Introduction
It is increasingly recognized that the prognosis of
patients with cancer is influenced by not only the oncological
characteristics of the tumor, but also the conditions of the host.
The relationship between the inflammatory and immunonutritional
status and the prognosis of patients with cancer has been reported
in several studies (1–4) in recent years. A number of indicators,
including the neutrophil-to-lymphocyte ratio (NLR), the prognostic
nutritional index (PNI), and the Glasgow prognostic score (GPS),
have been reported to be useful predictors of overall or
disease-free survival in numerous types of cancers (1–6). The NLR
was first shown to correlate with short-term outcome in patients in
an intensive care unit by Zahorec (7) in 2001. Recently, other studies
(8–11) have also shown that these parameters
are good predictors of postoperative complications in patients with
colorectal or otolaryngological cancer. Therefore, these indicators
may be effective markers of short-term outcome in various
fields.
Distal cholangiocarcinoma is associated with a high
morbidity rate. The majority of patients with these tumors already
show signs of liver dysfunction, jaundice, and malnutrition (e.g.,
hypoalbuminemia due to obstruction of the biliary duct) at the
first visit to the hospital (12,13).
Moreover, some patients exhibit an inflammatory reaction, such as
cholangitis, and require biliary drainage during the preoperative
period. Therefore, the biochemical data of patients with distal
cholangiocarcinoma are likely to show abnormal values to some
extent before surgery.
The only curative treatment for distal
cholangiocarcinoma is surgical resection, such as
pancreaticoduodenectomy (PD), which is a highly invasive and
complicated procedure. Although the mortality rate of PD has
decreased satisfactorily to 5.0% in high volume centers, due to
improvements in surgical techniques and perioperative management,
the postoperative morbidity rate remains high, with values ranging
from 30.0–65.0% (14,15). Several studies (16–18) have
reported risk factors for postoperative complications after PD.
However, the patients recruited to these studies included those
with distal cholangiocarcinoma, as well as, pancreatic head cancer.
A recent study of a large cohort of patients with resected
periampullary cancer (13) showed
that patients with cholangiocarcinoma had a higher morbidity rate
than those with pancreatic head cancer and that apparent biological
differences existed between the 2 groups. Few studies have
evaluated the risk of morbidity associated with only distal
cholangiocarcinoma (19) and risk
factors for postoperative complications after PD in patients with
distal cholangiocarcinoma have not yet been established.
We hypothesized that systemic inflammatory,
immunological, and nutritional status would influence the morbidity
rate of patients with distal cholangiocarcinoma. The aim of this
study was to determine the effectiveness of the NLR, the PNI, and
the GPS in evaluating risk factors for postoperative complications
after PD in patients with distal cholangiocarcinoma.
Materials and methods
Patients
We conducted a retrospective analysis of 84 patients
who underwent PD for pathologically confirmed distal
cholangiocarcinoma, including distal cholangiocellular and
ampullary carcinoma, at Kitasato University Hospital (Kitasato,
Japan) between January 2008 and December 2016. Patients who had
undergone simultaneous hepatic resection were excluded. Clinical,
surgical, and tumor characteristics, including age, sex, body mass
index (BMI), biliary drainage, bile culture, laboratory data,
operating times, blood loss and transfusion, International Union
Against Cancer (UICC) tumor-node-metastasis (TNM) stage, and
postoperative hospital stay, were collected and analyzed from the
clinical database.
All patients underwent pylorus-preserving (PP),
subtotal stomach-preserving (SSP), or conventional PD with regional
lymph node dissection. Portal vein resection was performed when the
tumor had invaded to achieve resection-free margins. Reconstruction
was a modified Child's reconstruction. Pancreaticojejunostomy was
performed according to the Kakita et al (20) method, as described previously (i.e.,
duct-to-mucosa anastomosis with a lost pancreatic stent and
end-to-side anastomosis with 4 stitches of transpancreatic suture
in all patients). Two closed drains were routinely inserted at the
Winslow's space in front of the pancreaticojejunostomy. Drain
management was as follows, the drains were removed on day 4 under
condition that the amylase level of drain fluid were less than
4,000 U/ml or the volume of drain fluid were less than 30 ml. If
this condition was not satisfied or drain fluid were infected, the
drains were continued to drainage.
Postoperative complications
Postoperative complications, such as anastomotic
leakage, postpancreatectomy hemorrhage, liver abscess,
intraperitoneal abscess, allergic reaction, and pneumonia, were
classified according to the Clavien-Dindo (CD) classification
(21). Patients with CD
classification grade III or higher postoperative complications were
assigned to the major complications group, and the remainder
assigned to the non-major complications group.
NLR, PNI, and GPS evaluation
Blood test results from the day immediately prior to
surgery were used. The NLR was calculated as the absolute
neutrophil count divided by the absolute lymphocyte count. The PNI
was calculated as 10 × albumin + 0.05 × the lymphocyte count. The
GPS was determined based on C-reactive protein (CRP) and albumin.
Patients with elevated CRP (>0.3 mg/dl) and hypoalbuminemia
(<3.5 mg/dl) were assigned a score of 2. Patients with either
one of these 2 biochemical outliers were assigned a score of 1.
Patients with neither of these biochemical outliers were assigned a
score of 0 (22).
Statistical analyses
Categorical variables were compared using the
Chi-square test or Fisher's exact test. Continuous variables were
compared using the Student's t-test or Mann-Whitney U test.
Continuous variables were presented as the mean and standard
deviation. Receiver operating characteristic (ROC) curve analysis
was performed to determine the optimal cut-off values for several
variables to predict postoperative complications. Variables
associated with postoperative complications in the univariate
analysis were included in the multivariate logistic regression
analysis. Odds ratio (OR) and 95.0% confidence intervals (CIs) were
used to quantify the strength of the association between predictors
and morbidity. All statistical analyses were conducted using
JMP® software (version 11.2.0; SAS Institute Inc., Cary,
NC, USA). A P<0.05 was considered statistically significant.
Results
In total, 84 patients were enrolled in the study.
The characteristics of the patients are summarized in Table I. The mean age was 68.1±8.7 years.
Sixty-three patients were male and 21 patients were female.
Fifty-seven patients had distal cholangiocellular carcinoma and 27
patients had ampullary carcinoma. Seventy-four patients underwent
PPPD, 7 patients underwent SSPPD, and 3 patients underwent
conventional PD. Thirty-nine patients (46.4%) experienced
postoperative complications of CD classification grade III or
higher. The most common postoperative complication was anastomotic
leakage (n=31; 36.9%) [pancreaticojejunostomy leakage (n=27),
choledochojejunostomy leakage (n=2), and duodenojejunostomy leakage
(n=2)]. Other complications included bleeding (n=2),
intraperitoneal abscess (n=2), pneumonia (n=2), liver abscess
(n=1), and anaphylactic shock (n=1).
 | Table I.Patient characteristics (n=84). |
Table I.
Patient characteristics (n=84).
| Patient
characteristic | Data |
|---|
| Age (years) | 68.1±8.7 |
| Sex | Male, 63 (75%) |
|
| Female, 21 (25%) |
| Diagnosis | Ampullary carcinoma,
27 (32.1%) |
|
| Distal
cholangiocellular carcinoma, |
|
| 57 (67.9%) |
| Operative
procedure | PPPD, 74 (88.1%) |
|
| SSPPD, 7 (8.3%) |
|
| PD, 3 (3.6%) |
| Complication
(≥CDIII) | Anastomotic
leakage, 31 (36.9%) |
|
| Intra-abdominal
abscess, 2 (2.4%) |
|
| Bleeding, 2
(2.4%) |
|
| Pneumonia, 2
(2.4%) |
|
| Liver abscess, 1
(1.2%) |
|
| Anaphylactic shock,
1 (1.2%) |
First of all, we compared patients with ampullary
carcinoma and those with distal cholangiocelluler carcinoma. The
clinical characteristics of each group are summarized in Table II. Biliary drainage rate was over
90% in both groups. Patients with distal cholangiocellular
carcinoma showed high frequency of bile culture positive (88%) and
slightly high CRP (1.44±2.14) rather than ampullary carcinoma (70%
and 0.60±0.64, respectively), but with no statistically significant
difference (P=0.06 and 0.051, respectively). Other preoperative
biochemical variables and operative parameters including operative
times, blood loss volumes, the incidence of transfusion or portal
vein resection were comparable between the 2 groups. There was no
significant difference in UICC TNM stage, NLR and GPS, however
patients with ampullary carcinoma showed higher level of PNI rather
than distal cholangiocellular carcinoma with statistically
significant difference. The complication rate over grade III in CD
classification and postoperative hospital stays were comparable
between the 2 groups.
 | Table II.Characteristics of patients with
ampullary carcinoma and patients with distal cholangiocellular
carcinoma. |
Table II.
Characteristics of patients with
ampullary carcinoma and patients with distal cholangiocellular
carcinoma.
| Variable | Ampullary carcinoma
(n=27) | Distal
cholangiocellular carcinoma (n=57) | P-value |
|---|
| Age (years) | 65.8±10.3 | 69.2±7.7 | 0.092 |
| Sex (M/F) | 18/9 | 45/12 | 0.704 |
| Total bilirubin
(mg/dl) | 0.87±0.62 | 1.02±0.61 | 0.326 |
| ALP (U/l) | 448.6±65.9 | 546.6±45.3 | 0.224 |
| GTP (U/l) | 149.8±53.1 | 267.7±6.6 | 0.071 |
| Platelets,
×104/µl | 26.2±10.3 | 24.6±6.6 | 0.385 |
| Neutrophils/µl | 3,261±1,228 | 3,504±1,269 | 0.411 |
| Lymphocytes/µl | 1,558±552 | 1,437±479 | 0.308 |
| Albumin (g/dl) | 3.79±0.07 | 3.62±0.05 | 0.066 |
| CRP (mg/dl) | 0.60±0.64 | 1.44±2.14 | 0.051 |
| BMI | 21.94±3.35 | 22.43±3.39 | 0.541 |
| Biliary drainage
(+) | 25 (93%) | 56 (98%) | 0.212 |
| Bile culture
positive | 19 (70%) | 50 (88%) | 0.060 |
| Operation time
(min) | 505±130 | 537±117 | 0.263 |
| Blood loss
(ml) | 1,293±1,142 | 1,196±899 | 0.682 |
| Transfusion
(+) | 7 (26%) | 10 (18%) | 0.379 |
| PV resection
(+) | 1 (4%) | 6 (11%) | 0.259 |
| Stage |
|
| 0.195 |
| IA | 7 | 5 |
|
| IB | 3 | 8 |
|
|
IIA | 4 | 18 |
|
|
IIB | 12 | 24 |
|
|
III | 0 | 0 |
|
| IV | 1 | 1 |
|
| NLR | 2.40±1.60 | 2.68±1.32 | 0.392 |
| PNI | 45.64±4.82 | 43.36±4.39 | 0.034 |
| GPS |
|
| 0.388 |
| 0 | 16 | 26 |
|
| 1 | 9 | 22 |
|
| 2 | 2 | 9 |
|
| Complication
(≥CDIII) | 14 (52%) | 25 (44%) | 0.493 |
| Postoperative
hospital stay (days) | 25.4±17.7 | 26.9±20.7 | 0.743 |
Next, the patients were divided into 2 groups
depending on the presence of complication or not for evaluating
risk factor of postoperative complication. The 39 patients with
postoperative complications of CD classification grade III or
higher were assigned to the major complications group. The
remaining 45 patients were assigned to the non-major complications
group. The clinical characteristics of each group are summarized in
Table III. Preoperative
biochemical variables, including platelet count and total
bilirubin, alkaline phosphatase, gamma-glutamyltransferase,
albumin, and CRP levels, were comparable between the 2 groups.
There were no significant differences in mean operative times,
blood loss volumes, the incidence of blood transfusions or portal
vein resection, UICC TNM stage, the frequencies of biliary drainage
and bile culture positive. The major complications group had a
significantly higher neutrophil count (P<0.05), NLR (P<0.01),
and BMI (P<0.01) than the non-major complications group. In
contrast, the lymphocyte count was significantly lower in the major
complications group than in the non-major complications group
(P<0.05). Other indicators, such as the PNI and the GPS, did not
differ significantly between the 2 groups. Understandably, the
postoperative hospital stay was statistically long in the major
complications group rather than in the non-major complications
group.
 | Table III.Comparison between clinical
characteristics and perioperative outcomes between the major and
non-major complications groups. |
Table III.
Comparison between clinical
characteristics and perioperative outcomes between the major and
non-major complications groups.
| Variable | Major complications
group (n=39) | Non-major
complications group (n=45) | P-value |
|---|
| Age (years) | 69.2±8.5 | 67.3±8.9 | 0.325 |
| Sex (M/F) | 30/9 | 33/12 | 0.704 |
| Total bilirubin
(mg/dl) | 0.86±0.10 | 1.06±0.65 | 0.131 |
| ALP (U/l) | 491.7±55.2 | 535.4±51.4 | 0.563 |
| GTP (U/l) | 232.5±45.1 | 227.5±42.0 | 0.935 |
| Platelets,
×104/µl | 24.1±7.5 | 26.0±8.4 | 0.293 |
| Neutrophils/µl | 3,758±1,138 | 3,138±1,290 | 0.023 |
| Lymphocytes/µl | 1,353±375 | 1,582±576 | 0.037 |
| Albumin (g/dl) | 3.68±0.06 | 3.67±0.06 | 0.905 |
| CRP (mg/dl) | 1.15±1.57 | 1.18±2.06 | 0.943 |
| BMI | 23.3±3.67 | 21.35±2.80 | 0.007 |
| Biliary drainage
(+) | 38 (97%) | 43 (96%) | 0.639 |
| Bile culture
positive | 33 (85%) | 36 (80%) | 0.580 |
| Operation time
(min) | 535±130 | 519±115 | 0.553 |
| Blood loss
(ml) | 1,359±1,201 | 1,119±743 | 0.282 |
| Transfusion
(+) | 11 (28%) | 6 (13%) | 0.090 |
| PV resection
(+) | 1 (3%) | 6 (13%) | 0.060 |
| Stage |
|
| 0.327 |
| IA | 5 | 7 |
|
| IB | 6 | 5 |
|
|
IIA | 13 | 9 |
|
|
IIB | 15 | 21 |
|
|
III | 0 | 0 |
|
| IV | 0 | 2 |
|
| NLR | 3.04±1.46 | 2.21±1.26 | 0.007 |
| PNI | 43.54±4.82 | 44.58±4.46 | 0.306 |
| GPS |
|
| 0.846 |
| 0 | 19 | 23 |
|
| 1 | 14 | 17 |
|
| 2 | 6 | 5 |
|
| Postoperative
hospital stays (days) | 38.3±21.9 | 16.1±9.1 | <0.0001 |
Variables for which significant differences were
identified in the univariate analysis were further analyzed using
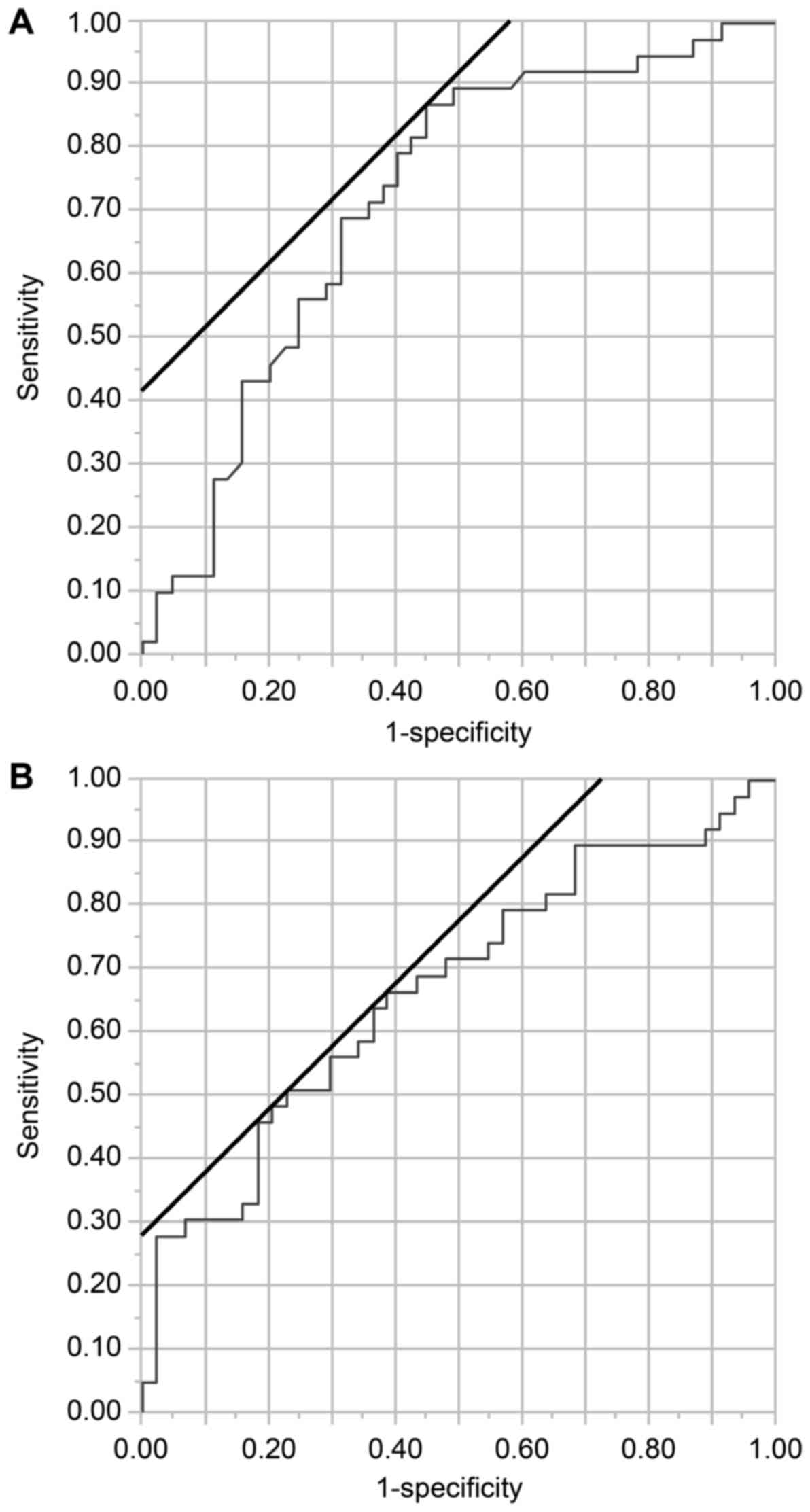
ROC curve analysis to determine the optimal cut-off values. The
optimal cut-off value for the NLR was 2.0 [area under curve (AUC),
0.72; Fig. 1A]. The optimal cut-off
value for the BMI was 23.0 kg/m2 (AUC, 0.67; Fig. 1B). The optimal cut-off values for the
neutrophil count and lymphocyte count were 2,727 (AUC, 0.67) and
1,870 (AUC, 0.59), respectively. These parameters were included in
the multivariate logistic regression analysis. A BMI of >23.0
kg/m2 (OR: 3.83, 95.0% CI: 1.35–11.83; P=0.011) and a
NLR of >2.0 (OR: 6.77, 95.0% CI: 2.44–21.13; P<0.001) were
independent risk factors for major postoperative complications
(Table IV).
 | Table IV.Multivariate logistic regression
analysis of the influence of clinical parameters on postoperative
complications. |
Table IV.
Multivariate logistic regression
analysis of the influence of clinical parameters on postoperative
complications.
| Variable | OR | 95% CI | P-value |
|---|
| Neutrophil
>2,727 | 2.08 | 0.68–6.49 | 0.201 |
| Lymphocyte
<1,870 | 2.70 | 0.44–22.81 | 0.290 |
| NLR >2.0 | 6.77 | 2.44–21.13 | <0.001 |
| BMI >23.0
kg/m2 | 3.83 | 1.35–11.83 | 0.011 |
Discussion
The present study highlights the usefulness of the
NLR in predicting postoperative major complications after PD in
patients with distal cholangiocarcinoma, providing surgeons with
important information. Surgeons sometimes confront the situation
that patients with biliary carcinoma experience acute cholangitis
immediately prior to surgery. Acute cholangitis activates
inflammatory responses, whilst suppressing immunological and
nutritional responses, resulting in elevated neutrophil counts and
CRP levels and reduced lymphocyte counts and albumin levels. In
such cases, antibiotic treatment or biliary drainage (stenting)
were performed. However, we do not know how long we should wait for
the patient's condition to improve. Our findings suggest that
surgeons may be able to determine treatment strategies based on the
NLR.
Over the past few decades, many studies (14–18,23–25) have
reported that the risk factors for postoperative complications
after PD include a soft pancreatic texture, a small pancreatic duct
diameter, a high BMI, preoperative biliary drainage, operative
blood loss, and sarcopenia. However, the patients enrolled in these
studies have included a mixture of those with distal
cholangiocarcinoma and pancreatic head cancer. Andrianello et
al (13) reported that the
postoperative morbidity rate was significantly higher in patients
with distal cholangiocarcinoma than in those with pancreatic ductal
adenocarcinoma (75.9% vs. 52.1%, respectively; P<0.01).
Preoperative variables [e.g., the incidence of jaundice (94.4% vs.
72.0%, respectively; P<0.01) and biliary stent insertion (79.6%
vs. 54.4%, respectively; P<0.01)] also differed significantly
between the 2 groups. In this study, we compared patients with
ampullary carcinoma and those with distal cholangiocellular
carcinoma. Both groups showed the same frequencies of biliary
drainage (93% vs. 98%, respectively; P=0.212). Concerning bile
juice infection, slightly higher incidence was observed in patients
with distal cholangiocellular carcinoma (88%) compared to patients
with ampullary carcinoma (70%), however the frequency of
postoperative complications was observed equally between 2 groups
(44% vs. 52%; P=0.493).
Finally, a high NLR and a high BMI were extracted as
independent risk factors for postoperative complications after PD
for distal cholangiocarcinoma. Yamashita et al (26) reported that a BMI of ≥25.0
kg/m2 is an independent risk factor for clinically
relevant pancreatic fistulas after PD for pancreatic ductal
adenocarcinoma. The authors speculate that this may be because the
pancreas of patients with a high BMI contains a large fatty
component, which results in a fragile parenchyma (so called ‘soft
pancreas’) (27–29). It seems acceptable that the BMI
threshold in our study was lower than that of the Yamashita et
al (26) study because
pancreatic exocrine function is well preserved in patients with
cholangiocarcinoma compared to those with pancreatic ductal
adenocarcinoma (30).
In the last decade, the NLR has emerged as an
attractive proxy to characterize systemic inflammatory status.
Several studies (1,3,31) have
suggested that a high NLR is a poor prognostic marker because
neutrophilia accelerates tumor vessel angiogenesis and lymphopenia
diminishes immune protection against tumor invasion. Therefore, a
high NLR is associated with favorable tumor conditions. However,
the reason why the NLR is concerning for postoperative
complications may be related to quite different mechanisms. The
mechanisms underlying associations between systemic inflammation
and postoperative complications remain to be elucidated. Josse
et al (10) reported that a
high NLR of >2.3 correlated with postoperative complications,
specifically anastomotic leakage and not infections, in patients
with colorectal cancer. Kudo et al (32) reported an association between the
perioperative NLR and postoperative complications, specifically
infections, in patients with colorectal cancer. Considering these
findings, one potential explanation is that subclinical
inflammation (e.g., latent cholangitis) may be exaggerated by
surgery in a second attack. Excessive postoperative systemic
inflammatory response syndrome causes a cytokine storm that results
in microcirculatory disturbances in whole organs. In this study,
the most common complication was anastomotic leakage. Nakanishi
et al (33) reported that
systemic inflammatory responses modified endothelial function. An
inability of the endothelium to produce nitric oxide and
prostacyclin could result in depletion of the vasodilator and
antithrombotic properties of the vascular endothelium. Yao et
al (34) also reported that a
high NLR was associated with vascular thrombosis. Thus, a high NLR
seems to drive microvascular impairment, which is unfavorable for
wound healing.
A value of 2.0, as determined by ROC curve analysis,
was selected as the threshold for the NLR in this study. This value
was relatively low compared to that reported in the literature
(5.0) (1). We speculate that this
may be because systemic inflammatory responses need to be sustained
for a prolonged period and with sufficient power to influence
prognosis compared to postoperative complications. Other studies
(8,29) have reported thresholds of 2.3 for
postoperative complications in colorectal surgery. It is reasonable
that the threshold is 2.0 for PD and 2.3 for colorectal surgery
because PD is a more invasive procedure than colorectal
surgery.
A high preoperative NLR of >2.0 is a novel
indicator for identifying patients at high risk of postoperative
complications after PD. As the NLR can be calculated from routine
blood test results, it is cost effective and a convenient biomarker
for surgeons to determine the best time to operate or to select
different strategies for positive surgical outcomes.
This study has several limitations associated with
its single-center retrospective design, limited sample size, and
8-year enrollment period. Although the perioperative management and
surgical procedures have remained relatively constant, the skills
and experiences of the surgeons were not included in the analysis.
As cholangiocarcinoma is a rare disease, large multicenter studies
would be desirable to validate our findings in a prospective
manner.
In conclusion, a high NLR and a high BMI are risk
factors of morbidity after PD in patients with distal
cholangiocarcinoma. To our knowledge, this is the first study to
report the effectiveness of the NLR for predicting postoperative
complications in the pancreato-biliary field. Based on our
findings, aggressive therapeutic intervention and/or waiting for a
reduction in the NLR are recommended for reducing morbidity in
patients with distal cholangiocarcinoma and a high preoperative
NLR.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
YK designed this study and wrote the whole draft of
the manuscript. TK and HT contributed to the preparation of the
clinical data. NN and SE contributed to analysis the data. KI
contributed to the preparation of documents for the local ethics
committee and search literatures. MW supervised the study. All
authors read and approved the final manuscript.
Ethics approval and consent to
participate
The study was approved by the Ethics Committee of
Kitasato University School of Medicine (Kitasato, Japan). Research
was conducted in accordance with the 1964 Declaration of Helsinki
and its later amendments.
Patient consent for publication
Not applicable
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
AUC
|
area under the curve
|
|
BMI
|
body mass index
|
|
CD
|
clavien-dindo
|
|
CI
|
confidence interval
|
|
CRP
|
C-reactive protein
|
|
GPS
|
glasgow prognostic score
|
|
NLR
|
neutrophil-to-lymphocyte ratio
|
|
OR
|
odds ratio
|
|
PD
|
pancreaticoduodenectomy
|
|
PNI
|
prognostic nutritional index
|
|
PP
|
pylorus-preserving
|
|
PPPD
|
pylorus-preserving
pancreaticoduodenectomy
|
|
ROC
|
receiver operating characteristic
|
|
SSP
|
subtotal stomach-preserving
|
|
SSPPD
|
substomach-preserving
pancreaticoduodenectomy
|
|
TNM
|
tumor-node-metastasis
|
|
UICC
|
International Union Against Cancer
|
References
|
1
|
Paramanathan A, Saxena A and Morris DL: A
systematic review and meta-analysis on the impact of pre-operative
neutrophil lymphocyte ratio on long term outcomes after curative
intent resection of solid tumours. Surg Oncol. 23:31–39. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Nozoe T, Ninomiya M, Maeda T, Matsukuma A,
Nakashima H and Ezaki T: Prognostic nutritional index: A tool to
predict the biological aggressiveness of gastric carcinoma. Surg
Today. 40:440–443. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Absenger G, Szkandera J, Stotz M,
Postlmayr U, Pichler M, Ress AL, Schaberl-Moser R, Loibner H,
Samonigg H and Gerger A: Preoperative neutrophil-to-lymphocyte
ratio predicts clinical outcome in patients with stage II and III
colon cancer. Anticancer Res. 33:4591–4594. 2013.PubMed/NCBI
|
|
4
|
Zhang P, Xi M, Li QQ, He LR, Liu SL, Zhao
L, Shen JX and Liu MZ: The modified glasgow prognostic score is an
independent prognostic factor in patients with inoperable thoracic
esophageal squamous cell carcinoma undergoing chemoradiotherapy. J
Cancer. 5:689–695. 2014. View
Article : Google Scholar : PubMed/NCBI
|
|
5
|
He C, Mao Y, Lao X, Li S and Lin X:
Neutrophil-to-lymphocyte ratio predicts overall survival of
patients with combined hepatocellular cholangiocarcinoma. Oncol
Lett. 15:4262–4268. 2018.PubMed/NCBI
|
|
6
|
Han F, Shang X, Wan F, Liu Z, Tian W, Wang
D, Liu Y, Wang Y, Zhang B and Ju Y: Clinical value of the
preoperative neutrophil-to-lymphocyte ratio and red blood cell
distribution width in patients with colorectal carcinoma. Oncol
Lett. 15:3339–3349. 2018.PubMed/NCBI
|
|
7
|
Zahorec R: Ratio of neutrophil to
lymphocyte counts-rapid and simple parameter of systemic
inflammation and stress in clinically ill. Bratisl Lek Listy.
102:5–14. 2001.(In English, Slovak). PubMed/NCBI
|
|
8
|
Nozoe T, Kimura Y, Ishida M, Saeki H,
Korenaga D and Sugimachi K: Correlation of pre-operative
nutritional condition with post-operative complications in surgical
treatment for oesophageal carcinoma. Eur J Surg Oncol. 28:396–400.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Maruyama Y, Inoue K, Mori K, Gorai K,
Shimamoto R, Onitsuka T, Iguchi H, Okazaki M and Nakagawa M:
Neutrophil-lymphocyte ratio and platelet-lymphocyte ratio as
predictors of wound healing failure in head and neck
reconstruction. Acta Otolaryngol. 137:106–110. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Josse JM, Cleghorn MC, Ramji KM, Jiang H,
Elnahas A, Jackson TD, Okrainec A and Quereshy FA: The
neutrophil-to-lymphocyte ratio predicts major perioperative
complications in patients undergoing colorectal surgery. Colorectal
Dis. 18:O236–O242. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Kang WM, Zhu CZ, Yang XX, Yu JC, Ma ZQ, Ye
X, Li K and Liu D: Application of the Onodera prognostic nutrition
index and neutrophil-to-lymphocyte ratio in risk evaluation of
postoperative complications in Crohn's disease. Sci Rep.
7:84812017. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
El Nakeeb A, Askar W, Atef E, Hanafy EE,
Sultan AM, Salah T, Shehta A, Sorogy ME, Hamdy E, Hemly ME, et al:
Trends and outcomes of pancreaticoduodenectomy for periampullary
tumors: A 25-year single-center study of 1000 consecutive cases.
World J Gastroenterol. 23:7025–7036. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Andrianello S, Marchegiani G, Malleo G,
Rusev BC, Scarpa A, Bonamini D, Maggino L, Bassi C and Salvia R:
Over 700 whipples for pancreaticobiliary malignancies:
Postoperative morbidity is an additional negative prognostic factor
for distal bile duct cancer. J Gastrointest Surg. 21:527–533. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Kawai M, Tani M, Hirono S, Ina S, Miyazawa
M and Yamaue H: How do we predict the clinically relevant
pancreatic fistula after pancreaticoduodenectomy?-an analysis in
244 consecutive patients. World J Surg. 33:2670–2678. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Yamamoto Y, Sakamoto Y, Nara S, Esaki M,
Shimada K and Kosuge T: A preoperative predictive scoring system
for postoperative pancreatic fistula after pancreaticoduodenectomy.
World J Surg. 35:2747–2755. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Yamane H, Abe T, Amano H, Hanada K, Minami
T, Kobayashi T, Fukuda T, Yonehara S, Nakahara M, Ohdan H and
Noriyuki T: Visceral adipose tissue and skeletal muscle index
distribution predicts severe pancreatic fistula development after
pancreaticoduodenectomy. Anticancer Res. 38:1061–1066.
2018.PubMed/NCBI
|
|
17
|
Mungroop TH, van Rijssen LB, van Klaveren
D, Smits FJ, van Woerden V, Linnemann RJ, de Pastena M, Klompmaker
S, Marchegiani G, Ecker BL, et al: Alternative fistula risk score
for pancreatoduodenectomy (a-FRS): Design and international
external validation. Ann Surg. Dec 12–2017.(Epub ahead of print).
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Gagnière J, Abjean A, Franz M, Aumont O,
Pereira B, Dupré A, Veziant J, Le Roy B, Boyer L, Pezet D and Buc
E: A normal preoperative lipase serum level is an easy and
objective risk factor of pancreatic fistula after
pancreaticoduodenectomy. Pancreas. 46:1133–1140. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Uemetsu S, Wakiya T, Ishido K, Kudo D,
Kimura N, Miura T, Toyoki Y and Hakamada K: Effect of sarcopenia on
the outcomes after pancreaticoduodenectomy for distal
cholangiocarcinoma. ANZ J Surg. Feb 1–2018.(Epub ahead of print).
View Article : Google Scholar
|
|
20
|
Kakita A, Yoshida M and Takahashi T:
History of pancreaticojejunostomy in pancreaticoduodenectomy:
Development of a more reliable anastomosis technique. J
Hepatobiliary Pancreat Surg. 8:230–237. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Dindo D, Demartines N and Clavien PA:
Classification of surgical complications: A new proposal with
evaluation in a cohort of 6336 patients and results of a survey.
Ann Surg. 240:205–213. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Proctor MJ, Morrison DS, Talwar D, Balmer
SM, O'Reilly DS, Foulis AK, Horgan PG and McMillan DC: An
inflammation-based prognostic score (mGPS) predicts cancer survival
independent of tumour site: A glasgow inflammation outcome study.
Br J Cancer. 104:726–734. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Fujii T, Yamada S, Suenaga M, Kanda M,
Takami H, Sugimoto H, Nomoto S, Nakao A and Kodera Y: Preoperative
internal biliary drainage increases the risk of bile juice
infection and pancreatic fistula after pancreatoduodenectomy: A
prospective observational study. Pancreas. 44:465–470.
2015.PubMed/NCBI
|
|
24
|
Fang CH, Chen QS, Yang J, Xiang F, Fang ZS
and Zhu W: Body mass index and stump morphology predict an
increased incidence of pancreatic fistula after
pancreaticoduodenectomy. World J Surg. 40:1467–1476. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Nishida Y, Kato Y, Kudo M, Aizawa H, Okubo
S, Takahashi D, Nakayama Y, Kitaguchi K, Gotohda N, Takahashi S and
Konishi M: Preoperative sarcopenia strongly influences the risk of
postoperative pancreatic fistula formation after
pancreaticoduodenectomy. J Gastrointest Surg. 20:1586–1594. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Yamashita Y, Yoshizume T, Fukuzawa K,
Nishizaki T, Tsujita E, Kajiyama K, Soejima Y, Yamagata M, Yamamoto
K, Adachi E, et al: Surgical results of pancreaticoduodenectomy for
pancreatic ductal adenocarcinoma: A multi-institutional
retrospective study of 174 patients. Anticancer Res. 36:2407–2412.
2016.PubMed/NCBI
|
|
27
|
Gaujoux S, Cortes A, Couvelard A, Noullet
S, Clavel L, Rebours V, Lévy P, Sauvanet A, Ruszniewski P and
Belghiti J: Fatty pancreas and increased body mass index are risk
factors of pancreatic fistula after pancreaticoduodenectomy.
Surgery. 148:15–23. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Rosso E, Casnedi S, Pessaux P,
Oussoultzoglou E, Panaro F, Mahfud M, Jaeck D and Bachellier P: The
role of ‘fatty pancreas’ and of BMI in the occurrence of pancreatic
fistula after pancreaticoduodenectomy. J Gastrointest Surg.
13:1845–1851. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Olsen TS: Lipomatosis of the pancreas in
autopsy material and its relation to age and overweight. Acta
Pathol Microbiol Scand A. 86A:1–373. 1978.
|
|
30
|
Callery MP, Pratt WB, Kent TS, Chaikof EL
and Vollmer CM Jr: A prospectively validated clinical risk score
accurately predicts pancreatic fistula after pancreatoduodenectomy.
J Am Coll Surg. 216:1–14. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Walsh SR, Cook EJ, Goulder F, Justin TA
and Keeling NJ: Neutrophil-lymphocyte ratio as a prognostic factor
in colorectal cancer. J Surg Oncol. 91:181–184. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Kubo T, Ono S, Ueno H, Shinto E, Yamamoto
J and Hase K: Impact of the perioperative neutrophil-to-lymphocyte
ratio on the long-term survival following an elective resection of
colorectal carcinoma. Int J Colorectal Dis. 29:1091–1099. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Nakanishi N, Sato M, Shirai K, Suzuki K
and Tatara K: White blood cell count as a risk factor for
hypertension; a study of Japanese male office workers. J Hypertens.
20:851–857. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Yao C, Zhang Z, Yao Y, Xu X, Jiang Q and
Shi D: Predictive value of neutrophil to lymphocyte ratio and
platelet to lymphocyte ratio for acute deep vein thrombosis after
total joint arthroplasty: A retrospective study. J Orthop Surg Res.
13:402018. View Article : Google Scholar : PubMed/NCBI
|