Introduction
The first case of SCCP was reported in 1977
(1). It has since been reported that
a number of patients have a history of a hormonally treated acinar
adenocarcinoma. SCCP is histologically similar to small-cell
carcinoma of the lung (SCCL) (2). In
terms of distinguishing between SCCP and a metastatic tumor from
the lung, however, the results are conflicting as to whether SCCP
is positive for thyroid transcription factor-1 (TTF-1). As the pure
SCCP component predominates, as reported among different studies,
the serum prostate-specific antigen (PSA) level may be
undetectable. There is no difference in prognosis between patients
with pure and mixed (with an acinar adenocarcinoma component) SCCP
(1). Surgery and clinical staging
are not correlated with prognosis. We herein present a rare case of
a patient who was diagnosed with pure SCCP, with a serum PSA level
of 56.78 ng/ml. To the best of our knowledge, among the 21 cases of
pure SCCP published on PubMed, our patient had the highest serum
PSA level reported to date for this type of tumor (1,3).
Case report
A 66-year-old man was admitted to the Department of
Urology of Peking University Shenzhen Hospital in March 2015 due to
elevated serum PSA level.
The transrectal ultrasound examination revealed an
irregularly enlarged prostate. The findings on chest X-ray and
laboratory biochemical tests, including routine complete blood
count, serum biochemical analysis and urinalysis, were
unremarkable, except for an abnormally elevated PSA value (56.78
ng/ml; normal, <4 ng/ml). The patient subsequently underwent
transrectal ultrasound-guided prostate biopsy, which revealed a
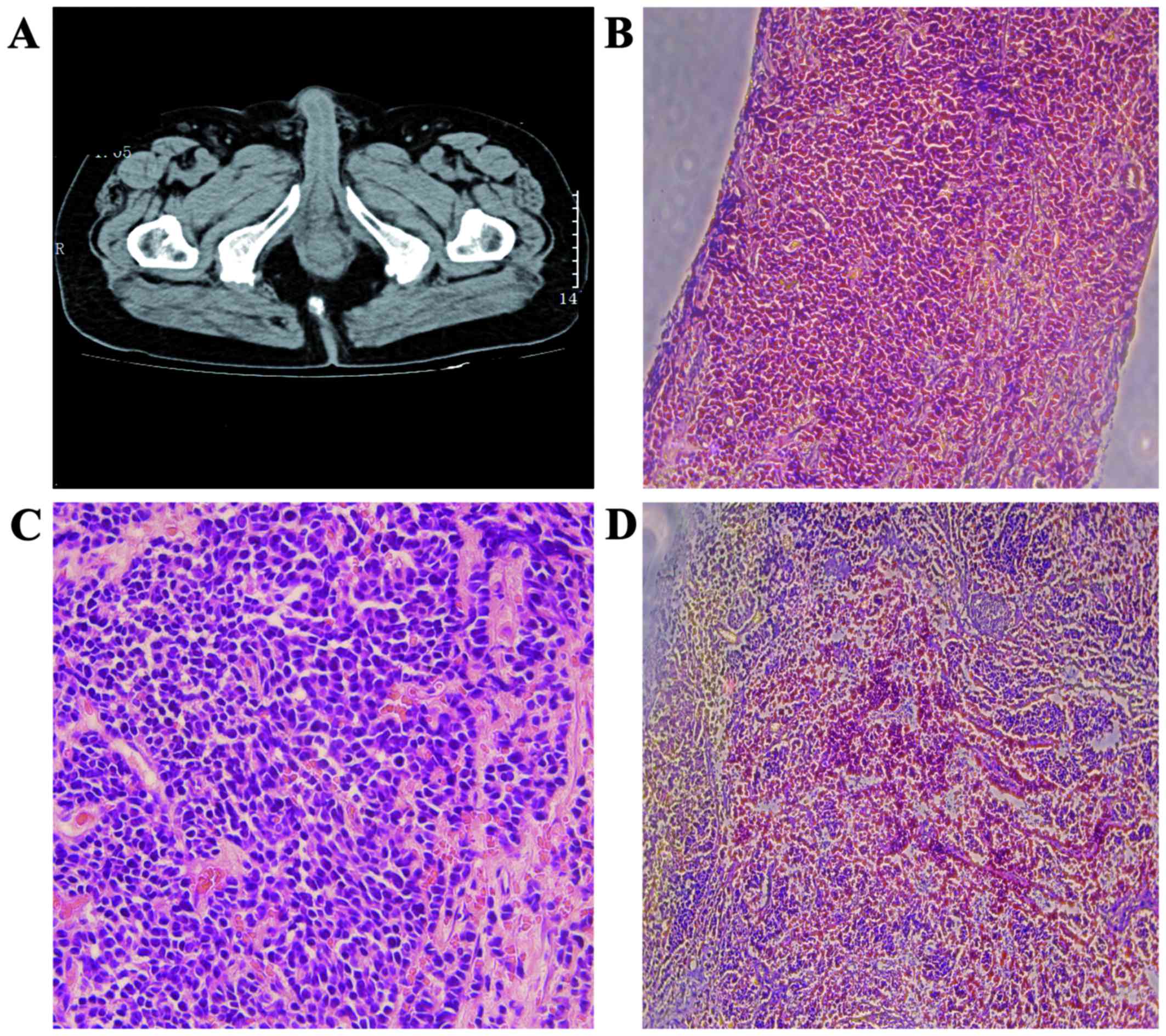
Gleason score of 3+4=7. An abdominal and pelvic computed tomography
examination was performed, which revealed a prostatic mass, without
signs of distant metastasis (Fig.
1A). The preoperative stage in our case was T2N0M0. The patient
was diagnosed with a preinvasive PCa and radical prostatectomy
surgery was immediately initiated. Radical prostatectomy with
regional lymph node dissection was performed straight after
surgery. Macroscopic cancer invasion of periprostatic tissue was
not detected intraoperatively.
The patient was discharged on postoperative day 7
without major complications (although minor complications included
bleeding, pain and sleeplessness). The microscopic examination of
the surgical specimen revealed the presence of pure (without foci
of adenocarcinoma) SCCP (Fig. 1B and
C). The immunohistochemical examination further confirmed the
diagnosis, with a positive expression of TTF-1 (Fig. 1D). After the recommended
postoperative treatment options were explained in detail, the
patient refused further chemotherapy, radiotherapy and endocrine
therapy. The serum PSA level was measured on a 3-month basis, while
abdominal and pelvic contrast-enhanced CT was performed at 6 and 12
months postoperatively. On the multiple follow-up visits (the last
in March 2016), the patient exhibited no signs of disease
recurrence.
The publication of this case was approved by the
Ethics Committee of Peking University Shenzhen Hospital, and
written informed consent was obtained from the patient.
Discussion
SCCP is a rare entity, accounting for <0.5% of
all PCa cases (4). SCCP has an
aggressive behavior and it tends to metastasize early to distant
organs, such as the liver, bones, skin, bladder, rectum, lymph
nodes, and even the lungs (4). SCCP
usually arises from the peripheral zone of the prostate and may
occur without obstructive symptoms of the urinary tract. SCCP may
be divided into two subtypes (pure and mixed SCCP), according to
the presence of an adenocarcinomatous element (1). Pure SCCP is an uncommon pathological
pattern, with only few cases reported in the literature to date.
The diagnosis is easily missed due to the normal PSA level
(5). The main finding in our patient
was the abnormal PSA level, which was inconsistent with previous
studies.
The clinicopathological characteristics of pure SCCP
are similar to those of SCCL (Table
I). As in SCCL, vascular invasion, high-grade malignancy, high
mitotic index and necrosis are common characteristics (6). Two possibilities regarding the
histogenesis of SCCP were recently proposed (7). The most persuasive hypothesis is that
pure SCCP is derived from totipotential stem cells, which may
easily differentiate into neuroendocrine and epithelial types
(7). Another theory is that
small-cell cancer may arise from the amine precursor uptake or
decarboxylation cells of the endoderm. The latter depends on the
hypothesis that SCCP is part of the huge spectrum of the prostatic
adenocarcinomas (7). Primary pure
SCCP and metastatic carcinoma may be distinguished by the
expression of TTF-1 (8). The
confirmation of pure SCCP mainly relies on pathological
examination. Microscopically, SCCP cells are round or short
spindle-shaped, arranged in a flaky and nest-like pattern. The
carcinoma cells contain scat cytoplasm, with nuclei situated far
from mitochondria or endoplasmic reticulum (8). The nucleolus is blurry and mitotic
figures may be observed; necrosis is also a common accompanying
finding. Positive expression of synaptophysin, chromogranin A and
CD56 on immunohistochemical examination is valuable for the
diagnosis (9). The aforementioned
criteria may be useful in differentiating pure SCCP from its mixed
counterpart (9).
 | Table I.Clinicopathological characteristics of
22 cases of pure SCCP. |
Table I.
Clinicopathological characteristics of
22 cases of pure SCCP.
| Case no. | Age, years | PSA | Diagnostic
procedure | Treatment | Follow-up,
months | Survival status |
|---|
| 1 | 76 | 0.81 | TURP | None | 6 | Deceased |
| 2 | 32 | 0.45 | Radical | Chemotherapy | 10 | Deceased |
| 3 | 61 | 2.4 | Biopsy | None | 1 | Lost |
| 4 | 43 | 8.8 | Biopsy | Chemotherapy | 1 | Alive |
| 5 | 56 | <4 | Radical | Endocrine
therapy | 17 | Alive |
| 6 | 32 | <4 | Biopsy | Chemotherapy | 3 | Lost |
| 7 | 65 | <4 | Biopsy |
Chemoradiotherapy | 11 | Deceased |
| 8 | 21 | 0.69 | Biopsy | Endocrine
therapy | 3 | Deceased |
| 9 | 82 | 2.26 | TURP | Endocrine
therapy | 3 | Deceased |
| 10 | 61 | 0.32 | Radical | Chemotherapy | 3 | Alive |
| 11 | 55 | <4 | Radical | Endocrine
therapy | 3 | Deceased |
| 12 | 34 | <4 | Biopsy | Chemotherapy | 13 | Deceased |
| 13 | 62 | <4 | Radical | None | 5 | Alive |
| 14 | 65 | 1.92 | Biopsy | Chemotherapy +
endocrine therapy +radiotherapy | 11 | Deceased |
| 15 | 55 | <4 | Radical | Endocrine
therapy | 3 | Deceased |
| 16 | 34 | <4 | Biopsy | Chemotherapy | 12 | Alive |
| 17 | 50 | 0.31 | Radical | None | 1 | Deceased |
| 18 | 82 | 2.61 | TURP | Chemotherapy +
endocrine therapy +radiotherapy | 17 | Alive |
| 19 | 81 | 39.26 | Biopsy | None | 5 | Deceased |
| 20 | 77 | 25.02 | Biopsy |
Chemoradiotherapy | 2 | Alive |
| 21 | 76 | <4 | Biopsy | None | 8 | Deceased |
| Present | 66 | 56.78 | Biopsy | Surgery | 14 | Alive |
As the endocrine component of pure SCCP is similar
to SCCL, paraneoplastic syndromes may be observed in a proportion
of the patients, such as thyrotoxicosis, syndrome of inappropriate
antidiuretic hormone secretion, hypercalcemia and Cushing's
syndrome. The present case did not exhibit any symptoms or signs of
paraneoplastic syndromes (10). The
results of biochemical examinations were normal, except for the
increased PSA level. A large proportion of cases diagnosed with
pure SCCP usually have a PSA level within the normal range
(11); however, the serum PSA level
in the present case was significantly higher than normal (56.78
ng/ml; normal, <4 ng/ml).
The mean survival of patients with pure SCCP ranges
between 4 and 12 months, with <2% of the patients surviving
beyond 12 months (1). The difficulty
in the treatment of pure SCCP is due to its uncommon aggressive
characteristics, similar to patients with SCCL. Pure SCCP is more
common among older adults, with a mean age of 70 years at the time
of diagnosis (7). However, the
patient in this case was aged 66 years, which was younger compared
with the mean reported age, and he survived beyond 12 months. The
general rationale of SCCP treatment mainly includes radical
surgery, chemotherapy, radiotherapy and endocrine therapy. Among
all published cases, there is only one disease-free survival case
that did not undergo radical surgery and was only treated with
chemotherapy, without receiving other treatments, such as
radiochemotherapy or endocrine therapy (2). Other researchers have suggested that
surgery may not be the optimal choice, as patients with pure SCCP
usually have distant metastasis at the time of the initial
diagnosis (12). Furthermore, there
remains the question of whether pure SCCP should be treated with
only a combination of the chemotherapeutic agents that are applied
in other cases of small-cell cancers. It has been reported that
endocrine therapy or systemic chemotherapy may have some effect on
the natural history of the disease (13).
To conclude, pure SCCP is rare, and the patient in
the present case had a serum PSA level that was the highest
reported to date. The patient has remained disease-free 14 months
postoperatively, as determined at the last follow-up appointment
during March, 2016. The findings of the present case raise the
question of whether the PSA level is a trustworthy marker for the
screening of the SCCP. Therefore, this case may be valuable for
future studies.
Acknowledgements
Not applicable.
Funding
The present study was supported by the Science and
Technology Development Fund Project of Shenzhen (grant nos.
JCYJ20150403091443329 and JCYJ20170307111334308), the fund of the
‘San-ming’ Project of Medicine in Shenzhen (no.SZSM201612066), and
the fund of Guangdong Key Medical Subject.
Availability of data and materials
The datasets used or analysed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
XMM approved the use of the patient for the case
report. JH analysed patient data and was involved in drafting the
manuscript as well as its critical revision for important
intellectual content. TH performed follow-up appointments and
collected patient data. LJ, YZ and WL performed data analysis. BW
performed post-operative clinical examination of the patient. YL
performed literature searches and collected patient data. YQL
revised the manuscript critically for important intellectual
content. LCN provided final approval of the manuscript version to
be published. All authors read and approved the final
manuscript.
Ethics approval and consent to
participate
The present study was approved by the Ethics
Committee of Peking University Shenzhen Hospital, and written
informed consent was obtained from the patient.
Consent for publication
The patient provided informed consent for the use of
the data in this study.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Guo A, Wen S, Ma Y, Wei L and Liu A:
Clinicopathological analysis on small cell carcinoma of the
prostate in chinese patients. J Cancer. 5:797–803. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Horie K, Kameyama K, Mizutani K, Sugawara
T, Seike K, Tsuchiya T, Yasuda M, Yokoi S, Nakano M, Deguchi T, et
al: Small cell carcinoma of the prostate effectively treated for
relatively long term: A case report. Hinyokika Kiyo. 60:517–521.
2014.(In Japanese). PubMed/NCBI
|
|
3
|
Addeo A, Rinaldi C and Panades M: A case
of small cell carcinoma of the prostate and review of the
literature. Tumori. 98:76e–78e. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Hirai M, Konishi T, Saito K, Washino S,
Kobayashi Y, Nokubi M and Miyagawa T: Small cell carcinoma of the
prostate: A case report of relative long-term survival. Nippon
Hinyokika Gakkai Zasshi. 106:280–284. 2015.(In Japanese).
PubMed/NCBI
|
|
5
|
Kimura H, Uegaki M, Aoyama T, Kawai J,
Hamano T and Hashimura T: Carboplatin plus irinotecan induced
partial response in a patient with small cell carcinoma of the
prostate; a case report. Hinyokika Kiyo. 60:39–43. 2014.(In
Japanese). PubMed/NCBI
|
|
6
|
Lee WY, Butt M, Campbell A and Greenstone
M: Small cell carcinoma of the prostate and the syndrome of
inappropriate antidiuretic hormone: A rare entity and presentation.
Isr Med Assoc J. 16:458–460. 2014.PubMed/NCBI
|
|
7
|
Dixit S, Coup A, Hunt C and Coombs L:
Small cell cancer of the prostate. Urology. 80:e58–e60. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Terada T: Primary small cell carcinoma of
prostate without immunoreactive neuroendocrine proteins but with
expressions of KIT and platelet-derived growth factor-α. Int J
Urol. 22:122–124. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Wang L, Davidson DD, Montironi R,
Lopez-Beltran A, Zhang S, Williamson SR, MacLennan GT, Wang C, Wang
M, Emerson RE, et al: Small cell carcinoma of the prostate:
Molecular basis and clinical implications. Histol Histopathol.
30:413–424. 2015.(In Japanese). PubMed/NCBI
|
|
10
|
Ishii G, Omono H, Kasai K, Hata K, Kimura
T, Suzuki M and Egawa S: Docetaxel for small cell carcinoma of the
prostate with a metastatic pelvic tumor: A case report. Hinyokika
Kiyo. 60:641–644. 2014.(In Japanese). PubMed/NCBI
|
|
11
|
Weiner AB, Patel SG, Richards KA,
Szmulewitz RZ and Eggener SE: Population-based analysis of
treatment modalities and survival for clinically localized
small-cell carcinoma of the prostate. Prostate Cancer Prostatic
Dis. 17:286–291. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Cohen A, Richards KA, PATEL S, et al:
Metastatic small cell carcinoma of the prostate: Population-based
analysis of patient characteristics and treatment paradigms. Urol
Oncol. 33:70 e1–7. 2015. View Article : Google Scholar
|
|
13
|
Wang L, Williamson SR, Zhang S, Huang J,
Montironi R, Davison DD, Wang M, Yao JL, Lopez-Beltran A, Osunkoya
AO, et al: Increased androgen receptor gene copy number is
associated with TMPRSS2-ERG rearrangement in prostatic small cell
carcinoma. Mol Carcinog. 54:900–907. 2015. View Article : Google Scholar : PubMed/NCBI
|