Introduction
Endometrial carcinoma is the most common of all the
gynecologic malignancies and its incidence is rising (1,2).
Surgical staging is an important determinant of adjuvant therapy.
Whilst most patients with early stage disease have a good
prognosis, the prognosis for patients with recurrent or advanced
disease at presentation, i.e., advanced stage, incurable by surgery
or radiotherapy, is poor. The most important parameter to elaborate
the surgical stage is the depth of myometrial invasion (3,4). The
percentage of myometrial invasion (%MI) is routinely determined in
hysterectomy specimens and categorized as greater than or less than
50% and it is one of the parameters used in the determination of
the need for adjuvant therapy (5).
However, more recently, two other alternative methods of measuring
myometrial invasion have been proposed: Absolute depth of invasion
(DMI, the distance in millimeters between the endomyometrial
junction and the deepest point of myometrial invasion) and
tumour-free distance from serosa (TFD, the distance in millimeters
between the deepest point of invasion and the serosal surface)
(6). Previous studies comparing the
predictive value of TFD and DMI were underpowered (7).
Our objectives were: i) to compare the predictive
prognostic value of TFD, DMI and 50% MI; and ii) to evaluate the
relationship of these parameters with clinicopathologic factors and
progression free survival.
Patients and methods
The present study is retrospective carried out over
a 4 and half-year period (January 2003 and June 2007) and conducted
at the Pathology Department of Salah Azaeiz Institute, Tunisia.
Patients treated surgically by hysterectomy and bilateral
salpingo-oophorectomy with or without pelvic lymphadenectomy for
primary endometrial carcinoma were recorded. A total of 62 cases
were identified. Clinical information including: Age at diagnosis,
follow-up and survival data (date of recurrence, date of death and
the cause of death) were collected from medical records. Surgical
data were abstracted from operatory reports. Stage of disease was
defined according to the 2009 International Federation of
Gynecology and Obstetrics (FIGO) staging system (8). Adjuvant curietherapy was indicated for
all stages. External radiotherapy was indicated for stage IA type 2
or with vascular invasion, stage IB grade 3 and stages II, III and
IV.
The slides of the primary endometrial carcinoma of
all patients were reviewed by a specialist gynaecological
pathologist to determine histological type, tumor grade, myometrial
thickness, DMI, TFD, %MI categorized as more or less than 50% (50%
MI), lymphovascular space invasion (LVSI), cervical involvement,
serosal and adnexal involvement and lymph node status. Myometrial
thickness was measured from the endomyometrial junction to the
serosal surface. TFD was measured from the deepest point of
invasion to the serosal surface. DMI was defined as the distance
between the endomyometrial junction and the deepest point of
myometrial invasion. If there confounding factors were present such
as the irregularity of the endomyometrial junction, the extension
of the tumor into adenomyotic foci, exophytic tumors and extensive
smooth muscle metaplasia within the stroma of the neoplasm, slides
were reviewed by another specialist gynecological pathologist,
blind to any outcome data. Between 3 and 6 slides of tumor were
reviewed for each case. The %MI was calculated from the equation:
MI=(DMIx100)/(DMI+RFD). Follow up was to the last date seen in the
outpatients' clinics.
Statistical analysis was performed using the
software package SPSS 16.0 for Microsoft Windows. Receiver
operating characteristics (ROC) analyses were established to
evaluate the performance (sensitivity and specificity) of DMI and
TFD to predict recurrence disease. Univariate and multivariate cox
regression analyses were performed to correlate between DMI, TFD,
50% MI and clinicopathologic characteristics (age, histologic type,
tumor grade, FIGO stage, LVSI, cervical involvement, extra-uterine
involvement and lymph node status). Survival analysis was
calculated using the method of Kaplan Meier and was then correlated
with DMI, TFD, 50% MI and other clinicopathologic characteristics
by Log Rank test.
Results
Patient characteristics are summarized in Table I. The mean age of 62 patients
enrolled in the study was 60 years and the majority of patients
(86%) were in the postmenopausal period. According to the last WHO
Classification of endometrial cancer (9), histology revealed endometrioid
carcinoma in 52 cases (84%), serous carcinoma (6 cases) and clear
cell carcinoma (4 cases). Thirty one patients (60%) had grade 1
cancer, 18 patients (34%) had grade 2, 13 patients (20%) had grade
3. Myometrial invasion was found in 87 cases (92%). The deepest
myometrial invasion was less than 50% in 32 patients (52%). Median
DMI was 2,7 mm (range 0–15 mm). Median TFD was 3 mm (range 0–19
mm). There was LVSI in 11 patients (17,5%), cervical involvement in
11 patients (17,5%), extra-uterine extension in 9 cases (14%) and
lymph node metastasis in 12 patients (22%). Patient characteristics
are shown in Table I. The majority
was diagnosed with FIGO stage I (35 patients). Total hysterectomy
with bilateral annexectomy was performed in all patients.
Lymphadenectomy was indicated in 54 cases. Forty four patients
received adjuvant therapy. The median follow-up time was 3, 8 years
(1–9 years), with 10 deaths (16%) and 12 recurrences (19,3%).
 | Table I.Clinical and pathologic
characteristics of all patients (n=62). |
Table I.
Clinical and pathologic
characteristics of all patients (n=62).
| Variable | N/median | Range (%) |
|---|
| Age | 60 | 40–87 |
| Stade (FIGO
2009) |
|
|
| IA | 26 | 42 |
| IB | 9 | 14 |
| II | 10 | 16 |
| III | 11 | 18 |
| IV | 6 | 10 |
| Histologic type
Endometrioid | 52 | 84 |
|
Serous | 6 | 10 |
| Clear
cell | 4 | 6 |
| Histogenetic
type |
|
|
| Type
1 | 52 | 84 |
| Type
2 | 10 | 16 |
| Histologic grade |
|
|
| G1 | 31 | 50 |
| G2 | 18 | 29 |
| G3 | 13 | 21 |
| Tumour size, mm |
|
|
|
<20 | 9 | 14 |
| ≥20 | 34 | 56 |
| Non
precised | 19 | 30 |
| Myometrial invasion
Absent, % | 5 | 8 |
| ≥50 | 25 | 40 |
|
<50 | 32 | 52 |
| DMI, mm |
|
|
| ≥3 | 28 | 47 |
|
<3 | 34 | 53 |
| TFD, mm |
|
|
| ≥3 | 40 | 64,5 |
|
<3 | 22 | 35,5 |
| Cervical
involvement |
|
|
| Yes | 17 | 27 |
| No | 45 | 73 |
| LVSI |
|
|
| Yes | 11 | 17.5 |
| No | 51 | 82.5 |
| Lymph node
involvement |
|
|
| Yes | 12 | 22 |
| No | 47 | 73 |
| Extrauterine
involvement |
|
|
| Yes | 9 | 14 |
| No | 53 | 86 |
| Adjuvant therapy |
|
|
| No | 18 | 29 |
|
Curietherapy | 23 | 37 |
|
Radiotherapy | 8 | 13 |
|
Radio+Curietherapy | 13 | 21 |
| Recurrence |
|
|
| Yes | 4 | 6.5 |
| No | 58 | 93.5 |
| Death |
|
|
| Yes | 12 | 19.5 |
| No | 50 | 80.5 |
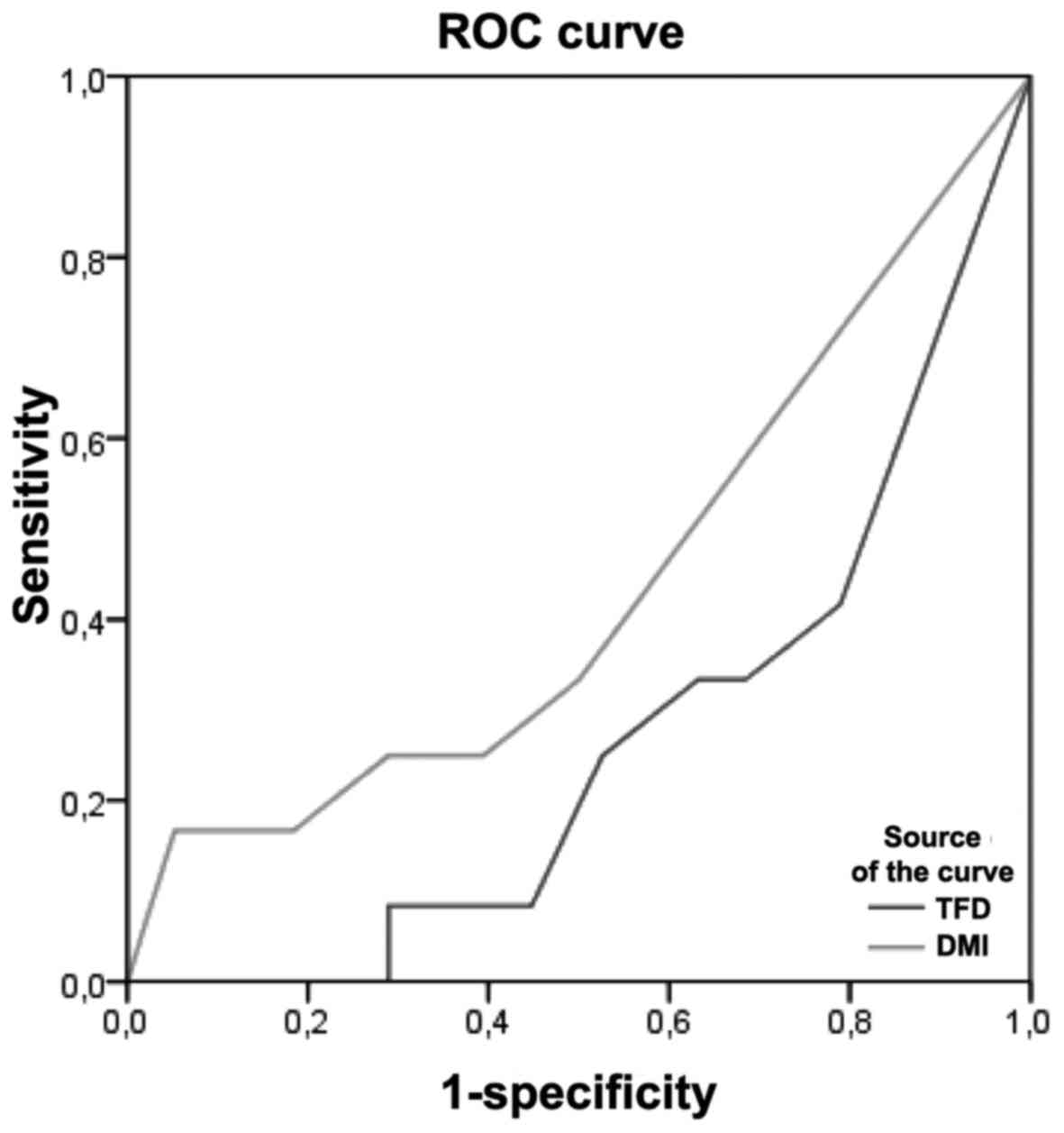
ROC curve
DMI showed a larger area under the ROC curve (AUC)
than TFD for prediction of recurrence of disease: 0, 43 and 0,26
respectively. Thus, The importance of DMI in predicting recurrence
of disease was found to be highest in terms of sensitivity and
specificity (Fig. 1). The cut-off
value with the highest sensitivity and specificity crossing the ROC
curve was calculated to be 3 mm for DMI and 2,5 mm for TFD.
Correlation between 50% MI, DMI and
TFD with clinicopathologic parameters
In univariable analysis, >50% MI was
significantly associated with cervical involvement, type II
carcinomas and LVSI (Table II). No
significant association was found between DMI and TFD with
clinicopathologic parameters.
 | Table II.The association between presence of
≥1/2 myometrial invasion (MI), the depth of myometrial invasion
(DMI), tumor-free distance from Serosa and prognostic. |
Table II.
The association between presence of
≥1/2 myometrial invasion (MI), the depth of myometrial invasion
(DMI), tumor-free distance from Serosa and prognostic.
| Variable | >% MI | DMI |
|---|
| Stage | P=0.000 | P=0.002 |
| Histologic grade | P=0.077 | P=0.56 |
| Histologic type | P=0.032 | P=0.13 |
| Lymphovascular | P=0.003 | P=0.82 |
| space invasion |
| P=0.38 |
| Cervical
involvement | P=0.004 | P=0.29 |
| Lymphadenectomy | P=0.29 | P=0.42 |
| Lymph node
involvement | P=0.19 |
|
| Extrauterine
extension | P=0.24 | P=0.39 |
Survival analyses
In univariate analysis, only percentage of
myometrial invasion (50% MI) was significant predictor of the
5-year overall survival rate and recurrence-free survival (P=0.05,
P=0.01) (Tables III and IV). No significant association was found
between DMI and TFD with survival rates.
 | Table III.Correlation between 5-year overall
survival and clinicopathologic parameters. |
Table III.
Correlation between 5-year overall
survival and clinicopathologic parameters.
| Variable | 5-year overall
survival (%) | P-value |
|---|
| Myometrial
invasion |
|
|
|
≥50% | 87 | 0.05 |
|
<50% | 72 |
|
| DOI, mm |
|
|
| ≥3 | 83 | 0.57 |
|
<3 | 79 |
|
| TFD, mm |
|
|
| ≥3 | 88 | 0.17 |
|
<3 | 74 |
|
 | Table IV.Correlation between 5-year
recurrence-free survival and clinicopathologic parameters. |
Table IV.
Correlation between 5-year
recurrence-free survival and clinicopathologic parameters.
| Variable | 5-year
recurrence-free survival (%) | P-value |
|---|
| Age, years |
|
|
|
≤50 | 72 | 0.81 |
|
>50 | 69 |
|
| Stage |
|
|
| IA | 81 | 0.000 |
| IB | 100 |
|
| II | 80 |
|
|
III | 63 |
|
| IV | 0 |
|
|
Lymphadenectomy |
|
|
|
Yes | 46 | 0.017 |
| No | 77 |
|
| Histologic type
Endometrioid | 74 | 0.66 |
|
Serous | 60 |
|
| Clear
cell | 75 |
|
| Lymphovascular
space invasion |
|
|
|
Yes | 54 | 0.09 |
| No | 77 |
|
| Histologic
grade |
|
|
| G1 | 74 | 0.078 |
| G2 | 84 |
|
| G3 | 53 |
|
| Myometrial
invasion, % |
|
|
|
≥50 | 55 | 0.01 |
|
<50 | 82 |
|
| DOI, mm |
|
|
| ≥3 | 65 | 0.17 |
|
<3 | 85 |
|
| Median TFD, mm |
|
|
| ≥3 | 64 | 0.17 |
|
<3 | 82 |
|
| Cervical
involvement |
|
|
|
Yes | 70 | 0.79 |
| No | 75 |
|
| Lymph node
involvement |
|
|
|
Yes | 56 | 0.002 |
| No | 82 |
|
| Radiotherapy |
|
|
|
Yes | 59 | P=0.14 |
| No | 78 |
|
| Curietherapy |
|
|
|
Yes | 82 | 0.003 |
| No | 51 |
|
Discussion
Our study show different result from earlier studies
in proposing TFD and DMI as a useful measure of myometrial
invasiveness.
The prognostic significance of MI is well documented
(10). It is associated with
recurrence, overall survival, and lymph node involvement (11). In this study, the ability of 50% MI,
DMI and TFD to predict recurrence and death from disease was
investigated. DMI showed a larger area under the ROC curve for
prediction of recurrence than TFD. Our results revealed that a
cut-off value of 3 mm DMI and 2,5 mm TFD led to the most effective
balance between sensitivity and specificity. For DMI, only one
study proposed a cut-off value of 4 mm and showed that DMI was a
better predictor of recurrence than TFD, and was more strongly
correlated with clinicopathologic parameters than TFD and 50% MI
(12). The concept of DMI as a
better prognostic indicator than TFD and 50% MI has been documented
in the study of Kondalsamy-Chennakesavan et al (6). In fact, authors showed that depth of MI
can predict nodal involvement independently when compared with TFD.
On the other hand, several studies have shown that TFD is superior
than DMI and 50% MI in predicting disease extension as well as
outcome (11,13). Chattopadhyay et al (10) demonstrated that TFD is an independent
predictor of disease specific survival, recurrence and pelvic lymph
node involvement. Data regarding the cut-off value of TFD in
patients with endometrial cancer which revealed the best predictive
performance value varied between 1,75 and 10 mm (7,14). In
the current study, only >50% MI was found to have a predictive
value for cervical involvement, type 2 and LVSI. There was no
statistically significant result for the correlation between DOI,
TFD and clinicopathologic parameters. As far as the
reproductibility of measurement of myometrial invasion is
concerned, the best agreement is reached when measuring %MI and TFD
according to van der Putten et al (14). Myometrial invasion is sometimes
affected by confounding factors, including the irregularity of the
endomyometrial junction, the extension of the tumour into
adenomyotic foci, exophytic tumours, leiomyomas and different
patterns of myometrial invasion (15,16). On
the other hand, TFD, defined as the distance from deepest
myometrial invasion to the serosal surface, is a way of assessing
the myometrial invasiveness of the tumour that is not dependent on
myometrial thickness and consequently is associated with low
inter-observer variability (13). In
our study, a discrepancy between %MI and DMI was found in 11 cases
(17,7%). The most common reason for underestimation of myometrial
invasion (7 cases) was intramural leiomyomas, and for
overestimation (4 cases) was an exophytic tumour growth.
To our knowledge, this is the first study
demonstrating the superiority of MI compared to TFD and DMI in
predicting EC outcomes. This can be explained by the reduced number
of patients included in this study compared with other study
(11,14). Besides, the lack of equitable
distribution of histological types with a large predominance of
endometroid carcinoma seems to influence the results of the study.
Another confounding factor that must be mentioned is the short
duration of follow up which influenced survival rates.
The shortcoming of this study is retrospectivity,
while the evaluation requires larger prospective studies to clarify
the impact of DMI and TFD.
In conclusion, our results indicate that DMI is a
superior predictive factor of recurrence of disease compared to
TFD. We need further studies to prove the prognostic usefulness of
these parameters and then to improve management of endometrial
cancer.
Acknowledgements
The authors would like to thank the Ministry of
Health of Tunisia for its support.
Funding
No funding was received.
Availability of data and materials
Not applicable.
Authors' contributions
RD, revision of the manuscript and statistical
analysis; SC, redaction the manuscript; YH, redaction of the
manuscript; LC, pathological diagnosis; NB, revision of the
manuscript; MD, pathological diagnosis; KM, general
supervision.
Ethics approval and consent to
participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Siegel RL, Miller KD and Jemal A: Cancer
statistics, 2016. CA Cancer J Clin. 66:7–30. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Amant F, Mirza MR, Koskas M and Creutzberg
CL: Cancer of the corpus uteri. Int J Gynaecol Obstet. 131 Suppl
2:S96–S104. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Vargas R, Rauh-Hain JA, Clemmer J, Clark
RM, Goodman A, Growdon WB, Schorge JO, Del Carmen MG, Horowitz NS
and Boruta DM II: Tumor size, depth of invasion and histologic
grade as prognostic factors of lymph node involvement in
endometrial cancer: A SEER analysis. Gynecol Oncol. 133:216–220.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Rahatli S, Dizdar O, Kucukoztas N, Oguz A,
Yalcin S, Ozen O, Reyhan NH, Tarhan C, Yildiz F, Dursun P, et al:
Good outcomes of patients with stage IB endometrial cancer with
surgery alone. Asian Pac J Cancer Prev. 15:3891–3893. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
ASTEC/EN.5 Study Group, . Blake P, Swart
AM, Orton J, Kitchener H, Whelan T, Lukka H, Eisenhauer E, Bacon M,
Tu D, et al: Adjuvant external beam radiotherapy in the treatment
of endometrial cancer (MRC ASTEC and NCIC CTG EN.5 randomised
trials): Pooled trial results, systematic review and meta-analysis.
Lancet. 373:137–146. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Kondalsamy-Chennakesavan S, van Vugt S,
Sanday K, Nicklin J, Land R, Perrin L, Crandon A and Obermair A:
Evaluation of tumor-free distance and depth of myometrial invasion
as prognostic factors for lymph node metastases in endometrial
cancer. Int J Gynecol Cancer. 20:1217–1221. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
AJCC: AJCC Cancer Staging Manual. 6th
edition. Springer Verlag; New York: 2002
|
|
8
|
Zaino R, Carinelli SG, Ellenson LH, Eng C,
Katabuchi H, Konishi I, Lax S, et al: Tumours of the uterine
corpus: Epithelial tumours and precursorsWHO classification of
tumours of female reproductive organs. 6th edition. Carcangiu ML,
Herrington CS and Young RH: World Health Organization; Kurman, RJ:
pp. 126–132. 2014
|
|
9
|
Schwab KV, O'Malley DM, Fowler JM,
Copeland LJ and Cohn DE: Prospective evaluation of prognostic
significance of the tumor-free distance from uterine serosa in
surgically staged endometrial adenocarcinoma. Gynecol Oncol.
112:146–149. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Chattopadhyay S, Galaal KA, Patel A,
Fisher A, Nayar A, Cross P and Naik R: Tumour-free distance from
serosa is a better prognostic indicator than depth of invasion and
percentage myometrial invasion in endometrioid endometrial cancer.
BJOG. 119:1162–1170. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Geels YP, Pijnenborg JM, van den Berg-van
Erp SH, Snijders MP, Bulten J and Massuger LF: Absolute depth of
myometrial invasion in endometrial cancer is superior to the
currently used cut-off value of 50%. Gynecol Oncol. 129:285–291.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Lindauer J, Fowler JM, Manolitsas TP,
Copeland LJ, Eaton LA, Ramirez NC and Cohn DE: Is there a
prognostic difference between depth of myometrial invasion and the
tumor-free distance from the uterine serosa in endometrial cancer?
Gynecol Oncol. 91:547–551. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Lee KB, Ki KD, Lee JM, Lee JK, Kim JW, Cho
CH, Kim SM, Park SY, Jeong DH and Kim KT: The risk of lymph node
metastasis based on myometrial invasion and tumor grade in
endometrioid uterine cancers: A multicenter, retrospective Korean
study. Ann Surg Oncol. 16:2882–2887. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
van der Putten LJ, van de Vijver K,
Bartosch C, Davidson B, Gatius S, Matias-Guiu X, McCluggage WG,
Toledo G, van der Wurff AA, Pijnenborg JM, et al: Reproducibility
of measurement of myometrial invasion in endometrial carcinoma.
Virchows Arch. 470:63–68. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Quick CM, May T, Horowitz NS and Nucci MR:
Low-grade, low-stage endometrioid endometrial adenocarcinoma: A
clinicopathologic analysis of 324 cases focusing on frequency and
pattern of myoinvasion. Int J Gynecol Pathol. 31:337–343. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Ali A, Black D and Soslow RA: Difficulties
in assessing the depth of myometrial invasion in endometrial
carcinoma. Int J Gynecol Pathol. 26:115–123. 2007. View Article : Google Scholar : PubMed/NCBI
|