Introduction
The incidence of colorectal cancer has increased
4-fold over the last 25 years in Japan. A recent study showed that
25% of patients with colorectal cancer had distant disease at
diagnosis. The most common site of distant metastases was liver.
During the natural course of colorectal carcinoma, liver metastasis
develops in about half of the patients, and metastatic liver tumors
are responsible for death in about two-thirds of those patients
(1). For patients with untreated
liver metastasis, the median survival is reported to be around 8
months (2). Recently, impressive
development in systemic chemotherapy has improved the clinical
response and survival rates of patients with colorectal liver
metastasis. Nevertheless, it remains shorter than survival of
patients who received curative resections. Therefore, when hepatic
metastatic lesions are diagnosed as resectable, hepatectomy is the
first treatment modality (3,4). Although the rate of cure with the
initial hepatic resection is <25% (5), the 5-year survival rate of patients
treated with complete resections of liver metastases was reported
to be 40–50% (6,7).
For patients with colorectal liver metastasis
involving adjacent organs, hepatectomy combined with a resection of
the involved adjacent organ is selected to achieve negative
surgical margins. Adjacent organs involved with metastatic liver
tumors are mainly the diaphragm and inferior vena cava (IVC).
Previously, a liver resection with a simultaneous diaphragm
excision was associated with a greater incidence of perioperative
morbidity and a significantly worse long-term outcome than liver
resection alone (8,9). In addition, the only treatment modality
for curing a primary or metastatic liver tumor that invaded IVC was
an aggressive hepatectomy combined with resection and
reconstruction of IVC, which is a challenging treatment with high
morbidity and mortality rates (10,11).
Recently, surgical techniques have been developed
(12,13), and recent advances in combination
chemotherapy have improved patients' survival. To our knowledge,
few previous reports have studied short and long-term outcomes of
patients who received a hepatectomy combined with resection of an
adjacent organ for treating colorectal liver metastasis. In this
study, we compared patients' characteristics between two groups:
the extended liver resection group, which included patients that
received a hepatectomy combined with resection of adjacent organs,
and the non-extended resection group, which included patients that
received a simple hepatectomy. We also evaluated the surgical
outcomes and prognosis of patients that received extended liver
resections.
Patients and methods
Patients
Between February 2000 and November 2015, 190
patients with a diagnosis of colorectal liver metastasis were
treated with a liver resection in the Department of
Gastroenterological Surgery at Osaka University Hospital. Written
informed consent to receive perioperative management and surgery
was obtained from all patients. The inclusion criteria were
follows; the patients of colorectal liver metastasis who received a
liver resection and elective surgery. The exclusion criteria were
as follows: emergency or urgent surgery; viable metastatic lesion
in the remnant liver after the resection and missing details in the
medical records. Patients who met at least one of these exclusion
criteria were excluded. The patients underwent routine
pre-operative imaging studies, including enhanced chest and abdomen
computed tomography (CT), superparamagnetic iron oxide magnetic
resonance imaging (MRI), and angiography. More recently, patients
have been assessed with multiphasic dynamic CT, with ethoxybenzyl
MRI, and 18F-Fluorodeoxyglucose positron emission tomography
(FDG-PET), without angiography. Out of 190 patients with colorectal
liver metastasis, twelve patients were excluded for further
analysis; of these, seven patients had viable metastatic lesions in
the remnant liver after the liver resection, and five patients had
missing details in the medical records. Consequently, 178 eligible
patients were included in the final analysis.
The 178 patients were divided into two groups: the
extended resection group (n=20) and the non-extended resection
group (n=158). We defined a non-extended resection as a hepatectomy
alone, and an extended resection as a liver resection combined with
a resection of adjacent organs that were directly invaded by the
metastatic lesions of the liver. These two groups were analyzed and
compared for clinical features, including sex, age, body weight,
number of resections, timing of metastasis (synchronous or
metachronous), tumor number, neoadjuvant therapy, distribution of
hepatic lobes, Child-Pugh score, tumor size, stage of primary
colorectal carcinoma (based on the International Union Against
Cancer, 7th edition), adjuvant chemotherapy, usage of molecular
targeted drugs in perioperative chemotherapy and the conversion
cases from the unresectable liver metastasis to the resectable
liver metastasis by intense systemic chemotherapy. The
perioperative chemotherapy was defined as neoadjuvant chemotherapy
and adjuvant chemotherapy in this study. Two groups were also
compared for surgical outcomes, including operation time, blood
loss, blood transfusion volume, resected liver weight,
postoperative complications, histology, and postoperative hospital
stay. The surgical curability of the resection was categorized as
follows: R0 indicated that all gross disease was removed and the
margins were histologically free of disease; R1 indicated that all
gross disease was removed but the margins were histologically
positive for disease; and R2 indicated that some residual gross
disease remained after the resection (14). Complications were graded according to
an extension of the Clavien-Dindo classification of surgical
complications, known as the Japan Clinical Oncology Group
postoperative complications criteria. This system describes
complications more precisely than the original Clavien-Dindo
classification criteria (15,16).
Operative procedure and follow-up
For each patient, the operative procedure was
determined based on an extensive, preoperative evaluation of the
primary tumor location and the extent of invasion into adjacent
organs. When an invasion was detected, resection of the primary
tumor was accompanied by a removal of the involved adjacent organ.
A lymphadenectomy was not performed, unless obvious findings of
lymph node metastasis were observed in the preoperative
radiological examination and confirmed during the operation. For
each organ removed, both cut ends were examined by preparing frozen
sections for histological analysis. Furthermore, microscopic
examinations confirmed the final diagnosis of the surgical margin
and the presence of invasion into extrahepatic adjacent organs.
After discharge from the hospital, patients had been
followed at least for 5 years. Follow-up included a radiological
examination with CT or MRI every 6 months and a check for blood
test every 3 months. After the liver resection, up to 2003,
selected patients received adjuvant treatment with transcatheter
hepatic infusion chemotherapy. After 2003, systemic chemotherapy
was provided in a clinical trial setting.
Statistics
All data are expressed as the mean±standard
deviation. Statistical differences between groups were analyzed
with the Mann-Whitney U test (continuous variables) or the
chi-square test (categorical variables). Disease-free survival and
overall survival curves were estimated with the Kaplan-Meier method
and analyzed with the log rank test. All statistical analyses were
conducted with JMP@11 (SAS Institute Inc., Cary, NC, USA). P-values
<0.05 were considered as statistically significant. This
retrospective study protocol was approved by the institutional
reviewer board of the Osaka University Graduate School of Medicine.
(No. 15145)
Results
One hundred seventy-eight patients received liver
resections. There were 89 synchronous metastases and 89
metachronous metastases. Ninety-four patients had a single
metastasis and 84 patients had multiple metastases. For the
synchronous metastases, 53 patients were treated with resections of
both the primary colorectal cancer and the metastatic liver tumor
in one operation; the other 36 patients were treated with a
resection of the primary lesion in first operation and resection of
the metastatic lesion in a second operation. There are eight
conversion cases from the unresectable liver metastasis to the
resectable cases; two cases in the extended resection group and six
cases in the non-extended resection group. Table I showed the characteristics of all
178 patients in the extended and the non-extended resection groups.
The groups were well-matched in terms of sex, age, body weight,
Child-Pugh score, tumor size, and stage of colorectal carcinoma.
There is a significant difference in the proportion of synchronous
or metachronous metastases (P=0.0306). As to the number of
resections, patients who received repeated liver resections were
more frequently observed in the extended resection group than in
the non-extended resection group (P=0.0002). The two groups showed
no significant differences in the proportions of single or multiple
tumors, unilobar or bilobar distributions in hepatic lobes, the
presence of perioperative chemotherapy, the uase of molecular
targeted drugs (bevacizumab, cetuximab and panitumumab) in
perioperative chemotherapy. There are eight conversion cases from
the unresectable liver metastasis to the resectable cases; two
cases in the extended resection group and six cases in the
non-extended resection group, although there was no significant
difference between the extended group and non-extended group
(P=0.2224).
 | Table I.Patient characteristics in liver
metastasis. |
Table I.
Patient characteristics in liver
metastasis.
| Variables | Types | Non-extended
(n=158) | Extended (n=20) | P-value |
|---|
| Sex | Male | 101 | 12 | 0.8067 |
|
| Female | 57 | 8 |
|
| Age (years) |
| 63.6± 0.8 | 63.5±2.4 | 0.9590 |
| Body weight (kg) |
| 58.5±0.8 | 58.8±2.6 | 0.9076 |
| Number of
resections | First | 156 | 15 | 0.0002 |
|
| Repeated | 2 | 5 |
|
| Timing of
metastasis | Synchronous | 84 | 5 | 0.0306 |
|
| Metachronous | 74 | 15 |
|
| Tumor number | Single | 82 | 12 | 0.6357 |
|
| Multiple | 76 | 8 |
|
| Neoadjuvant
therapy | Yes | 55 | 9 | 0.4591 |
|
| No | 103 | 11 |
|
| Distribution in
hepatic lobes | Unilobar | 109 | 13 | 0.7992 |
|
| Bilobar | 49 | 7 |
|
| Child-Pugh score | A | 156 | 19 | 0.3021 |
|
| B | 2 | 1 |
|
|
| C | 0 | 0 |
|
| Tumor size
(mm) |
| 33.0±1.8 | 40.9±5.2 | 0.1632 |
| Stage (primary
tumor) | I | 5 | 3 | 0.0762 |
|
| II | 30 | 5 |
|
|
| III | 29 | 5 |
|
|
| IV | 88 | 5 |
|
|
| Unknown | 6 | 2 |
|
| Adjuvant
chemotherapy | Yes | 103 | 14 | 0.7463 |
|
| No | 55 | 4 |
|
|
| Unknown | 0 | 2 |
|
| Bevacizumab in | Yes | 16 | 2 | 1.0000 |
| perioperative
therapy | No | 142 | 18 |
|
| Cetuximab in
perioperative therapy | Yes | 3 | 2 | 0.0977 |
|
| No | 155 | 18 |
|
| Panitumumab in
perioperative therapy | Yes | 3 | 2 | 0.0977 |
|
| No | 155 | 18 |
|
| Conversion
case | Yes | 6 | 2 | 0.2224 |
|
| No | 152 | 18 |
|
Table II showed the
surgical outcomes of liver resections in the two groups. The mean
duration of the operation was significantly longer in the extended
resection group (414 min) than in the non-extended resection group
(308 min; P=0.0103). The volume of blood loss in the extended
resection group was significantly greater than that in the
non-extended group (P=0.0272), but the increased blood loss did not
influence the postoperative course or the length of the
postoperative hospital stay, which were similar between the groups.
Moreover, the resected liver weight was not significantly different
between groups. In both groups, most patients received an R0
resection; R0 resection rates were 90% in the extended resection
group and 95% in the non-extended resection group. Among all
patients, there were nine R2 resection cases due to lung
metastasis. Postoperative complications were observed in 26
patients, including 24 patients (15.2%) in the non-extended
resection group and 2 patients (10.0%) in the extended resection
group. No mortality case occurred in either group.
 | Table II.Surgical outcomes of liver
resections. |
Table II.
Surgical outcomes of liver
resections.
| Variables | Types | Non-extended
(n=158) | Extended
(n=20) | P-value |
|---|
| Operation time
(min) |
| 307.8±13.0 | 414.1±36.0 | 0.0103 |
| Blood loss
(ml) |
| 642.2±81.8 | 2025.0±573.8 | 0.0272 |
| Blood infusion
(ml) |
| 127±418 | 826±1528 | 0.0100 |
| Resected liver
weight (g) |
| 159.4±17.1 | 211.2±39.2 | 0.2385 |
| Postoperative
complication | Yes | 24 | 2 | 1.0000 |
|
| No | 134 | 18 |
|
| Histology | tub1/2 | 143 | 19 | 0.6113 |
|
| por | 4 | 0 |
|
|
| others | 7 | 0 |
|
|
| Unknown | 4 | 1 |
|
| Postoperative
hospital stay (days) |
| 24.1±1.4 | 23.8±2.5 | 0.9247 |
Concerning about complications, there were 23
patients with postoperative complications. Of the two patients in
the extended resection group with postoperative complications, one
had wound infection (grade IIIa) and one had bile leakage (grade
IIIa). In the non-extended resection group, half the complications
(n=12) were wound infections (n=7 grade I, n=1 grade II, and n=4
grade IIIa). Three patients had bile leakage (grades I, II, and
IIIa). Four patients had a paralytic ileus after the hepatic
resection (grade I, grade II, and 2 grade IIIa); one patient with a
grade IIIb intestinal obstruction required a re-operation. Two
patients had intra-abdominal abscesses (grades II and IIIa). Two
patients had ascites (grade II). One patient with a grade II
pulmonary embolism required anticoagulant therapy with warfarin.
One with a grade II urinary tract infection and one with
drug-induced erythema multiform. Three patients developed two
complications; thus, these patients were counted twice.
The organs resected in the extended resection group
was shown in Table III, and ten
patients had metastatic lesions that invaded the diaphragm, and
they received the extended resection of the diaphragm and with a
direct closure; no patients required an artificial patch to repair
the diaphragm. Five patients received combined resections of IVC.
Of these, three patients received continuous suture repairs, and
two patients needed reconstructions with ringed
polytetrafluoroethylene (PTFE) tube grafts. Three patients received
combined resections that included the bile duct. One patient
received a direct duct closure, and did not require an anastomosis.
Another patient received a bile duct reconstruction with an end to
end anastomosis of each bile duct. In two patients, the bile duct
resections were combined with a hepaticojejunostomy and a Roux-en-Y
anastomosis. Other organs resected included the right hepatic vein,
adrenal gland, small intestine, pericardium, and abdominal wall.
Three patients received resections of multiple adjacent organs;
thus, these patients were counted multiple times.
 | Table III.Organs of extended resection. |
Table III.
Organs of extended resection.
| Organs | n |
|---|
| Diaphragm | 10 |
| Inferior vena
cava | 5 |
| Bile duct | 3 |
| Hepatic vein | 1 |
| Adrenal gland | 1 |
| Small
intestine | 1 |
| Pericardium | 1 |
| Abdominal wall | 1 |
In the non-extended resection group, 149 patients
received R0 resections, but 86 had tumor recurrences. Tumor
relapses were observed in the remnant liver (n=23), in an
extra-hepatic organ (n=30), or in both (n=30). In the extra-hepatic
organs, most frequent organ was lung (n=33) and secondly lymph
nodes. In the extended resection group, 12 patients had tumor
recurrences after R0 resections. Of these, 7 affected the remnant
liver, 3 affected extra-hepatic organs, and 2 affected both. Lung
metastasis was frequently observed in the extended resection group
(n=3). Recurrences at the surgical margin were found in 3 patients
in the non-extended resection group, and only one patient in
extended resection group. There was no significant difference about
local recurrence between two groups (P=0.2170). About treatments
for the recurrence after hepatectomy, surgical resections were
performed in 5 cases in extended resection group and 33 patients in
non-extended resection group. A total of 4 patients in extended
resection group and 49 patients in non-extended resection group
were treated with systemic chemotherapy. Three patients in extended
resection group and 4 patients in non-resection group were
supported by best supportive care. We could not follow 2 patients
in non-extended resection group about treatment after recurrence.
There was no significant difference in the treatment for the
recurrence after hepatectomy in two groups (P=0.2046).
Fig. 1 showed the
disease-free survival curves and overall survival curves for both
groups. The median disease-free survivals were 1.5 years in the
non-extended resection group and 0.8 years in the extended
resection group. The 1- and 3-year disease-free survival rates were
60.9 and 36.3% in the non-extended resection group, respectively,
and 35.6 and 17.8% in the extended resection group, respectively.
Although the difference of rates was not significant (P=0.1338),
the disease-free survival rates in the extended resection group
tended to be lower than in the non-extended resection group. The 3-
and 5-year overall survival rates were 80.7 and 67.9% in the
non-extended resection group, respectively, and 67.5% and 45.0% in
the extended resection group, respectively. The overall survival
rates in extended resection group were not inferior to those in the
non-extended resection group, and the difference between two groups
was not significant (P=0.4887).
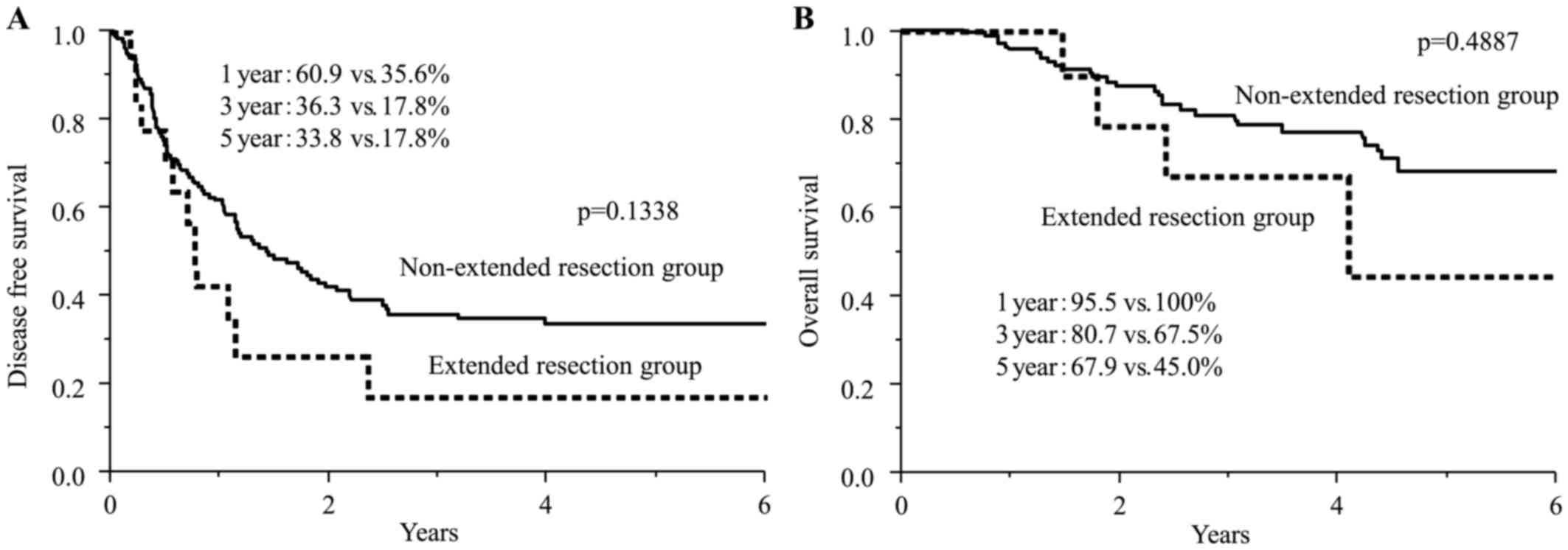 | Figure 1.(A) Disease-free survival curves for
patients that received non-extended resections and extended
resections for treating liver metastases of colorectal cancer. The
1-year, 3-year, and 5-year disease-free survival rates were 35.6,
17.8 and 17.8% in the extended resection group, and 60.9, 36.3 and
33.8% in the non-extended resection group. (B) The overall survival
curves for patients that received non-extended resections and
extended resections. The 1-year, 3-year, and 5-year overall
survival rates were 95.5, 80.7 and 67.9% in the non-extended
resection group, and 100, 67.5 and 45.0% in the extended resection
group. |
In the extended resection group, the patients with
the repeated resection were significantly frequent as shown in
Table I. Fig. 2 showed the disease-free survival
curves and overall survival curves of both groups from the timing
of first hepatectomy. The 1-year, 3-year, and 5-year disease-free
survival rates for the extended resection group were 45.6, 31.3 and
23.5%, and for the non-extended resection group were 61.8, 37.2 and
34.7%, respectively. These disease survival rates were not
significant (P=0.6524). The 1-year, 3-year, and 5-year overall
survival rates were 95.5, 80.8 and 68.0% in the non-extended
resection group, and 100, 74.6 and 63.9% in the extended resection
group. These survival rates were not significant (P=0.9167).
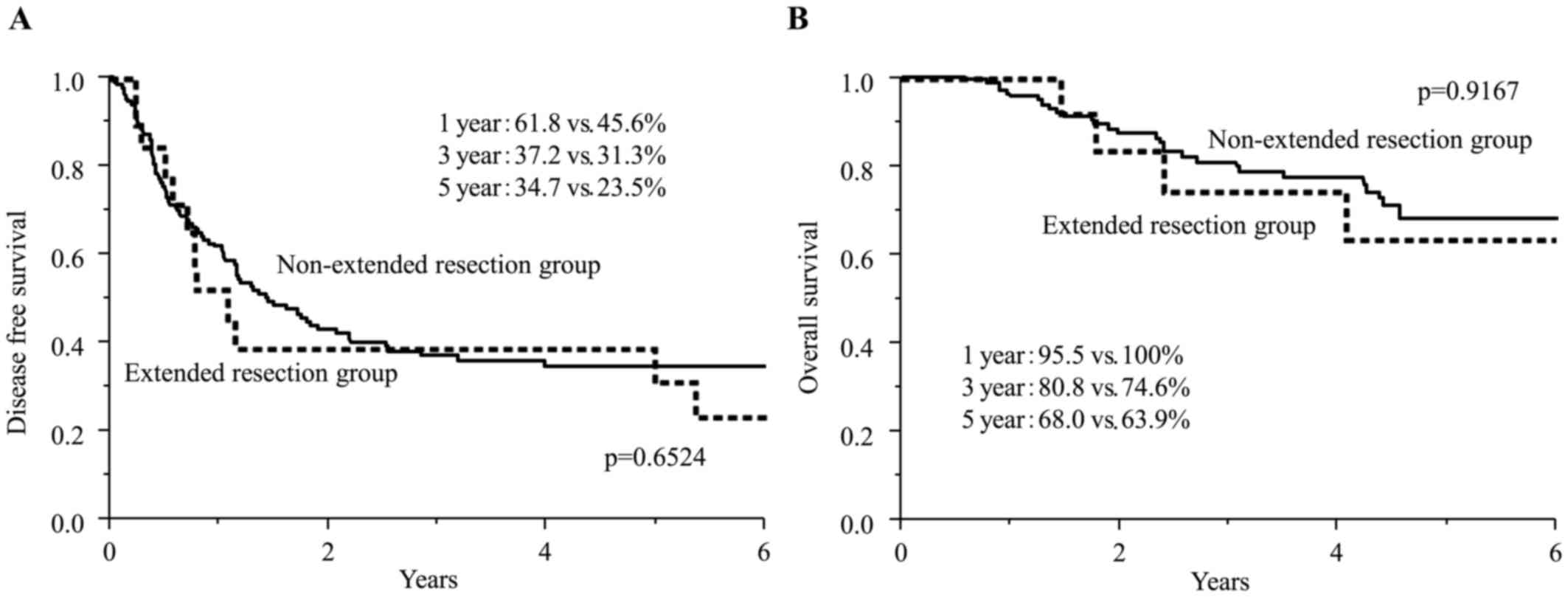 | Figure 2.the survival periods from the first
hepatectomy. (A) Disease-free survival curves from the first
hepatectomy in two groups. The 1-year, 3-year, and 5-year
disease-free survival rates were 45.6, 31.3 and 23.5% in the
extended resection group, and 61.8%, 37.2%, and 34.7% in the
non-extended resection group. (B) The overall survival curves from
the first hepatectomy. The 1-year, 3-year, and 5-year overall
survival rates were 95.5, 80.8 and 68.0% in the non-extended
resection group, and 100, 74.6 and 63.9% in the extended resection
group. |
Discussion
When colorectal liver metastasis involves an
adjacent organ, a hepatectomy combined with resection of the
involved adjacent organ is required for macroscopic curative
resection. It is important to obtain negative surgical margins,
because a positive margin after resection of hepatic colorectal
metastases was reported to be associated with increased risk of
local recurrence (17). In this
study, we analyzed the characteristics and surgical outcomes of
patients with colorectal liver metastasis that received a
hepatectomy combined with the resection of involved adjacent
organs. Among the resected adjacent organs, half involved the
diaphragm and one fourth involved IVC.
Previous studies focused on the diaphragm and IVC
when evaluating perioperative morbidity and mortality associated
with a hepatectomy combined with resection of adjacent organs.
Around 2010, several authors reported that liver resections
combined with a simultaneous diaphragm excision resulted in high
morbidity (44%) but relative low mortality (3–7%) compared to a
liver resection alone for advanced colorectal liver metastases
(8,9). In contrast, conscerning about
hepatectomy combined with a IVC resection, several studies reported
high morbidity and mortality (40–43 and 8–11% respectively)
(11,18,19).
Those findings suggested that a hepatectomy combined with a
diaphragm resection required additional attention to postoperative
complications, but it could be safely conducted currently. In
contrast, a hepatectomy combined with IVC resection remains a
challenging procedure due to the high risk of mortality. In our
study, the perioperative morbidity was low (10%) in the extended
resection group, and no mortality cases were observed. The recent
advances in surgical techniques and perioperative management might
explain the low perioperative morbidity and no mortality case in
the present study.
Previous studies reported that patients with liver
metastases who received a curative resection had a 5-year survival
rate of 35–58% (6,7). On the other hand, for patients with
unresectable liver metastases, the median overall survival was
18–36 months with systemic chemotherapies or hepatic arterial
infusion chemotherapy plus systemic chemotherapy (20,21).
Thus, hepatectomy is the first recommendation for patients with
colorectal liver metastasis lesions. Only a few studies have
reported on long-term outcomes for a hepatectomy combined with
either a diaphragm excision or IVC resection. Lordan et al
reported that a liver resection with a simultaneous diaphragm
resection had a worse long-term outcome than a liver resection
alone (5-year overall survival rate, 19.6 vs. 62%; 3-year
disease-free survival rate, 22.1 vs. 50.7%, respectively (8). However, those authors stated that the
prognosis for an extended resection was superior to chemotherapy
alone. Similarly, in 2012, Li et al reported that patients
that received a hepatectomy combined with diaphragm resections for
treating colorectal liver metastasis had unfavorable survival rates
compared to those with no diaphragm resection (9). About hepatectomy combined with
resection of IVC, Miyazaki et al reported a survival rate of
33% at 3 years after aggressive surgery for colorectal liver
metastasis involving IVC in 1999. They concluded that aggressive
surgical approaches might provide a favorable outcome in selected
patients (22). In 2004, Aoki et
al reported that, among patients with metastatic liver tumors
from colorectal cancer, patients that received IVC resection or a
hepatic venous confluence reconstruction had shorter survival rates
than patients without IVC reconstructions (23). From these reports, patients who
received a hepatectomy combined with a diaphragm excision or IVC
resection had long-term outcomes that were inferior to those of
patients that received a hepatectomy alone. In this study, we
demonstrated that the survival curves in two groups were overlapped
and the differences were not significant. And the 3-year and 5-year
survival rates in the extended resection group in our cohort were
68% and 45%, and the rates were suprerior to the previous
reports.
A total of 8 of 20 patients (40%) in our series
showed pathological invasion of an adjacent organ in this study.
Currently, most institutions conduct a preoperative examination
with CT and MRI, and these imaging modalities have been improved;
nevertheless, it remains difficult to determine whether a hepatic
tumor has invaded an adjacent organ or not. Previously, the rate of
proven pathological invasions into adjacent organs associated with
colorectal liver metastasis has ranged from 15 to 44% (8,24), and
the rate of confirmed pathological invasions in this study was
within that range. Previous studies have identified several
indicators for supporting a preoperative diagnosis of invasion into
an adjacent organs (24–26). Despite recent radiological advances,
it remains difficult to evaluate whether a hepatic tumor has
invaded an adjacent organ. We consider that it is necessary to
perform a hepatectomy combined with resection of the involved
adjacent organ to achieve negative surgical margins for colorectal
liver metstasis when there is a strong suspicion that a hepatic
tumor has invaded an adjacent organ. Additionally, evaluating the
prognosis between the pathologically invasion-positive proup and
the invasion-negative group, there was no significant difference in
overall survival and disease-free survival rates in this study.
When R0 resection was achieved, it was supposed that the presence
of pathological invasion to adjacent organ of colorectal liver
metastasis might not influence to the prognosis after
hepatectomy.
In this study, the disease-free survival rates and
overall survival rates from hepatectomy in extended resection group
was inferior to these in non-extended resection group. However, the
patients in extended resection group received the repeart
hepatectomy more frequently compared with those in non-extended
resection group (25 vs. 1.3%, P=0.0002). This high rate of the
repeated resection might influence the disease-free survival and
overall survival rate in extended resection group. With analysis of
the survival periods from the first hepatectomy in both groups,
there was no difference in both the disease-free survival and the
overall survival rate between two groups as shown in Fig. 2.
There is some limitations in this study. Main
limitation was that it was conducted in only one institution, and
the extended group comprised a small number of patients. A larger
number of patients might be needed to draw firm conclusions about
the long-term outcomes of hepatectomy combined with a resection of
adjacent organs for treating colorectal liver metastasis.
In conclusion, we showed that the surgical outcome
of a hepatectomy combined with adjacent organ resection was
acceptable with low perioperative morbidity although this
aggressive operation increased the operation time and the blood
loss compared to a hepatectomy alone. We found that the overall
survival of the hepatectomy combined with an adjacent organ
resection might not be inferior to that of a hepatectomy alone.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
GS made substantial contributions to acquisition of
data and drafting the manuscript. TN and HE made substantial
contributions to study conception and design, and revising of the
manuscript. HE, YI, DY, TA, KK and KG made contributions to
acquisition of data and revising manuscript. SK, YT and MT made
substantial contributions to the analysis and interpretation of
data. TM, KU, YD and MM revised the paper it critically for
important intellectual content, and approved the final version. All
authors read and approved the final manuscript.
Ethics approval and consent to
participate
Written informed consent was obtained from all
participants. This retrospective study protocol was approved by the
Institutional Review Board of the Osaka University Graduate School
of Medicine (Suita, Japan). (No. 15145)
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Stangl R, Altendorf-Hofmann A, Charnley RM
and Scheele J: Factors influencing the natural history of
colorectal liver metastases. Lancet. 343:1405–1410. 1994.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Petrelli NJ, Abbruzzese J, Mansfield P and
Minsky B: Hepatic resection: The last surgical frontier for
colorectal cancer. J Clin Oncol. 23:4475–4477. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Watanabe T, Itabashi M, Shimada Y, Tanaka
S, Ito Y, Ajioka Y, Hamaguchi T, Hyodo I, Igarashi M, Ishida H, et
al: Japanese Society for Cancer of the Colon and Rectum: Japanese
Society for Cancer of the Colon and Rectum (JSCCR) Guidelines 2014
for treatment of colorectal cancer. Int J Clin Oncol. 20:207–239.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Stättner S, Primavesi F, Yip VS, Jones RP,
Öfner D, Malik HZ, Fenwick SW and Poston GJ: Evolution of surgical
microwave ablation for the treatment of colorectal cancer liver
metastasis: Review of the literature and a single centre
experience. Surg Today. 45:407–415. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Bismuth H, Adam R, Levi F, Farabos C,
Waechter F, Castaing D, Majno P and Engerran L: Resection of
nonresectable liver metastases from colorectal cancer after
neoadjuvant chemotherapy. Ann Surg. 224:509–522. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Weiss L, Grundmann E, Torhorst J, Hartveit
F, Moberg I, Eder M, Fenoglio-Preiser CM, Napier J, Horne CH, Lopez
MJ, et al: Haematogenous metastatic patterns in colonic carcinoma:
An analysis of 1541 necropsies. J Pathol. 150:195–203. 1986.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Kopetz S, Chang GJ, Overman MJ, Eng C,
Sargent DJ, Larson DW, Grothey A, Vauthey JN, Nagorney DM and
McWilliams RR: Improved survival in metastatic colorectal cancer is
associated with adoption of hepatic resection and improved
chemotherapy. J Clin Oncol. 27:3677–3683. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Lordan JT, Riga A, Worthington TR and
Karanjia ND: Early and long-term outcomes of patients undergoing
liver resection and diaphragm excision for advanced colorectal
liver metastases. Ann R Coll Surg Engl. 91:483–488. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Li GZ, Turley RS, Lidsky ME, Barbas AS,
Reddy SK and Clary BM: Impact of simultaneous diaphragm resection
during hepatectomy for treatment of metastatic colorectal cancer. J
Gastrointest Surg. 16:1508–1515. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Delis SG, Madariaga J and Ciancio G:
Combined liver and inferior vena cava resection for hepatic
malignancy. J Surg Oncol. 96:258–264. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Malde DJ, Khan A, Prasad KR, Toogood GJ
and Lodge JP: Inferior vena cava resection with hepatectomy:
Challenging but justified. HPB. 13:802–810. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Colvin H, Mizushima T, Eguchi H, Takiguchi
S, Doki Y and Mori M: Gastroenterological surgery in Japan: The
past, the present and the future. Ann Gastroenterological Surg.
1:5–10. 2017. View Article : Google Scholar
|
|
13
|
Wakayama K, Kamiyama T, Yokoo H, Kakisaka
T, Orimo T, Shimada S, Tsuruga Y, Kamachi H and Taketomi A: Our
technique of preceding diaphragm resection and partial mobilization
of the hepatic right lobe using a vessel sealing device (LigaSure™)
for huge hepatic tumors with diaphragm invasion. Surg Today.
46:1224–1229. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Nelson H, Petrelli N, Carlin A, Couture J,
Fleshman J, Guillem J, Miedema B, Ota D and Sargent D; National
Cancer Institute Expert Panel: Guidelines 2000 for colon and rectal
cancer surgery. J Natl Cancer Inst. 93:583–596. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Dindo D, Demartines N and Clavien P-A:
Classification of surgical complications: A new proposal with
evaluation in a cohort of 6336 patients and results of a survey.
Ann Surg. 240:205–213. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Katayama H, Kurokawa Y, Nakamura K, Ito H,
Kanemitsu Y, Masuda N, Tsubosa Y, Satoh T, Yokomizo A, Fukuda H, et
al: Extended Clavien-Dindo classification of surgical
complications: Japan Clinical Oncology Group postoperative
complications criteria. Surg Today. 46:668–685. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Pawlik TM, Scoggins CR, Zorzi D, Abdalla
EK, Andres A, Eng C, Curley SA, Loyer EM, Muratore A, Mentha G,
Capussotti L and Vauthey JN: Effect of surgical margin status on
survival and site of recurrence after hepatic resection for
colorectal metastases. Ann Surg. 241:715–722. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Arii S, Teramoto K, Kawamura T, Takamatsu
S, Sato E, Nakamura N, Iwai T, Mori A, Tanaka J and Imamura M:
Significance of hepatic resection combined with inferior vena cava
resection and its reconstruction with expanded
polytetrafluoroethylene for treatment of liver tumors. J Am Coll
Surg. 196:243–249. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Hemming AW, Mekeel KL, Zendejas I, Kim RD,
Sicklick JK and Reed AI: Resection of the liver and inferior vena
cava for hepatic malignancy. J Am Coll Surg. 217:115–124;
discussion 124–115. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Kemeny N, Jarnagin W, Paty P, Gönen M,
Schwartz L, Morse M, Leonard G, D'Angelica M, DeMatteo R, Blumgart
L, et al: Phase I trial of systemic oxaliplatin combination
chemotherapy with hepatic arterial infusion in patients with
unresectable liver metastases from colorectal cancer. J Clin Oncol.
23:4888–4896. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Cascinu S, Berardi R, Salvagni S, Beretta
GD, Catalano V, Pucci F, Sobrero A, Tagliaferri P, Labianca R,
Scartozzi M, et al: A combination of gefitinib and FOLFOX-4 as
first-line treatment in advanced colorectal cancer patients. A
GISCAD multicentre phase II study including a biological analysis
of EGFR overexpression, amplification and NF-kB activation. Br J
Cancer. 98:71–76. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Miyazaki M, Ito H, Nakagawa K, Ambiru S,
Shimizu H, Okuno A, Nukui Y, Yoshitomi H, Kusashio K, Furuya S, et
al: Aggressive surgical resection for hepatic metastases involving
the inferior vena cava. Am J Surg. 177:294–298. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Aoki T, Sugawara Y, Imamura H, Seyama Y,
Minagawa M, Hasegawa K, Kokudo N and Makuuchi M: Hepatic resection
with reconstruction of the inferior vena cava or hepatic venous
confluence for metastatic liver tumor from colorectal cancer. J Am
Coll Surg. 198:366–372. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Hashimoto T, Minagawa M, Aoki T, Hasegawa
K, Sano K, Imamura H, Sugawara Y, Makuuchi M and Kokudo N: Caval
invasion by liver tumor is limited. J Am Coll Surg. 207:383–392.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Lau WY, Leung KL, Leung TW, Liew CT, Chan
M and Li AK: Resection of hepatocellular carcinoma with
diaphragmatic invasion. Br J Surg. 82:264–266. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Yamashita Y, Morita K, Iguchi T, Tsujita
E, Soejima Y, Taketomi A and Maehara Y: Surgical impacts of an en
bloc resection of the diaphragm for hepatocellular carcinoma with
gross diaphragmatic involvement. Surg Today. 41:101–106. 2011.
View Article : Google Scholar : PubMed/NCBI
|
















