Introduction
An intraosseous calcaneal lipoma was first described
in 1976 (1). These are benign bone
tumors that are rather uncommon, despite the abundance of adipose
connective tissue in the bone marrow (1). One known site that intraosseous lipomas
may occur is within the calcaneus bone. Patients with intraosseous
lipomas are often asymptomatic, and several cases are incidentally
discovered (2). We herein report the
very rare case of intraosseous lipoma of thumb with bone
destruction.
Case report
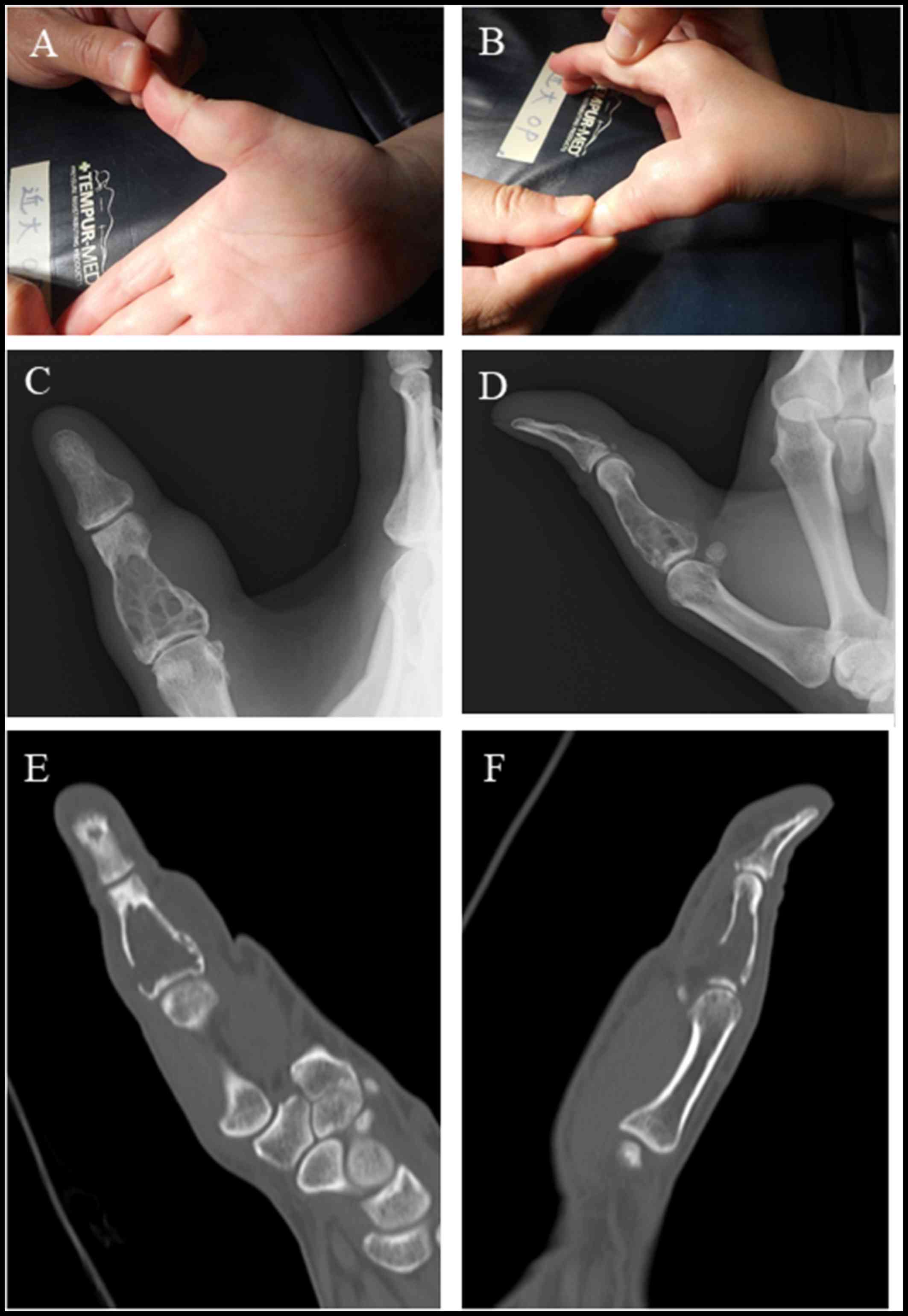
This is a case of a 47-year old woman who presented
with right thumb pain and swelling (Fig.
1A and B). Her past medical history did not include any trauma
or any other causative clinical condition. She also stated that
there was no family history of any type of cancer. There was no
limitation in the range of movement. A radiographic image showed a
soap-bubble appearance, indicating bone destruction and rupture of
the bone cortex of the intermediate phalanges of the thumb
(Fig. 1C and D). A computed
tomography (CT) image confirmed the presence of a tumor with fat
concentration, bone destruction in the intermediate phalanges, and
rupture of the bone cortex (Fig. 1E and
F). Magnetic resonance imaging (MRI) revealed a low-intensity
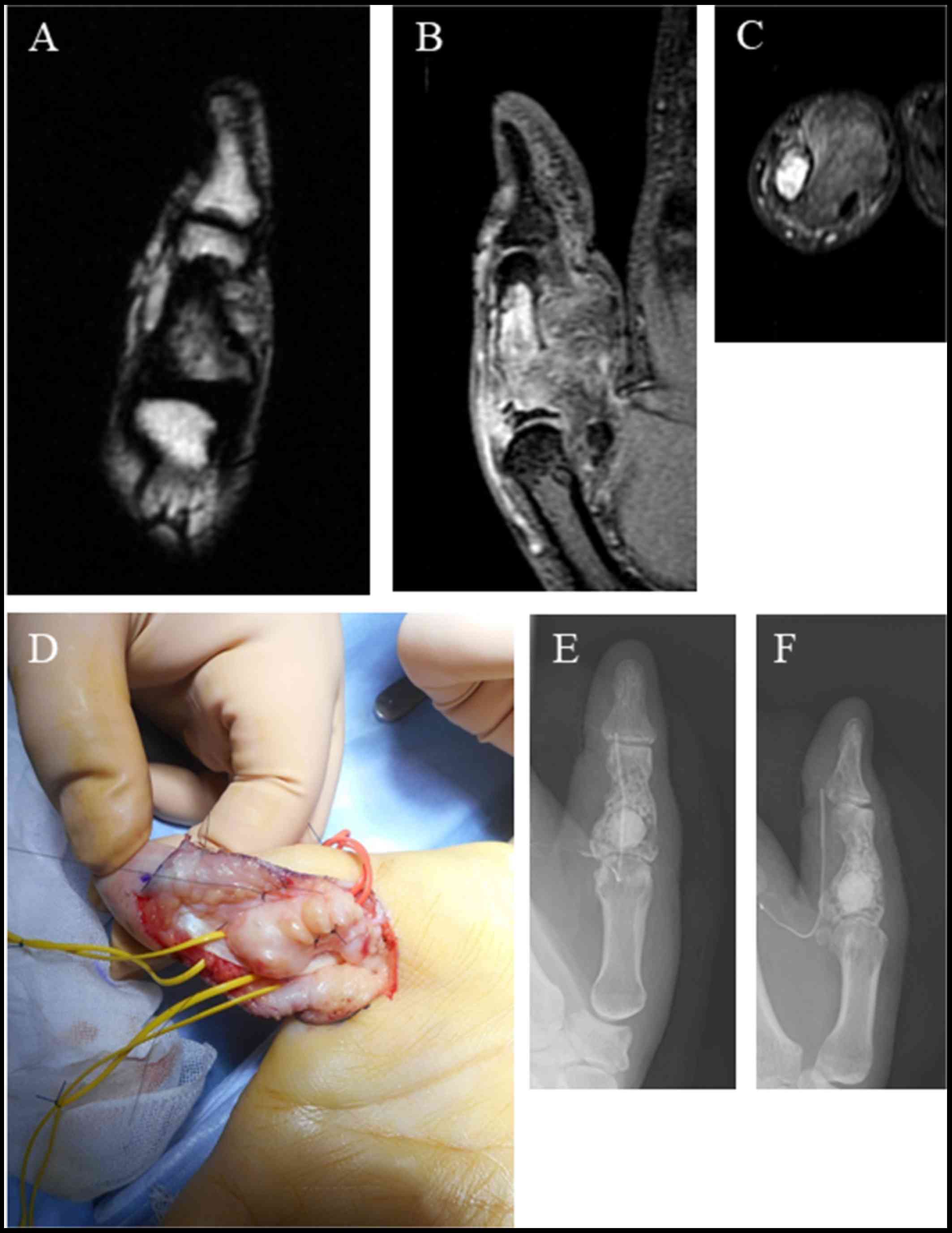
mass extending to the outer area of the phalangeal bone with inner
mosaic intensity in a T1-weighted image (Fig. 2A). A T2-weighted image also showed a
high-intensity mass with inner mosaic intensity (Fig. 2B), while a short TI inversion
recovery (STIR) image showed a low-intensity mass (Fig. 2C). The tumor was resect by making a
zigzag skin incision from the palm side. A further incision was
made from the A 0 pulley to the A 2 pulley, and the tumor was
identified by moving the flexor tendon to the side (Fig. 2D). The tumor was found adhered to the
bone wall, and had expanded by destroying several bone walls. To
remove the tumor, we scraped the bone wall where adhesion of the
tumor was evident. The tumor had also adhered to the surrounding
soft tissue. After washing, beta-tricalcium phosphate was used to
fill in the bone defect (Fig. 2E and
F). Sutures were placed extending from the A0 pulley to the A2
pulley. A Penrose drain was placed subcutaneously, and the
subcutaneous tissue and skin were sutured. The resected tumor
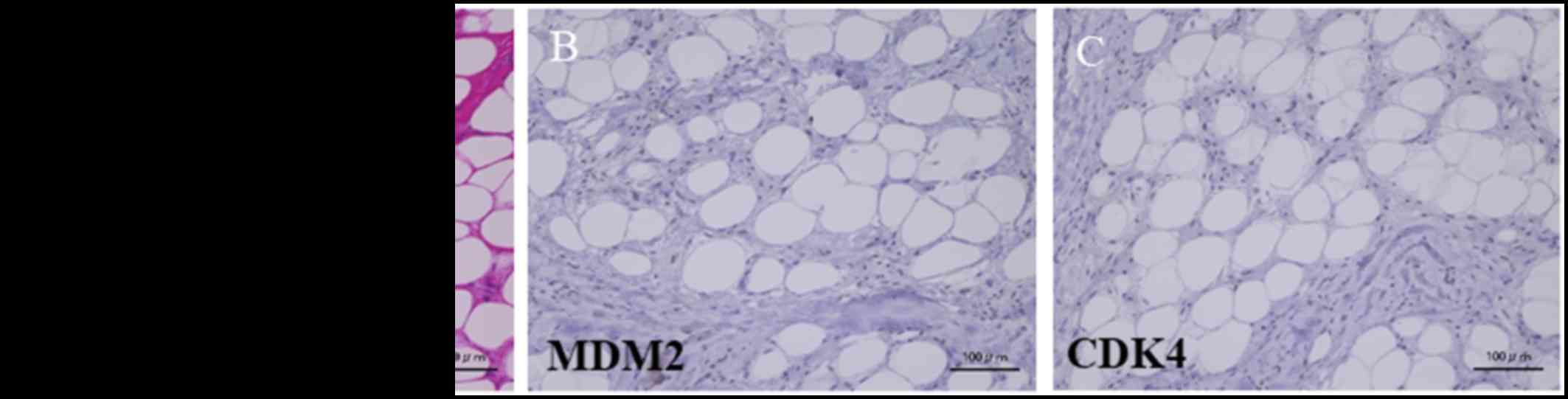
showed a lipomatous image profile and was elastic in texture.
Hematoxylin-eosin staining of the tumor section showed both
necrotic fat tissue and fibrous tissues (Fig. 3A). The immunohistochemical staining
was negative for murine double minute 2 (MDM2) and cyclin-dependent
kinase 4 (CDK4) (Fig. 3B and C). No
obvious abnormal findings were found in postoperative blood test
results (Table I). Currently, 6
months after surgery, tumor recurrence or any thumb malfunction has
not been observed.
 | Table I.Blood test results. |
Table I.
Blood test results.
| Test component | One month before
presentation | Time of
presentation | One day after
surgery |
|---|
| CRP level
(mg/dl) | 0.014 | 0.022 | 0.321 |
| eGFR | 90 | 95 | 93 |
| AST level (U/l) | 17 | 19 | 15 |
| ALT level (U/l) | 17 | 18 | 15 |
| WBC count
(×103 µl) | 6.27 | 5.75 | 7.68 |
| HGB count (g/dl) | 13.7 | 12.1 | 12.4 |
| PLT count
(×104 µl) | 28.5 | 23.5 | 24.5 |
Discussion
Intraosseous lipomas are a rare type of tumor.
Furthermore, tumors developing in the upper limbs are very rare
(7%) (3). Only one case of
intraosseous lipoma in the hand has been reported previously
(4). To the best of our knowledge,
this is the first reported case of an intraosseous lipoma occurring
from the intermediate phalanges of the thumb. Approximately 70% of
patients with intraosseous lipomas present with pain (5). Pathological fractures are a rare cause
of pain in intraooseous lipomas (3).
The cause of pain in this case was thought to be due to bone
destruction. An intraosseous lipoma with multiple region
involvement is also quite rare (6).
As in the present case, cases of single lesions account for the
majority of intraosseous lipomas. Discrete lipomas should be
distinguished from multiple lipomatosis (7), in which the fat deposition may be due
to associated endocrine abnormalities such as type IV
hyperlipidemia (8) or other
conditions such as macrodystrophia lipomatosa (3). The identification of fat density on CT
is usually considered diagnostic of an intraosseous lipoma
(9), although other lesions such as
osteomyelitis may demonstrate low attenuation values due to the
presence of fat-laden histiocytes (10). The fat component of the intraosseous
lipoma is easily recognized on MRI scans by high signal intensity
on both T1-weighted and T2-weighted scans, and fat suppression on
STIR or other fat suppression sequences. Cysts are commonly seen on
MRI scans and have well-demarcated borders (6). Signal intensity is intermediate on
T1-weighted sequences and very high on T2-weighted and
fat-suppressed scans (11). The
appearances of lipomas on radiography, CT, and MRI correspond to
the pathological staging system (2).
Milgram states that stage 1 lesions are purely radiolucent with
resorption of pre-existing bone and expansion or remodeling in 50%
of all cases (2). In stage 2
lesions, localized areas of calcification may be seen and are
typically central, but may also be peripheral. At stage 3, reactive
ossification is prominent around the calcified fat in the outer rim
of the lesion. Peripheral or central calcification fills much of
the lesion, but expansion is present in some cases (2). Clinical imaging indicated stage 2
disease in the current case. Bone expansion has been reported to be
typically minimal or absent, although prominent expansion has been
described in the fibula (12), spine
and sacrum (13), and skull
(14). One previous study reported
that smaller lesions in the long bones may lead to bone expansion
or focal infarction or may destroy the cortical bone (15). Interestingly, in the current case,
destruction of the bone wall was observed in addition to bone
expansion. Milgram also proposed three stages based on the
histological appearances of intraosseous lipomas (2). Stage 1 lesions are defined as
containing viable mature lipocytes interspersed with fine bony
trabeculae. The fat is identical to subcutaneous fat on
chromatography. There is no cellular atypia, mitosis, or capsular
tissue. Stage 2 lesions develop areas of infarction due to
expansion of fat cells within rigid trabeculae (2). The adipose tissue is partly necrotic
with loss of nuclei, and foamy macrophages may be present. Some
portions of necrotic fat become calcified. Reactive ossification
may develop adjacent to areas of necrotic fat, and resembles
primitive woven bone. Extension of infarction to involve the whole
lesion leads to a stage 3 lipoma, with necrotic fat, calcified fat,
cyst formation, and reactive peripheral or central new bone
formation, although these features may be seen to a lesser extent
in the stage 2 lesions. In this respect, lipomas resemble bone
infarcts, although the bony trabeculae are resorbed in stage 3
lipomas, and are considered to be a distinguishing feature
(2). Infarcts also typically have
peripheral calcification. Cysts have also been described in lesions
other than lipomas, such as bone infarcts and fibrous dysplasia. In
this case, histological findings resembled stage 2 of Milgram's
classification.
The interesting feature of the current case, in the
context of stage 2 of Milgram's classification, is that fibrosis
was observed. Most lipomas can be managed conservatively. The
primary indications for surgical intervention are suspicion or
evidence of malignancy (although rare) (16), or the risk of pathological fracture
(although there are no reports of this complication) (17). Other indications include cosmetic
deformity or pain. Surgical treatment usually consists of curettage
and packing with bone chips, similar to the treatment offered in
the current case. The limitation of the current study is that we
were not able to show a correlation between blood test findings and
the disease condition. Further research is needed to help identify
markers that can be used to help recognize the disease in
patients.
In conclusion, we report a case of a rare
intraosseous lipoma occurring from the intermediate phalanges of
the thumb. Surgical resection was successful. Bone tumors that
occur in the intermediate phalanges of the thumb should be screened
for intraosseous lipomas and treatment should be decided
accordingly. It should also be noted that even benign tumors may
cause bone destruction.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and material
The authors declare that data and material can be
made available on request.
Authors' contributions
KH, SN and RK were involved in the acquisition of
data. KH and SN analyzed the data. KH and MA prepared the
manuscript.
Ethics approval and consent to
participate
The Ethics Committee of the Kindai University
Faculty of Medicine approved the present study and patients
provided informed consent to participate.
Consent for publication
The patient provided written informed consent.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
CDK4
|
cyclin-dependent kinase 4
|
|
CT
|
computed tomography
|
|
MDM2
|
murine double minute 2
|
|
MRI
|
magnetic resonance imaging
|
|
STIR
|
short TI inversion recovery
|
References
|
1
|
Poussa M and Holmström T: Intraosseous
lipoma of the calcaneus. Report of a case and a short review of the
literature. Acta Orthop Scand. 47:570–574. 1976. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Milgram JW: Intraosseous lipomas. A
clinicopathologic study of 66 cases. Clin Orthop Relat Res.
277–302. 1988.PubMed/NCBI
|
|
3
|
Campbell RS, Grainger AJ, Mangham DC,
Beggs I, Teh J and Davies AM: Intraosseous lipoma: Report of 35 new
cases and a review of the literature. Skeletal Radiol. 32:209–222.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Bower G, Hosny S and Umarji SI:
Intraosseous lipoma of the scaphoid. J Hand Surg Eur Vol.
37:799–800. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Levin MF, Vellet AD, Munk PL and McLean
CA: Intraosseous lipoma of the distal femur: MRI appearance.
Skeletal Radiol. 25:82–84. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Rosenblatt EM, Mollin J and Abdelwahab IF:
Bilateral calcaneal intraosseous lipomas: A case report. Mt Sinai J
Med. 57:174–176. 1990.PubMed/NCBI
|
|
7
|
Rehani B and Wissman R: Multiple
intraosseous lipomatosis: A case report. Cases J. 2:73992009.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Freiberg RA, Air GW, Glueck CJ, Shikawa T
and Abrams NR: Multiple intraosseous lipomas with type-IV
hyperlipoproteinemia. A case report. J Bone Joint Surg Am.
56:1729–1732. 1974. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Ketyer S, Brownstein S and Cholankeril J:
CT diagnosis of intraosseous lipoma of the calcaneus. J Comput
Assist Tomogr. 7:546–547. 1983. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Ramos A, Castello J, Sartoris DJ, Greenway
GD, Resnick D and Haghighi P: Osseous lipoma: CT appearance.
Radiology. 157:615–619. 1985. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Propeck T, Bullard MA, Lin J, Doi K and
Marte W: Radiologic-pathologic correlation of intraosseous lipomas.
AJR Am J Roentgenol. 175:673–678. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Gero MJ and Kahn LB: Case report 498:
Intraosseous lipoma of the distal end of the fibula with focal
infarction. Skeletal Radiol. 17:443–446. 1988. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Hanelin LG, Sclamberg EL and Bardsley JL:
Intraosseous lipoma of the coccyx. Report of a case. Radiology.
114:343–344. 1975. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Yasuda Y, Tsukada S, Okada T and Haseda Y:
Intraosseous lipoma of the skull: A report of two cases. Ann Plast
Surg. 18:74–80. 1987. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Leeson MC, Kay D and Smith BS:
Intraosseous lipoma. Clin Orthop Relat Res. 186–190.
1983.PubMed/NCBI
|
|
16
|
Milgram JW: Malignant transformation in
bone lipomas. Skeletal Radiol. 19:347–352. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Richardson AA, Erdmann BB, Beier-Hanratty
S, Lautz D, Jacobs PM, Julsrud ME and Ringstrom JB: Magnetic
resonance imagery of a calcaneal lipoma. J Am Podiatr Med Assoc.
85:493–496. 1995. View Article : Google Scholar : PubMed/NCBI
|