Introduction
The oral cavity is an extremely rare site for
metastases, since metastases account for approximately 1% of all
malignant tumors in the oral cavity. The most common primary tumors
that metastasize to the oral cavity are lung carcinoma in males and
breast carcinoma in females, followed by renal cell carcinoma (RCC)
(1). Men between the ages of 30 and
60 years are the group most commonly affected by RCC (2). Metastases develop in approximately
one-third of RCC cases, and approximately one-half of RCC
metastases are distant metastases that are seen following the
initial diagnosis. Distant metastases from RCC most commonly affect
the lungs, bone, liver, adrenal glands, contralateral kidney, and
brain (3).
Myoepitheliomas are rare tumors of myoepithelial
differentiation, which account for 1.5% of all salivary gland
tumors. Malignant myoepithelioma is much rarer; <2% of all
salivary gland carcinomas are malignant myoepitheliomas (4). Distinguishing RCC from malignancies of
salivary gland origin is very important.
A case of RCC metastasis to the oral cavity that
initially presented with a left buccal submucosal swelling is
presented. In this patient, a malignant myoepithelioma was removed
surgically from buccal submucosa at the same site in another
hospital eleven years earlier.
Case report
A 75-year-old man was referred to our outpatient
clinic for an oral cavity lesion involving the left buccal
submucosa. The lesion had grown substantially over several weeks.
His family history was unremarkable. His past history included left
kidney cancer treated 26 years earlier and a malignant
myoepithelioma that was removed surgically from the buccal region
at the same site at another hospital eleven years earlier. He had
facial asymmetry, with diffuse swelling of the left side of the
cheek. On physical examination, a soft mass with a smooth surface
was seen in the oral cavity involving the left side buccal mucosa,
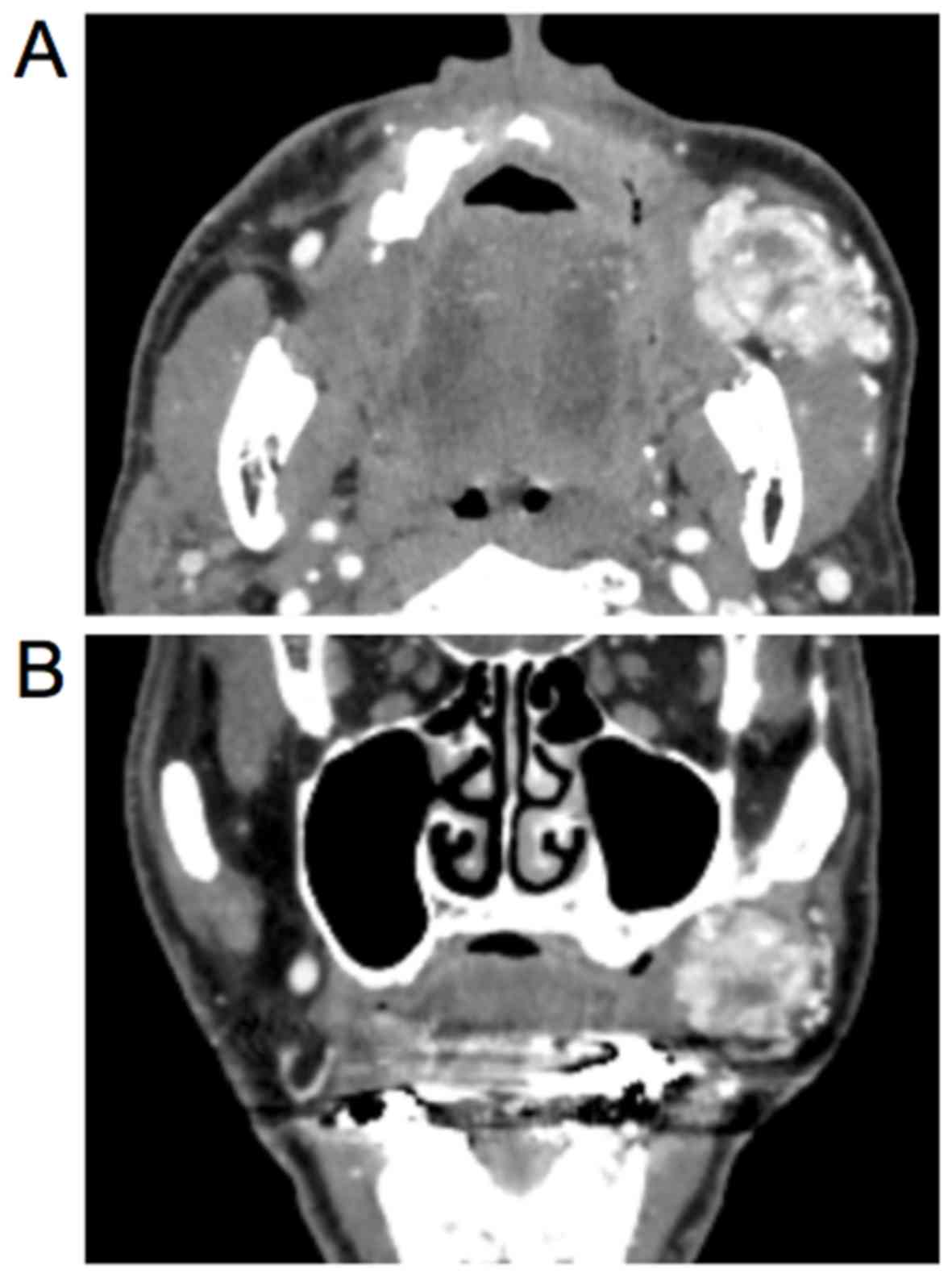
measuring 40×30 mm2. Contrast-enhanced computed
tomography (CECT) of the face showed a 40×35×35 mm3,
ill-defined, soft tissue mass lesion in the left side buccal
submucosa (Fig. 1A and B). On
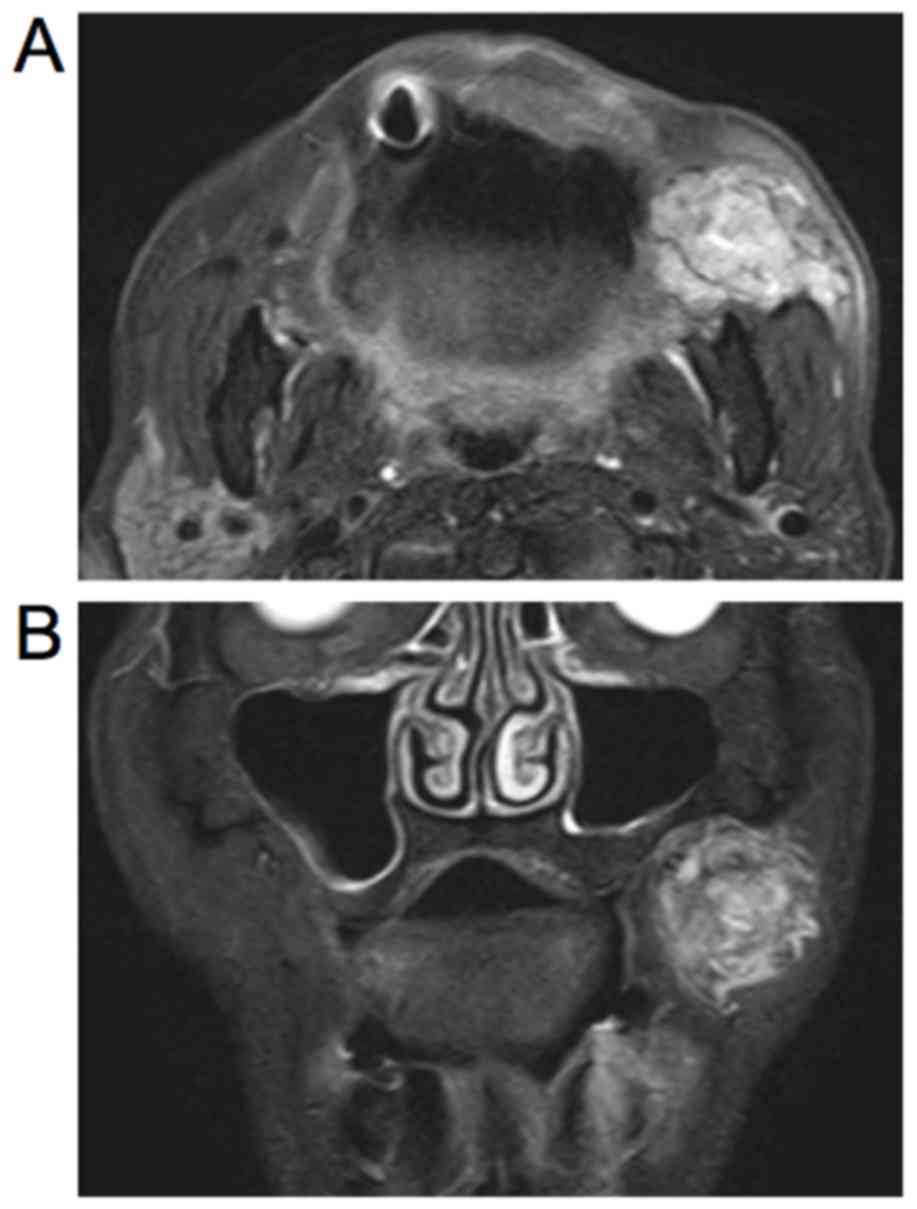
magnetic resonance imaging (MRI), there was a 40×40×35
mm3, well-circumscribed mass that showed high and
nonhomogeneous signal intensity on the left side under the buccal
mucous membrane (Fig. 2A and B).
Blood and serum biochemistry examinations were within normal
limits. Suspecting that this tumor was recurrent malignant
myoepithelioma, surgery was performed.
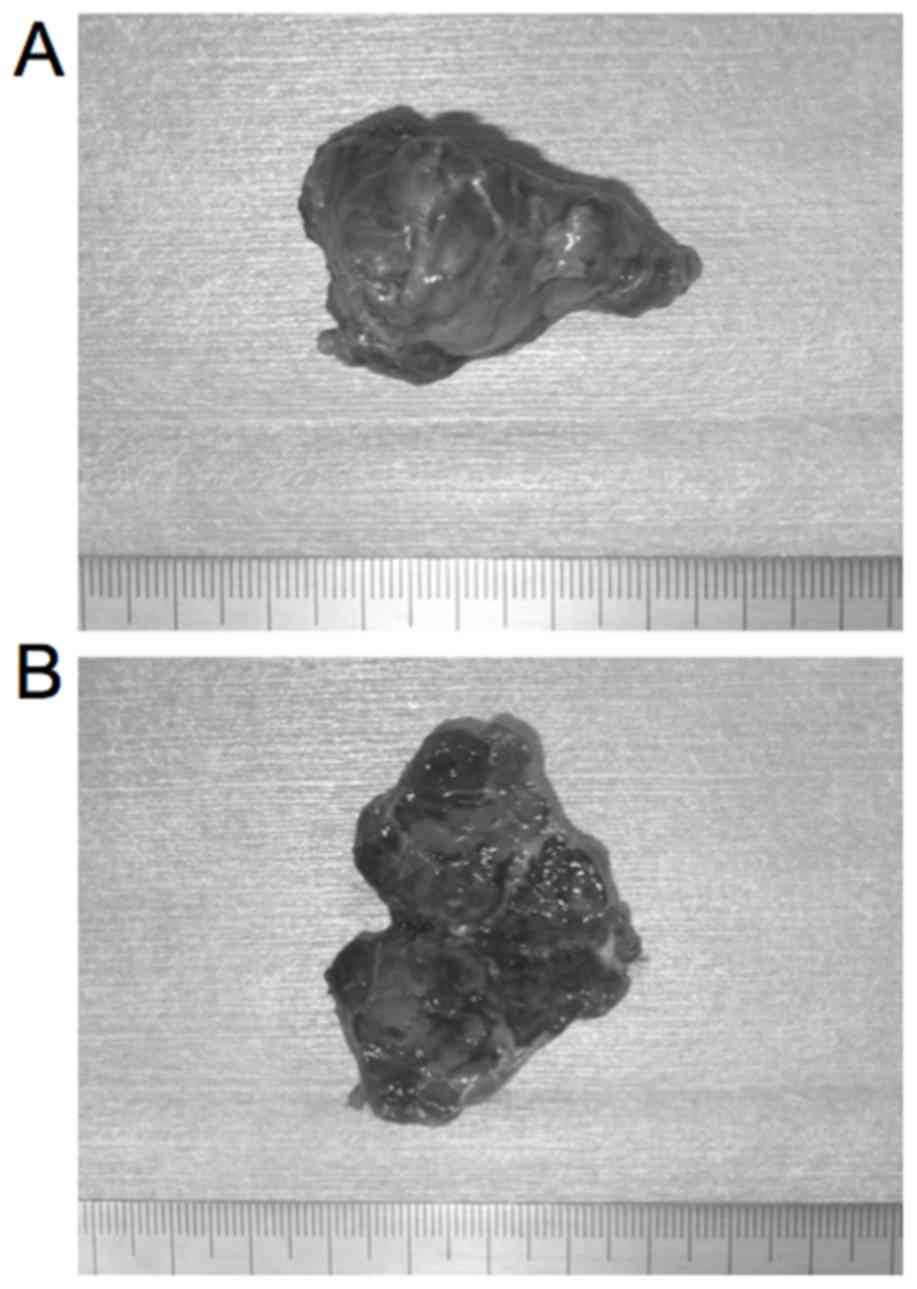
The lesion was removed via an intraoral incision of
the left buccal mucosa under general anesthesia. During surgery,
the mass was approached by a transverse 5-cm linear incision made
in the mucous lining overlying it. The irregular mass was carefully
excised with a 10 to 15-mm safety margin (Fig. 3A and B), and the wound was closed
using sutures.
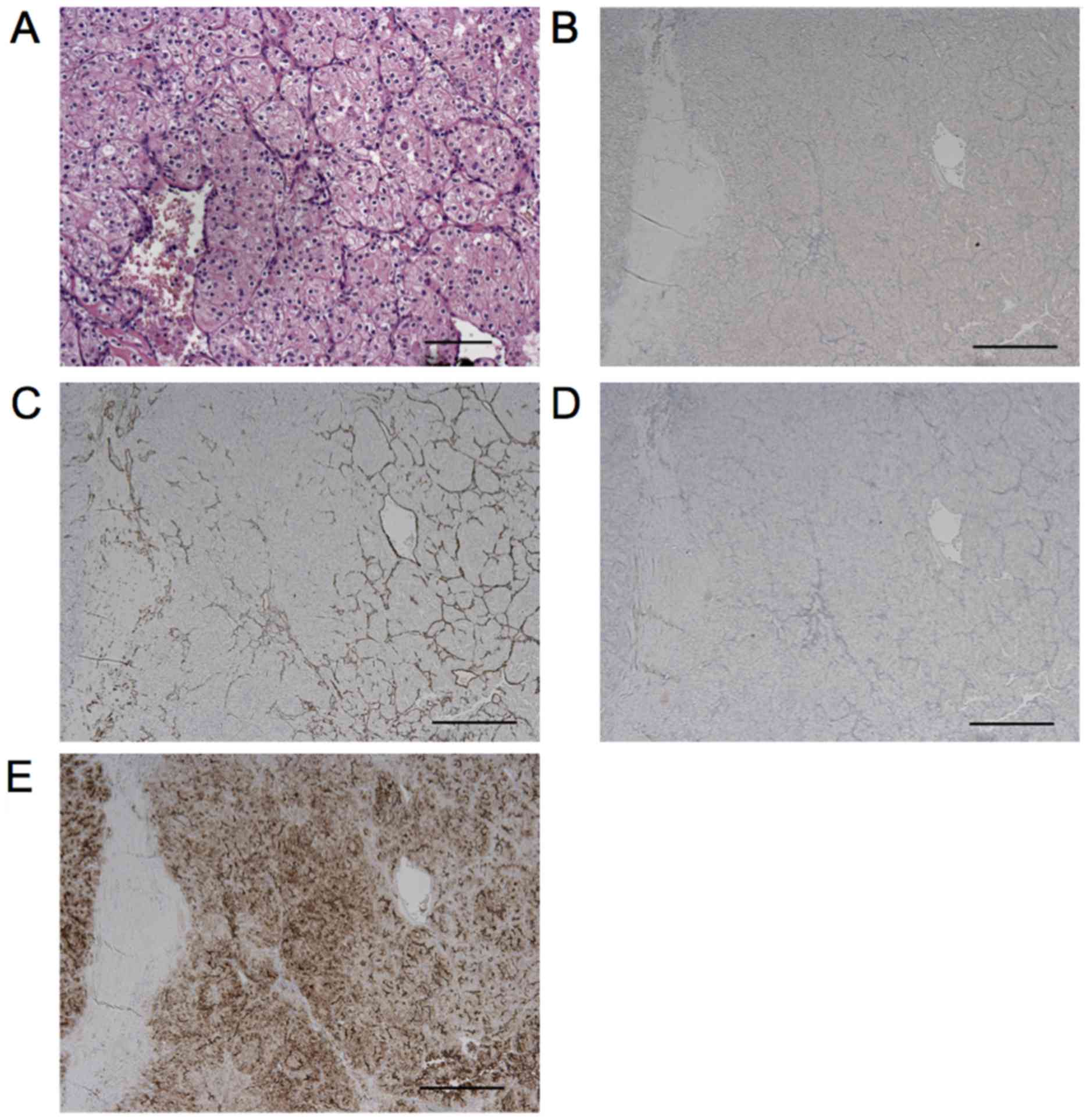
On histopathologic examination, the metastatic
origin of the submucous lesion was confirmed by images that were
compatible with clear-cell carcinoma (Fig. 4A). The use of immunohistochemical
techniques confirmed its renal origin (Fig. 4B-E). Histopathologic analysis was
performed on formalin fixed paraffin embedded sections (4 µm).
Hematoxylin and eosin (H&E) staining was performed at room
temperature (staining in hematoxylin for 3 min and eosin for 2
min). Immunohistochemical staining for S100 protein (cat. no.
N1517; 1:3 dilution; Dako Corporation, Carpinteria, CA, USA), αSMA
(cat. no. 712021; predilution antibody; Nichirei Biosciences Inc.,
Tokyo, Japan), p63 (cat. no. 713751, Nichirei biosciences Inc.,
predilution antibody), and CD10 (cat. no. 713261; predilution
antibody; Nichirei Biosciences Inc.) was performed, and it was
positive only for CD10. Therefore, the lesion was diagnosed as oral
metastasis of RCC. The symptoms resolved after the operation.
Postoperative follow-up at 22 months showed good healing without
evidence of recurrence. The patient has given his consent for this
case report to be published.
Discussion
Metastases to the oral cavity are extremely rare,
and they likely occur through the arterial, venous, and lymphatic
circulations. In the head and neck region, it has been reported
that RCC metastasizes to the nose, tongue, paranasal sinuses,
parotid glands, larynx, mandible, temporal bone, and thyroid gland
(3,5). Meanwhile, malignant myeoepithelioma is
also a much rarer lesion that can occur in all salivary glands. In
1975, Stromeyer described the first case of malignant
myoepithelioma in the parotid (4,6). This
malignant disease was defined by Ellis in 1991 and appeared as a
distinct clinicopathological entity for the first time in the WHO
classification in the same year (4,6). Since
that time, there have been reports of many cases affecting the
parotid gland. Patients' mean age at the time of diagnosis is 55
years (range 14–86 years), with no sex difference, and 75% of all
malignant myeoepitheliomas develop in the parotid, but they are
also found in the submandibular and other minor salivary glands
(4). In a previous study, all cases
of malignant myeoepithelioma were treated by surgical resection
(6).
If we suspect a salivary gland tumor, a fine-needle
aspiration biopsy is usually performed. However, the morphology and
histology of metastatic RCC are often very similar to the primary
renal lesion. There is a high risk of bleeding following
fine-needle aspiration biopsy of RCC involving the kidney; up to
90% of patients show evidence of perinephric bleeding on CT, with
clinically significant hemorrhage seen in 5–7% (3,7).
Therefore, when a biopsy is performed for clinical suspicion of RCC
metastasis, hemorrhage should be expected. In a previous study,
suggested measures to improve the prognosis of patients with RCC
included early diagnosis of metastases, nephrectomy, and
metastasectomy (8). In the present
case, fine-needle aspiration biopsy was not performed for several
reasons. Recurrent malignant myoepithelioma was strongly suspected
because the patient underwent surgery for a malignant
myoepithelioma from the same site in another hospital eleven years
earlier. This patient was an elderly person, 75 years old, and
there was a risk of bleeding with biopsy of a metastatic RCC
lesion. Whether it was a malignant myoepithelioma or metastatic
RCC, tumor removal was needed. Therefore, tumor removal was
performed without biopsy. After surgery, this lesion was
postoperatively diagnosed histologically as metastasis of RCC.
Differentiating among clear cell tumors
histologically is difficult by conventional light microscopy alone.
This is especially true when trying to distinguish RCC metastases
from clear cell malignancies of the salivary glands. Clear cell
carcinomas of the salivary glands are usually seen as nests of
clear cells divided by thin, fibrous connective septa and irregular
vascular tissue. However, immunohistochemical staining can help
make the diagnosis, since RCC metastases show a strong reaction to
vimentin and focal cytokeratin positivity, while minor salivary
gland cancers show diffuse cytokeratin positivity (9). Most malignant myoepitheliomas are
usually less monomorphic than benign myoepitheliomas. They
frequently have high mitotic activity and atypical forms (4). Variable expressions of vimentin,
broad-spectrum cytokeratin, and other myoepithelial markers,
including S100, αSMA, GFAP, CD10, calponin, maspin, and SMMHC
(smooth muscle myosin heavy chain) have been shown in various
immunohistological studies (4,6).
Immunohistochemical staining for p63 may be useful for
distinguishing mucoepidermoid carcinoma from some clear cell tumors
(10). CD10 expression in RCC may be
useful as a marker in the differential diagnosis of several tumors.
A chart of the differential diagnosis of clear cell tumors is shown
in Fig. 5. In the present case,
there was no differentiation to myoepithelial cells on
immunostaining because myoepithelial markers such as S100, αSMA,
and p63 were negative, excluding CD10. Moreover,
immunohistochemically, CD10 was positive, and the patient had a
past history of kidney cancer. Positive and negative controls were
used for immunostaining to evaluate staining status (data not
shown). Therefore, it was possible to diagnose the metastatic RCC.
In addition, although it could not be clearly confirmed, the lesion
that had been removed 11 years earlier might actually have also
been metastasis of RCC.
RCC is known to rarely metastasize to the head and
neck region. Therefore, in a patient with a history of RCC,
metastatic RCC should be considered in the differential diagnosis
of an oral or neck lesion. In patients with a clear cell carcinoma
of the mouth, immunohistochemical staining is important to
differentiate between metastatic RCC and malignant tumors of
salivary gland origin.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
YM and NM conceived and designed the present study.
YM, TI, CK and NM acquired the data. YM, YK and ANY performed data
analysis and interpreted the results. YM and TI drafted the
manuscript, and YM, TI, ANY and NM critically revised the
manuscript for important intellectual content. All authors gave
approval for the version of the manuscript to be published.
Ethics approval and consent to
participate
Not applicable.
Consent for publication
The patient provided written informed consent for
the publication of their data.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Pritchyk KM, Schiff BA, Newkirk KA,
Krowiak E and Deeb ZE: Metastatic renal cell carcinoma to the head
and neck. Laryngoscope. 112:1598–1602. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Cheng ET, Greene D and Koch RJ: Metastatic
renal cell carcinoma to the nose. Otolaryngol Head Neck Surg.
122:4642000. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Will TA, Agarwal N and Petruzzelli GJ:
Oral cavity metastasis of renal cell carcinoma: A case report. J
Med Case Reports. 2:3132008. View Article : Google Scholar
|
|
4
|
Richa, Ray JG, Mohanty SP and Vibha,
Richa, Ray JG, Mohanty SP and Vibha: Malignant myoepithelioma of
palate. Contemp Clin Dent. 3:370–372. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Torres-Carranza E, Garcia-Perla A,
Infante-Cossio P, Belmonte-Caro R, Loizaga-Iriondo J-M and
Gutierrez-Perez J-L: Airway obstruction due to metastatic renal
cell carcinoma to the tongue. Oral Surg Oral Med Oral Pathol Oral
Radiol Endod. 101:e76–e78. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Patrocinio LG, Damasceno PG and Patrocinio
JA: Malignant myoepithelioma of the hard palate: 9-year follow-up.
Rev Bras Otorrinolaringol (Engl Ed). 75:6202009.
|
|
7
|
Vassiliades VG and Bernardino ME:
Percutaneous renal and adrenal biopsies. Cardiovasc Intervent
Radiol. 14:50–54. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Naito S, Yamamoto N, Takayama T, Muramoto
M, Shinohara N, Nishiyama K, Takahashi A, Maruyama R, Saika T,
Hoshi S, et al: Prognosis of Japanese metastatic renal cell
carcinoma patients in the cytokine era: A cooperative group report
of 1463 patients. Eur Urol. 57:317–325. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Marioni G, Gaio E, Poletti A, Derosas F
and Staffieri A: Uncommon metastatic site of renal adenocarcinoma:
The oral tongue. Acta Otolaryngol. 124:197–201. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
O'Sullivan-Mejia ED, Massey HD, Faquin WC
and Powers CN: Hyalinizing clear cell carcinoma: Report of eight
cases and a review of literature. Head Neck Pathol. 3:179–185.
2009. View Article : Google Scholar : PubMed/NCBI
|