung cancer radiation study group:
The prognostic value of anaemia, thrombocytosis and leukocytosis at
time of diagnosis in patients with non-small cell lung cancer. Med
Oncol. 29:3176–3182. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Ray-Coquard I, Morère JF, Scotté F, Cals L
and Antoine EC: Management of anemia in advanced breast and lung
cancer patients in daily practice: Results of a French survey. Adv
Ther. 29:124–133. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Vannella L, Lahner E, Osborn J and
Annibale B: Systematic review: Gastric cancer incidence in
pernicious anaemia. Aliment Pharmacol Ther. 37:375–382. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Altman AD, Liu XQ, Nelson G, Chu P, Nation
J and Ghatage P: The effects of anemia and blood transfusion on
patients with stage III–IV ovarian cancer. Int J Gynecol Cancer.
23:1569–1576. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Banzet S, Sanchez H, Chapot R, Bigard X,
Vaulont S and Koulmann N: Interleukin-6 contributes to hepcidin
mRNA increase in response to exercise. Cytokine. 58:158–161. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Sun CC, Vaja V, Babitt JL and Lin HY:
Targeting the hepcidin-ferroportin axis to develop new treatment
strategies for anemia of chronic disease and anemia of
inflammation. Am J Hematol. 87:392–400. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Wu Y, Antony S, Meitzler JL and Doroshow
JH: Molecular mechanisms underlying chronic inflammation-associated
cancers. Cancer Lett. 345:164–173. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Aapro M, Jelkmann W, Constantinescu SN and
Leyland-Jones B: Effects of erythropoietin receptors and
erythropoiesis-stimulating agents on disease progression in cancer.
Br J Cancer. 106:1249–1258. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Tefferi A, Hudgens S, Mesa R, Gale RP,
Verstovsek S, Passamonti F, Cervantes F, Rivera C, Tencer T and
Khan ZM: Use of the functional assessment of cancer therapy-anemia
in persons with myeloproliferative neoplasm-associated
myelofibrosis and anemia. Clin Ther. 36:560–566. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Schwab LP, Peacock DL, Majumdar D, Ingels
JF, Jensen LC, Smith KD, Cushing RC and Seagroves TN:
Hypoxia-inducible factor 1α promotes primary tumor growth and
tumor-initiating cell activity in breast cancer. Breast Cancer Res.
14:R62012. View
Article : Google Scholar : PubMed/NCBI
|
|
26
|
Li S, Meng W, Guan Z, Guo Y and Han X: The
hypoxia-related signaling pathways of vasculogenic mimicry in tumor
treatment. Biomed Pharmacother. 80:127–135. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Milane L, Duan Z and Amiji M: Role of
hypoxia and glycolysis in the development of multi-drug resistance
in human tumor cells and the establishment of an orthotopic
multi-drug resistant tumor model in nude mice using hypoxic
pre-conditioning. Cancer Cell Int. 11:32011. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Ost D, Goldberg J, Rolnitzky L and Rom WN:
Survival after surgery in stage IA and IB non-small cell lung
cancer. Am J Respir Crit Care Med. 177:516–523. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Tanaka F, Yanagihara K, Otake Y, Miyahara
R, Kawano Y, Nakagawa T, Shoji T and Wada H: Surgery for non-small
cell lung cancer: Postoperative survival based on the revised
tumor-node-metastasis classification and its time trend. Eur J
Cardiothorac Surg. 18:147–155. 2000. View Article : Google Scholar : PubMed/NCBI
|