Introduction
Recently, chemotherapy for colorectal cancer has
progressed markedly. In particular, treatment for unresectable
metastatic colorectal cancer (mCRC) has notably improved with the
development of FOLFOX and FOLFIRI therapies (1). Furthermore, combination of monoclonal
antibody therapies, including anti-epidermal growth factor receptor
(EGFR) monoclonal antibody or anti-vascular endothelial growth
factor (VEGF) monoclonal antibody therapy with chemotherapeutic
cytotoxic drugs has made treatment more effective and useful for
patients with unresectable mCRC (2–6).
However, curing unresectable mCRC is difficult with chemotherapy
alone. At present, effective novel chemotherapeutic agents may now
convert unresectable mCRC with liver metastases into resectable
disease (conversion therapy) (7).
Case report
A 62-year-old female with occult blood in her stool
was referred to Gifu University Hospital (Gifu, Japan) for
evaluation and treatment. The patient had a previous history of
appendectomy and cesarean section. Colonoscopy revealed a
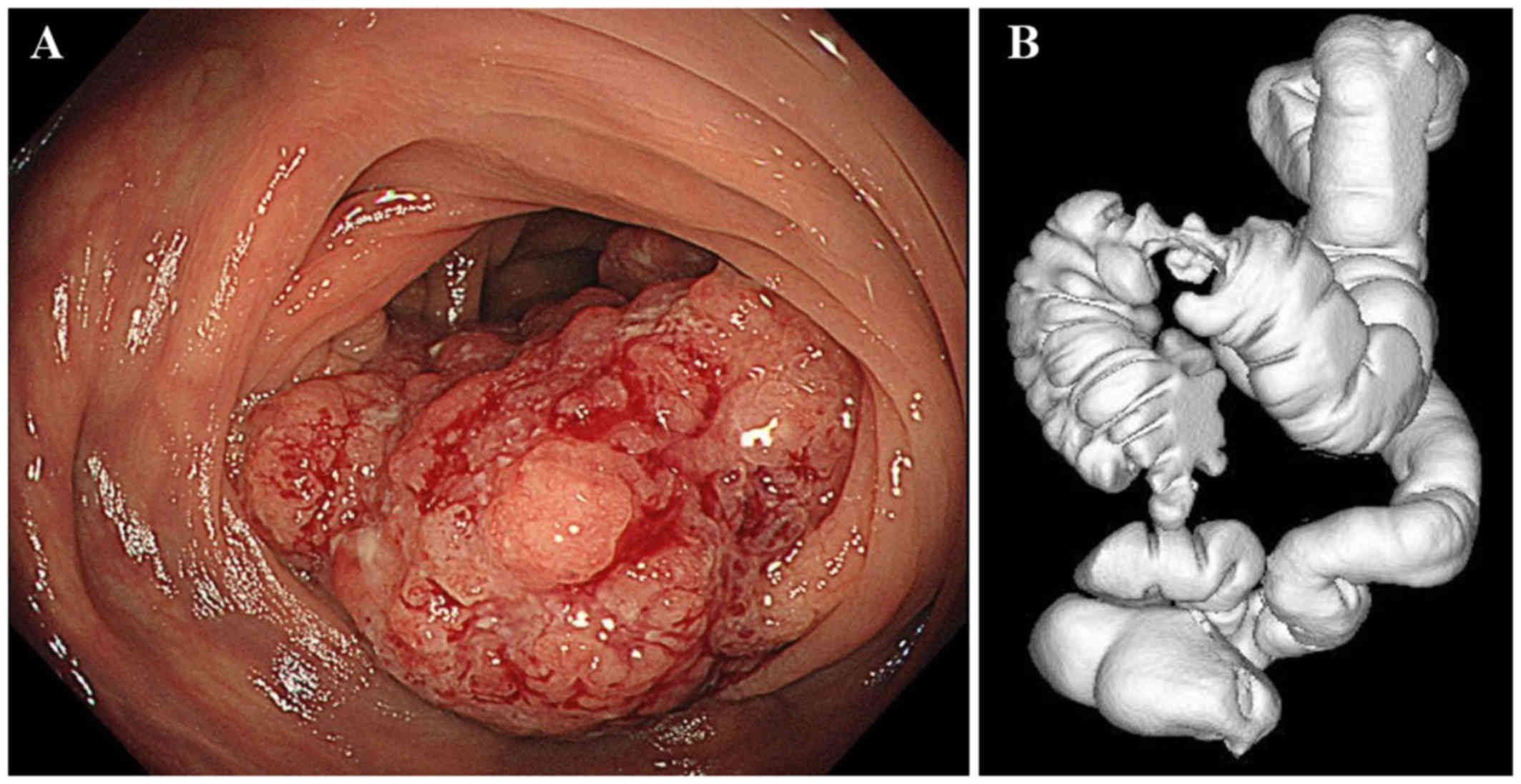
superficial elevated tumor in the transverse colon (Fig. 1), and biopsy results indicated
adenocarcinoma. An abdominal computed tomography (CT) scan revealed
increased transverse colon wall thickness and swollen lymph nodes
in the mesocolon, and along the superior mesenteric artery and
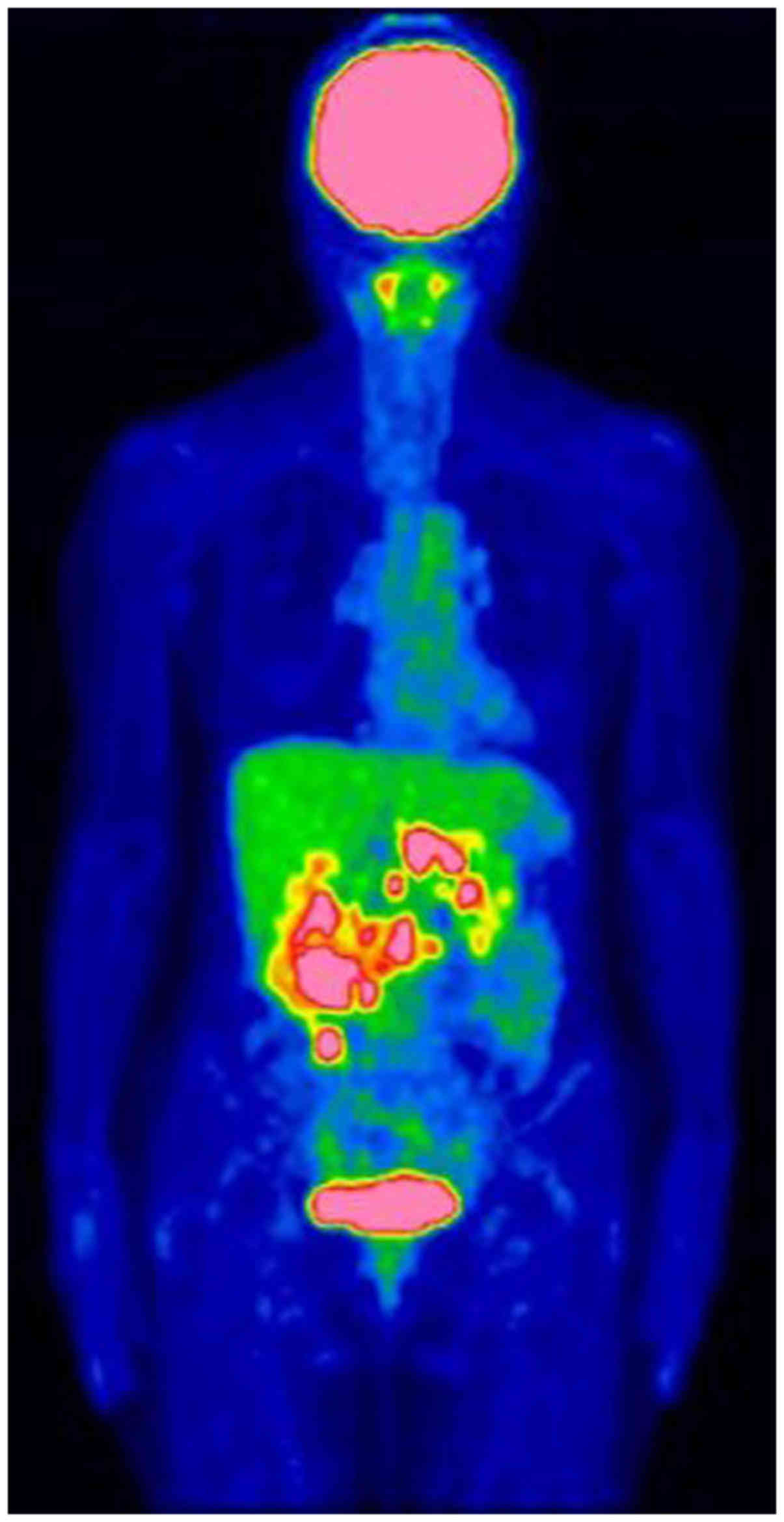
aorta. A fluorine-18 fluorodeoxyglucose (FDG) positron emission
tomography scan revealed FDG accumulations in the primary lesion
and multiple swollen lymph nodes (Fig.
2). The laboratory data revealed high levels of tumor markers,
CEA (1.5 ng/ml) and CA19-9 (221.4 U/ml). The initial diagnosis was
Stage IVA [T3 N2b M1a (lymph nodes)] according to the Union for
international cancer control TNM classification of malignant tumors
(8th edition) (8). Colonic stenosis
in this patient was not severe; therefore, chemotherapy without
surgery was planned for this unresectable case. The first course
consisted only of mFOLFOX6 [l-leucovorin 200 mg/m2
administered simultaneously with oxaliplatin 85 mg/m2,
followed by a 400-mg/m2 bolus of fluorouracil (5-FU) on
day 1 and then 2,400 mg/m2 5-FU as an intravenous
infusion over 46 h, every 2 weeks], but the tumor marker levels
increased markedly. As a genetic analysis revealed presence of the
wild-type KRAS gene, mFOLFOX6 plus cetuximab (400 mg/m2
loading dose on day 1 and then 250 mg/m2 weekly) were
administered for the second course. After 6 courses of chemotherapy
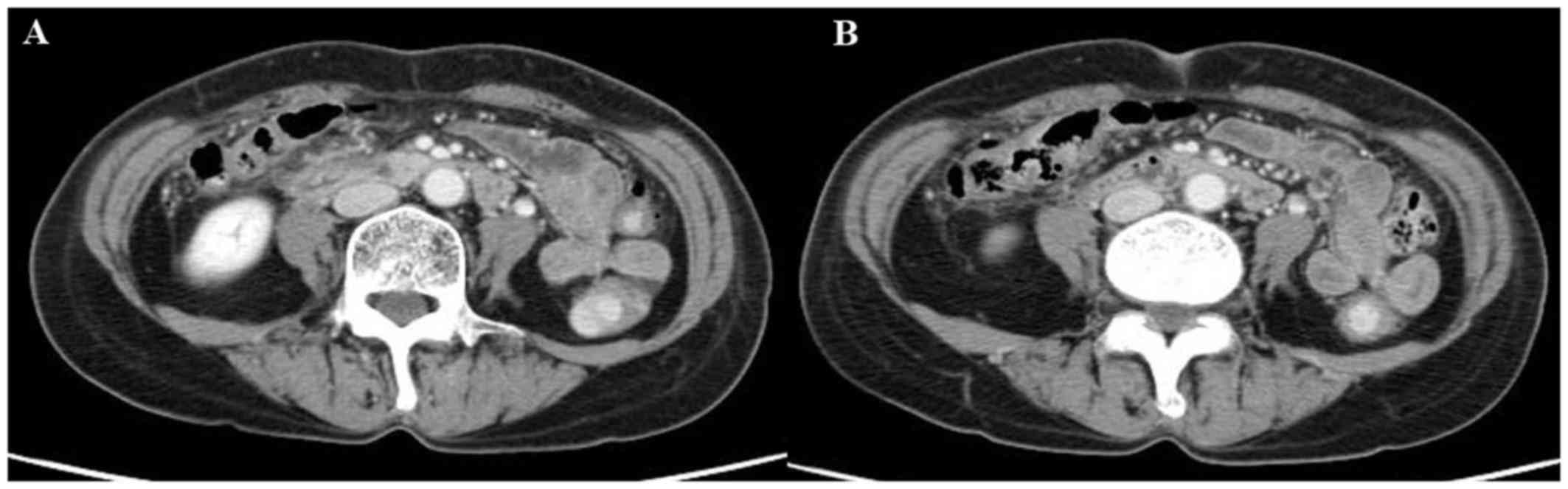
were performed, the tumor marker levels had declined markedly, and
all lymph node metastases had disappeared on enhanced CT scanning,
which indicated clinical complete response according to the
Response Evaluation Criteria in Solid Tumors (Fig. 3). Therefore, a conventional right
hemicolectomy with D3 lymph node dissection plus sampling excision
of the paraaortic lymph nodes was performed. The operative specimen
was fixed in 10% buffered formalin for 24 h, then processed to
paraffin embedded tissue. Paraffin sections were cut to 4 µm in
thickness. Sections were deparaffinized by xylene followed by
hydrolyzed with ethanol solution series. Antigen retrieval was
performed by heating in antigen retrieval solution (Ventana Medical
Systems, Inc., Tucson, AZ, USA) at 95°C for 35 min. Histological
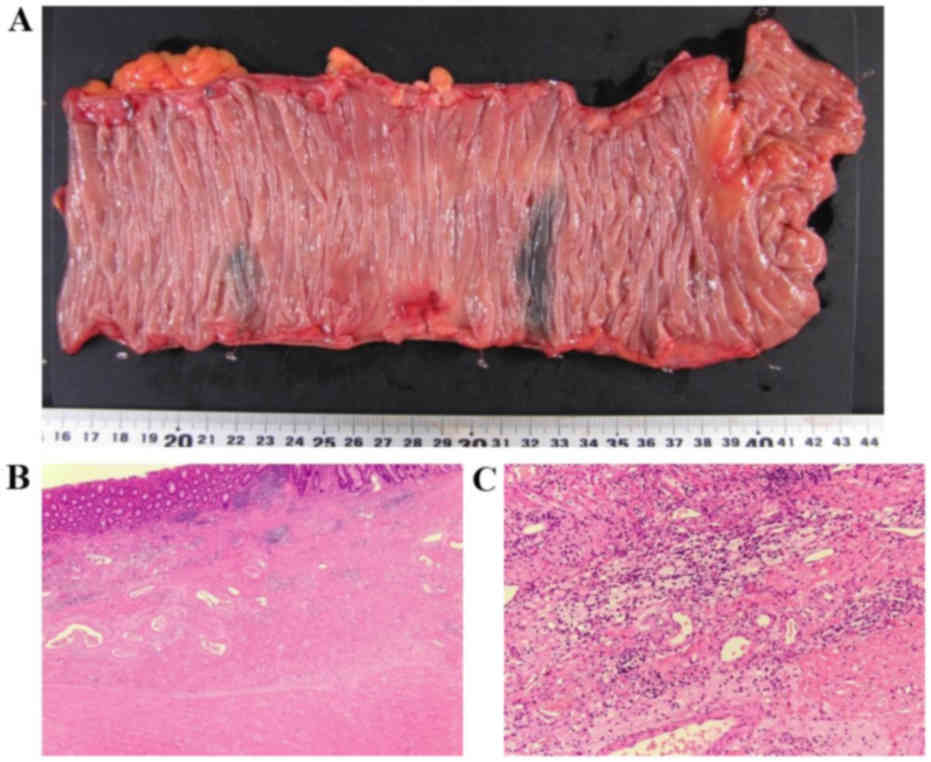
examination of the specimen did not reveal any malignant cells in
the colon wall or in the mesocolon lymph nodes (Fig. 4). The pathological diagnosis was a
complete response. The patient is currently alive 5 years after
surgery with no signs of recurrence.
Discussion
The prognosis of mCRC has significantly improved in
recent years with the development of more effective surgical
approaches, and more efficacious chemotherapy regimens, including
FOLFOX or FOLFIRI rendering more patients as surgical candidates
(9). Chemotherapy is now able to
convert unresectable colorectal liver metastasis into resectable
disease (conversion chemotherapy), and prior ‘rules of
resectability’ are being challenged (10). This has increased the rates of
resectability from 10–15% to up to 20–30% with 5- and 10-year
overall survival (OS) rates of ~33 and 23%, respectively. For
example, patients who undergo liver resection and survive beyond 10
years appear to be cured in almost all cases (11). In addition, CRC patients with lung
metastases or paraaortic lymph node metastases who undergo radical
resection are expected to have improved survival (12–16). The
concepts of early tumor shrinkage and deepness of response were
also previously assessed in first-line trials with anti-EGFR
monoclonal antibodies for patients with KRAS wild-type mCRC
(17,18). Therefore, we hypothesized that
anti-EGFR monoclonal antibodies are key drugs for the conversion of
unresectable and metastatic colorectal metastasis into resectable
disease.
Recently, primary tumor location, whether of right-
or left-sided origin, has been investigated for its role in aiding
in predicting outcomes. OS following anti-EGFR monoclonal antibody
treatment with CALGB/SWOG80405 (19)
was significantly different between right- and left-sided origins.
In particular, OS with anti-EGFR monoclonal antibody treatment was
significantly poorer for right-sided origins and significantly
improved for left-sided origins. However, Holch et al
(20) reported a meta-analysis of OS
and progression-free survival of patients with unresectable mCRC
treated with CALGB/SWOG80405, FIRE-3 or PEAK (19,21,22). In
a comparison of anti-EGFR and anti-VEGF therapy, patients with RAS
wild-type left-sided origins received a markedly greater benefit
from anti-EGFR-based therapy. These aforementioned studies
(19,21,22) also
reported the analysis of overall response rates in terms of the
impact of primary tumor location on therapy with either anti-EGFR
or anti-VEGF antibodies combined with standard chemotherapy. The
results demonstrated a significantly improved overall response rate
with anti-EGFR monoclonal antibody treatment for tumors of
right-sided origins. These results also indicated that BRAF mutant,
microsatellite instability (MSI)-high, and CpG island methylator
phenotype-1 tumors are expected to occur more frequently in colon
cancer of right-sided origin. In the present case, the treatment
regimen of mFOLFOX6 plus cetuximab was effective despite the
right-sided origin of the colon cancer.
Therefore, combination chemotherapy and surgical
resection may potentially cure transverse colon cancer with
multiple paraaortic lymph node metastases. It is important to
evaluate the rate of tumor shrinkage from the beginning of the
first-line treatment until 6 courses of anti-EGFR monoclonal
antibody have been administered and to determine whether conversion
therapy (surgery) is possible (23).
We hypothesized that patients with different types of mCRC of
right-sided origin may be effectively treated with anti-EGFR
monoclonal antibodies. At present, patients with poor clinical
outcomes can be expected to receive another treatment regimen of
anti-VEGF monoclonal antibodies (24,25).
In conclusion, the regimen of mFOLFOX6 plus
cetuximab was effective in treating the patient with mCRC in the
present study, despite its right-sided origin. We hypothesized that
even mCRC of right-sided origin may be effectively treated with
anti-EGFR monoclonal antibody treatment at uniform rates.
Anti-PDL-1 antibody treatment is recommended for patients with
MSI-high tumors in the National Comprehensive Cancer Network and
European Society for Medical Oncology guidelines (26,27).
Therefore, not only Ras- and BRAF-type colorectal tumors, but also
tumors of every genomic type, may be treated in this manner in the
future.
Acknowledgements
The authors would like to thank Natsuko Suzui and
Tatsuhiko Miyazaki of the Pathology Division of Gifu University
Hospital for providing assistance with the preparation of the
manuscript.
Funding
Funding information is not applicable.
Availability of data and material
All data generated or analyzed during this study are
included in this published article.
Authors' contributions
TS and NM were responsible for study conception and
design. TS, NM, TTak, TTan, SM, HI, YT and KY were responsible for
acquisition of data. TS, NM and TTak were responsible for analysis
and interpretation of data. TS and NM were responsible for drafting
of the manuscript. TS, NM, TTak and KY were responsible for
critical revision of the manuscript. KY was responsible for
supervision of the study.
Ethics approval and consent to
participate
Written informed consent for participation in the
study or use of their tissue was obtained from the participant.
Patient consent for publication
Written informed consent was obtained from the
patient for publication of this case report and accompanying
images.
Competing interests
All authors declare that they have no competing
interest.
Glossary
Abbreviations
Abbreviations:
|
CT
|
computed tomography
|
|
EGFR
|
epidermal growth factor receptor
|
|
FDG
|
fluorine-18 fluorodeoxyglucose
|
|
mCRC
|
metastatic colorectal cancer
|
|
MSI
|
microsatellite instability
|
|
OS
|
overall survival
|
|
VEGF
|
vascular endothelial growth factor
|
References
|
1
|
Tournigand C, André T, Achille E, Lledo G,
Flesh M, Mery-Mignard D, Quinaux E, Couteau C, Buyse M, Ganem G, et
al: FOLFIRI followed by FOLFOX6 or the reverse sequence in advanced
colorectal cancer: A randomized GERCOR study. J Clin Oncol.
22:229–237. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Hurwitz H, Fehrenbacher L, Novotny W,
Cartwright T, Hainsworth J, Heim W, Berlin J, Baron A, Griffing S,
Holmgren E, et al: Bevacizumab plus irinotecan, fluorouracil, and
leucovorin for metastatic colorectal cancer. N Engl J Med.
350:2335–2342. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Saltz LB, Clarke S, Díaz-Rubio E,
Scheithauer W, Figer A, Wong R, Koski S, Lichinitser M, Yang TS,
Rivera F, et al: Bevacizumab in combination with oxaliplatin-based
chemotherapy as first-line therapy in metastatic colorectal cancer:
A randomized phase III study. J Clin Oncol. 26:2013–2019. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
De Roock W, Jonker DJ, Di Nicolantonio F,
Sartore-Bianchi A, Tu D, Siena S, Lamba S, Arena S, Frattini M,
Piessevaux H, et al: Association of KRAS p.G13D mutation with
outcome in patients with chemotherapy-refractory metastatic
colorectal cancer treated with cetuximab. JAMA. 304:1812–1820.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Van Cutsem E, Köhne CH, Láng I, Folprecht
G, Nowacki MP, Cascinu S, Shchepotin I, Maurel J, Cunningham D,
Tejpar S, et al: Cetuximab plus irinotecan, fluorouracil, and
leucovorin as first-line treatment for metastatic colorectal
cancer: Updated analysis of overall survival according to tumor
KRAS and BRAF mutation status. J Clin Oncol. 29:2011–2019. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Douillard JY, Oliner KS, Siena S,
Tabernero J, Burkes R, Barugel M, Humblet Y, Bodoky G, Cunningham
D, Jassem J, et al: Panitumumab-FOLFOX4 treatment and RAS mutations
in colorectal cancer. N Engl J Med. 369:1023–1034. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Folprecht G, Gruenberger T, Bechstein W,
Raab HR, Weitz J, Lordick F, Hartmann JT, Stoehlmacher-Williams J,
Lang H, Trarbach T, et al: Survival of patients with initially
unresectable colorectal liver metastases treated with
FOLFOX/cetuximab or FOLFIRI/cetuximab in a multidisciplinary
concept (CELIM study). Ann Oncol. 25:1018–1025. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Brierley JD, Gospodarowicz MK and
Wittekind C: TNM Classification of Malignant Tumours. 8th. Wiley
-Blackwell; London: 2017
|
|
9
|
Kopetz S, Chang GJ, Overman MJ, Eng C,
Sargent DJ, Larson DW, Grothey A, Vauthey JN, Nagorney DM and
McWilliams RR: Improved survival in metastatic colorectal cancer is
associated with adoption of hepatic resection and improved
chemotherapy. J Clin Oncol. 27:3677–3683. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Adam R, Delvart V, Pascal G, Valeanu A,
Castaing D, Azoulay D, Giacchetti S, Paule B, Kunstlinger F,
Ghémard O, et al: Rescue surgery for unresectable colorectal liver
metastases downstaged by chemotherapy: A model to predict long-term
survival. Ann Surg. 240:644–657; discussion 657-658.
2004.PubMed/NCBI
|
|
11
|
Tomlinson JS, Jarnagin WR, DeMatteo RP,
Fong Y, Kornprat P, Gonen M, Kemeny N, Brennan MF, Blumgart LH and
D'Angelica M: Actual 10-year survival after resection of colorectal
liver metastases defines cure. J Clin Oncol. 25:4575–4580. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Yedibela S, Klein P, Feuchter K, Hoffmann
M, Meyer T, Papadopoulos T, Göhl J and Hohenberger W: Surgical
management of pulmonary metastases from colorectal cancer in 153
patients. Ann Surg Oncol. 13:1538–1544. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Iida T, Nomori H, Shiba M, Nakajima J,
Okumura S, Horio H, Matsuguma H, Ikeda N, Yoshino I, Ozeki Y, et
al; Metastatic Lung Tumor Study Group of Japan, . Prognostic
factors after pulmonary metastasectomy for colorectal cancer and
rationale for determining surgical indications: A retrospective
analysis. Ann Surg. 257:1059–1064. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Min BS, Kim NK, Sohn SK, Cho CH, Lee KY
and Baik SH: Isolated paraaortic lymph-node recurrence after the
curative resection of colorectal carcinoma. J Surg Oncol.
97:136–140. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Choi PW, Kim HC, Kim AY, Jung SH, Yu CS
and Kim JC: Extensive lymphadenectomy in colorectal cancer with
isolated para-aortic lymph node metastasis below the level of renal
vessels. J Surg Oncol. 101:66–71. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Nakai N, Yamaguchi T, Kinugasa Y, Shiomi
A, Kagawa H, Yamakawa Y, Numata M and Furutani A: Long-term
outcomes after resection of para-aortic lymph node metastasis from
left-sided colon and rectal cancer. Int J Colorectal Dis.
32:999–1007. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Tsuji A, Sunakawa Y, Ichikawa W, Nakamura
M, Kochi M, Denda T, Yamaguchi T, Shimada K, Takagane A, Tani S, et
al: Early tumor shrinkage and depth of response as predictors of
favorable treatment outcomes in patients with metastatic colorectal
cancer treated with FOLFOX plus cetuximab (JACCRO CC-05). Target
Oncol. 11:799–806. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Ye LC, Wei Y, Zhu DX, Chen T and Xu J:
Impact of early tumor shrinkage on clinical outcome in
wild-type-KRAS colorectal liver metastases treated with cetuximab.
J Gastroenterol Hepatol. 30:674–679. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Venook AP, Niedzwiecki D, Lenz HJ,
Innocenti F, Fruth B, Meyerhardt JA, Schrag D, Greene C, O'Neil BH,
Atkins JN, et al: Effect of first-line chemotherapy combined with
cetuximab or bevacizumab on overall survival in patients with KRAS
wild-type advanced or metastatic colorectal cancer: A randomized
clinical trial. JAMA. 317:2392–2401. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Holch JW, Ricard I, Stintzing S, Modest DP
and Heinemann V: The relevance of primary tumour location in
patients with metastatic colorectal cancer: A meta-analysis of
first-line clinical trials. Eur J Cancer. 70:87–98. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Heinemann V, von Weikersthal LF, Decker T,
Kiani A, Vehling-Kaiser U, Al-Batran S-E, et al: FOLFIRI plus
cetuximab versus FOLFIRI plus bevacizumab as first-line treatment
for patients with metastatic colorectal cancer (FIRE-3): a
randomised, open-label, phase 3 trial. Lancet Oncol. 15:1065–1075.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Schwartzberg LS, Rivera F, Karthaus M,
Fasola G, Canon JL, Hecht JR, et al: PEAK: a randomized,
multicenter phase II study of panitumumab plus modified
fluorouracil, leucovorin, and oxaliplatin (mFOLFOX6) or bevacizumab
plus mFOLFOX6 in patients with previously untreated, unresectable,
wild-type KRAS exon 2 metastatic colorectal cancer. Journal of
clinical oncology: J Clin Oncol. 21:2240–2247. 2014. View Article : Google Scholar
|
|
23
|
Poston G, Adam R and Xu J, Byrne B, Esser
R, Malik H, Wasan H and Xu J: The role of cetuximab in converting
initially unresectable colorectal cancer liver metastases for
resection. Eur J Surg Oncol. 43:2001–2011. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Cremolini C, Loupakis F, Antoniotti C,
Lonardi S, Masi G, Salvatore L, Cortesi E, Tomasello G, Spadi R,
Zaniboni A, et al: Early tumor shrinkage and depth of response
predict long-term outcome in metastatic colorectal cancer patients
treated with first-line chemotherapy plus bevacizumab: Results from
phase III TRIBE trial by the Gruppo Oncologico del Nord Ovest. Ann
Oncol. 26:1188–1194. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Tomasello G, Petrelli F, Ghidini M, Russo
A, Passalacqua R and Barni S: FOLFOXIRI plus bevacizumab as
conversion therapy for patients with initially unresectable
metastatic colorectal cancer: A systematic review and pooled
analysis. JAMA Oncol. 3:e1702782017. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
NCCN: National Cancer Guidelines for
Patients. Colon Cancer. https://www.nccn.org/patients/guidelines/colon/September
8–2018
|
|
27
|
Van Cutsem E, Cervantes A, Adam R, Sobrero
A, Van Krieken JH, Aderka D; Aranda Primary lesion has almost
disappeared, ; Aguilar E, Bardelli A, Benson A, Bodoky G, et al:
ESMO consensus guidelines for the management of patients with
metastatic colorectal cancer. Ann Oncol. 27:1386–1422. 2016.
View Article : Google Scholar : PubMed/NCBI
|