Introduction
Wegener's granulomatosis (WG), recently renamed as
granulomatosis with polyangiitis (GPA), is an antineutrophil
cytoplasmic antibody (ANCA)-associated vasculitis (AAV), usually
affecting small- and medium-sized vessels. GPA often involves the
upper and lower airways or the kidneys, but various other systems
may be affected (1), such as the
central nervous system (CNS), albeit infrequently (2). Involvement of the CNS occurs in 2–8% of
the patients and it is most commonly characterized by cranial
neuropathy. Lower cranial nerve palsies are reported as unusual
manifestations occurring in the later stages of the disease and
have been associated with pachymeningitis of the skull base
(3). Prior to the use of
cyclophosphamide (CYC), this disease was fatal (4); however, the use of CYC and rituximab
(RTX) have ameliorated prognosis, inducing a response or remission
in >80% of the patients (5).
Unfortunately, these agents may induce a profound and long-lasting
immunosuppression due to the depletion of mature B lymphocytes and
hypogammaglobulinemia, which may explain the negativity for c-ANCA,
mainly during disease relapse (6).
In addition, development of secondary cancers has been described
during the disease course and some reports have implicated these
therapies in cancer development (7,8). We
herein report the case of a heavily treated WG patient who
developed multiple low cranial nerve palsies during radiotherapy
for metachronous glottic cancer. Due to severe
hypogammaglobulinemia and profound B-cell depletion, the c-ANCA
titre was not detectable. The patient experienced a fatal
progressive WG flare complicated by aspiration pneumonia and
intestinal perforation, and succumbed to the disease 6 months
later.
Case report
In August 2011, a 60-year-old Caucasian male patient
presented to S. Giuseppe Moscati Hospital (Taranto, Italy)
complaining of severe dysphonia. The patient's family and
psychosocial history were not relevant, while his medical history
included diabetes and WG diagnosed 10 years earlier on a lobectomy
specimen of the left lung. The course of the disease had been
characterized by recurrent episodes of otitis, sinusitis and
febrile pneumonia with hemoptysis during a long chronic course
associated with ANCA titre positivity. No renal, dermal, ocular or
neurological manifestations had been priorly reported. The patient
had been previously treated with steroids, methotrexate, CYC and
RTX, obtaining a good clinical benefit and c-ANCA titre control.
RTX had been prescribed as off-label therapy outside clinical
trials, and had been administered between March 2007 and July 2011,
1 month prior to the diagnosis of glottic cancer. Although the
titre of c-ANCA was <20 UR/ml at that time, the C-reactive
protein (CRP) level was 25 mg/l (normal value <8 mg/l), while
the albumin level was 3.5 mg/l; therefore, the Glasgow Score for
this patient was 1. The blood tests revealed a white blood cell
(WBC) count of 8,000/mm3 and lymphopenia (18%
lymphocytes). Blood urea nitrogen was normal, while the protein
level was 6 g/ml, with hypogammaglobulinemia (12%). On clinical
examination, the patient had no palpable lymph nodes in the neck,
and reported an inexplicable weight loss of >5 kg over 3 months.
On suspicion of laryngeal involvement by WG, laryngoscopy was
conducted and revealed a right vocal fold palsy and bilateral
glottic thickening with oedema and nodular spots in the mucosal
surface, mainly on the right vocal fold. The biopsy revealed
infiltrating G2 squamous cell carcinoma (SCC) of the glottis,
mainly involving the right false vocal fold, the ventricle and the
infraglottic space. The magnetic resonance imaging (MRI) on T1 and
T1 STIR sequences confirmed enlargement of the right vocal padded
fold and glottic space reduction, without metastases to the neck
lymph nodes (Fig. 1). The total body
computed tomography (CT) scan did not reveal distant metastases; in
the left lung, a fibrotic cavity was identified. The disease was
staged T3N0M0 according to the American Joint Committee on Cancer
2011 criteria. Laryngectomy was recommended, but the patient
refused surgery; therefore, he was referred for definitive
radiotherapy. Concomitant chemoradiotherapy was excluded to
minimize the risk of more acute complications considering the
autoimmune vasculitis, although this disease was in clinical and
serological remission at that time. Radiotherapy consisted of a 3D
conformal multiportal technique with multileaf
collimator-customized 6–10 MV photon beams. The planning target
volume included the larynx and neck lymph nodes (bilateral levels
II-III-IV) treated to a total dose of 50 Gy at 2 Gy/fr, followed by
a boost on the larynx to a 70 Gy total prescribed dose delivered
over 7 weeks. During the second week of treatment, the patient
started to complain of worsening dysphagia and weight loss,
recurrent febrile episodes and otalgia. The diagnosis was bilateral
otitis media and mild mucositis of the soft palate, and antibiotics
with steroids were prescribed. A laryngoscopy revealed a mild
mucositis with an initial regression of the visible glottic cancer
with stagnant saliva in the pyriform sinuses. Radiotherapy was
temporarily discontinued at 30 Gy delivered dose. A few days later
the patient developed prolonged febrile episodes (temperature of
39°C); the weight loss worsened, so he was admitted to the
hospital. An X-ray and CT scan of the chest revealed pneumonia in
the right lung, and the blood tests revealed leukocytosis (WBC
count 9,000/mm3) with 80% neutrophils and 18%
lymphocytes. Blood chemistry results included hypogammaglobulinemia
(8% of total, 3 g/ml), ferritin 512 ng/ml (range 22–322 ng/ml),
increased erythrocyte sedimentation rate to 100 mm/h (normal value
<21 mm/h) and CRP 40 ml/l. Haemofilus influenzae was found in
the sputum. Steroids, antifungal and antibiotic therapy were
effective and, after 1 week, the CT scan of the chest revealed
partial resolution of the pneumonia and radiotherapy was resumed. A
weight loss of >10 kg during the following 3 weeks and a
progressive lack of appetite with poor oral intake warranted
administration of parenteral nutrition via central venous access
and nutritional hypercaloric oral supplementation, but no clinical
benefit was observed. The dysphagia persisted, and a percutaneous
endoscopic gastrectomy (PEG) was ultimately required. At 50 Gy
delivered dose the patient underwent laryngoscopy, which revealed
fixation of the right hemilarynx, mucosal oedema and a fibrinous
secretion on the epiglottis. The swallowing test revealed loss of
the oropharyngeal reflex due to IX nerve impairment; in addition,
the palpatory elevation of the larynx was absent due to X and XI
nerve impairment. The methylene blue test revealed loss of the
cough reflex; the swallowing test for semisolid food revealed
liquid passing through the larynx and the first tracheal rings.
Palsy of the cranial nerves IX, X and XI was diagnosed, and enteral
nutrition through PEG was deemed mandatory. The cough reflex was
not evocable, and diaphragmatic impairment was observed, requiring
tracheostomy. Radiotherapy was again interrupted at 60 Gy delivered
dose. An MRI scan of the larynx was performed 1 month later, which
revealed oedema of the glottis with shrinkage of the initial cancer
(Fig. 2), confirmed by fibroscopic
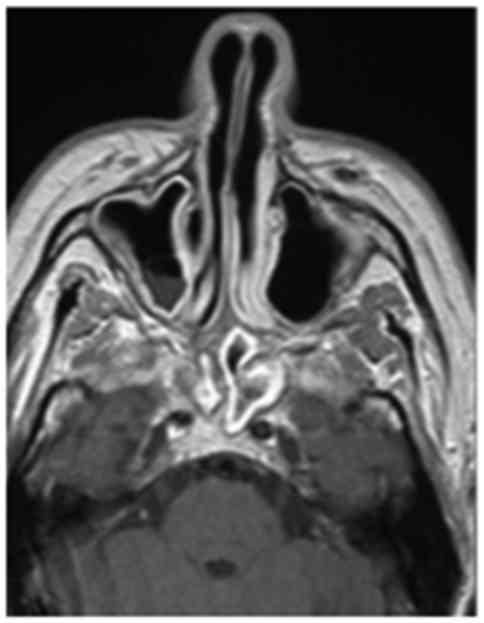
biopsy. The brain MRI with contrast enhancement performed at the
same time revealed sinusitis with concentric mucosal thickening of
the bilateral sphenoidal, ethmoidal and right maxillary sinuses,
with stagnant secretions (Fig. 3),
and a focal meningeal thickening of the skull base extending to the
mastoid processes below the jugular foramina (Fig. 4). The blood count revealed
leukocytosis (WBC 13,000/mm3) with absolute neutrophilia
and severe lymphopenia (14%). Blood biochemistry revealed severe
hypogammaglobulinemia (3.24% of total). The c-ANCA titre was 0.1
UR/ml. The immunophenotype of the lymphocytes involved complete
disappearance of the B-lymphocyte series; in particular, the value
of CD19+/CD20+ lymphocytes was zero. Three
months after radiotherapy was discontinued, the patient developed
aspiration pneumonia and later an intestinal perforation in the PEG
site. Finally, the patient succumbed at the end of April 2012 to
fatal complications of progressive ANCA-negative WG, including
peritonitis with ascites and thrombosis of the portal veins.
Discussion
WG is a rare autoimmune disease defined as a
systemic vasculitis affecting small- and medium-sized vessels
characterized by inflammatory granulomas with necrotizing
vasculitis (1). This disease is
usually associated with the presence of c-ANCA, which is an
established diagnostic marker of WG (9). ANCA-associated vasculitides affect both
sexes equally, with a mean age at diagnosis in the fifth decade of
life. Almost 93–98% of the patients are Caucasian or Hispanic,
although recent studies have reported an incidence in Japan similar
to that in the United Kingdom (10).
The estimated annual incidence varies from 2 to 12 cases per
million population, with a prevalence of 23–160 cases per million
population (11). According to the
Chapel Hill consensus conference in 2012, this disease has been
included in the group of small-vessel vasculitides as an
ANCA-associated vasculitis, and was renamed as GPA (12). As all ANCA-associated vasculitides,
GPA is a potentially life-threatening disease, depending on the
severity of the clinical manifestations. GPA predominantly affects
the upper and lower airways and the kidneys, but other organs may
be affected as well, such as the skin, CNS, eyeballs (often
presenting with proptosis), and ears (presenting with otitis with
progressive hearing loss). The systemic vasculitis form may be
lethal when renal or pulmonary involvement leads to alveolar
hemorrhage-related respiratory failure or necrotizing
glomerulonephritis (13). The
patient in the present case had a chronic disease lasting for
>10 years, with alternating relapses and remissions, with
initial respiratory involvement starting from the lung and
spreading to the paranasal sinuses. In GPA, the upper respiratory
tract symptoms, including rhinosinusitis or otitis media, are the
most frequent initial presentations, with a prevalence of >75%
(13). Moreover, the patient had
been heavily treated with the all immunosuppressive therapies
available until 2011, including steroids, methotrexate, CYC and
RTX. The latter was administered outside clinical trials as an
off-label modality for 5 years, until the diagnosis of metachronous
glottic cancer. It is unclear whether immunosuppressive therapy or
the granulomatosis per se were responsible for cancer development.
The patient was admitted to our institution with T3N0 glottic SCC
to receive definitive radiotherapy, as surgery had been rejected.
The testing for the gag reflex was normal and the patient did not
complain of systemic or local symptoms at the beginning of
radiation therapy. After delivery of 30 Gy within the first 3 weeks
of treatment, the patient developed clinical symptoms, such as
fever and inexplicable weight loss, which are described in 70–100%
of WG patients as the main initial symptoms (13); in the present case, there appeared to
be no association with the radiation treatment, as the delivered
dose was too low to explain the progressive weight loss. The
involuntary weight loss during head and neck cancer treatment is a
foreseeable event that may have different explanations, such as
tumor obstruction with swallowing difficulties, metabolic
alterations that may affect appetite, and tissue injury in the
irradiated area affecting the ability to eat (14). It has been reported that the weight
loss usually appears during the third week of radiotherapy, but
becomes most prominent towards the end of treatment in patients
treated with concomitant chemoradiotherapy for head and neck cancer
(15). Our patient had been treated
with radiotherapy alone, and complained of early progressive
dysphagia and marked weight loss during the 3rd week of treatment;
further specific tests revealed a swallowing impairment due to
lower cranial nerve palsies. These findings appeared too early to
be attributed to the radiation treatment; in addition, timing and
dosage were not supportive of this theory. However,
radiation-induced lower cranial nerve palsies (RINCP) may be
hypothesized in the present case, despite RINCP having been
reported as a long-term complication that appears more frequently
during treatment of nasopharyngeal carcinoma as a consequence of
radionecrosis of the temporal lobes, or a radiation dose to the
cranial nerves originating from the brain stem of >54 Gy
(16). In the present case,
neurological symptoms occurred at 30 Gy delivered dose as an acute
effect; moreover, after a revision of the treatment plan, the
maximum dose to the brain stem at the origin of the cranial nerves
was assessed, and was found to be <50 Gy.
Cranial nerve palsy due to CNS involvement by WG may
be hypothesized. The frequency of CNS involvement according to
various studies is 4–11% of WG cases (13). Dura mater infiltrates, cranial nerve
pathology and vasculitis are the most frequent CNS lesions
associated with clinical symptoms involving the cranial nerves,
such as paresthesia or motor function impairment (17). Our patient developed palsies of the
IX, X and XI cranial nerves originating from the bulbar brain stem
and crossing the neck through the jugular foramina (18). Lower cranial nerve palsies are
reported as an unusual occurrence, most commonly occurring in the
later stages of the disease, with unilateral distribution or
associated with pachymeningitis of the skull base (19). Severe dysphagia secondary to
paralysis of the lower cranial nerves and phrenic nerve involvement
followed by respiratory failure are described during the course of
this disease. The most possible cause of cranial nerve palsies in
our patient may be a direct extension of the granulomatosis into
the jugular foramina, possibly from the middle ear or paranasal
sinuses (20), resulting in
mechanical compression of the cranial nerves as documented by MRI.
As regards the role of c-ANCA in this patient, it remains
uncertain, as originally this patient had c-ANCA-positive disease,
but the c-ANCA titre was not detectable in the relapsed phase.
These antibodies are an established diagnostic tool for WG, more
easily used compared with biopsy (21), and are known to be a pathogenic
factor in inflammatory processes that underlie necrotizing
vasculitis (22). Although the
sensitivity of c-ANCA in active WG is up to 91%, with a specificity
of 99% (13), their prognostic role
as a marker of the disease remains unclear. Some authors have
reported a non-uniform response of the c-ANCA titre after RTX
treatment; remission with elevated c-ANCA titre or relapses with
undetectable c-ANCA have been recorded (23,24). In
the present case, the c-ANCA negativity may be attributed to the
past immunotherapies and the steroid administration during
radiation treatment (25). In fact,
after a long course of immunosuppressive therapies, such as CYC and
RTX, the c-ANCA titre may not be detectable due to the consequent
hypogammaglobulinemia and lymphopenia (26,27). In
our patient, the c-ANCA titre was not detectable, as there was
severe hypogammaglobulinemia and lymphopenia, and no mature B
lymphocytes (CD19+/CD20+) were found.
Furthermore, RTX is a chimeric monoclonal antibody against CD20
that depletes B-cells and was found to be effective in inducing and
maintaining remission of ANCA-associated vasculitis in randomized
controlled trials (28,29), so that it has been approved for the
treatment of rheumatoid arthritis and recently for induction
therapy of AAV. B lymphocytes are key to the pathogenesis of
autoimmune diseases by production of autoantibodies and
pro-inflammatory cytokines, and are targeted by RTX (30). As AAV frequently relapses, repeated
RTX infusions are used as maintenance treatment. However, as RTX
induces a long-lasting depletion of B-cells, it has been associated
with an altered B-cell maturation capacity (31). Furthermore, prolonged B-cell
depletion may lead to decreased antibody production, as assessed by
two retrospective studies in which severe infections and
hypogammaglobulinemia were frequent adverse events (26,27). Our
patient had been receiving RTX since 2007, albeit with an
unconventional schedule as an off-label therapy, developing severe
lymphopenia coinciding with radiotherapy and steroid
administration. Moreover, it is unclear whether the development of
glottic cancer should be considered a consequence of the heavy
immunosuppressive therapy or a late manifestation of WG. In fact,
there are some reports on rare cases of SCC of the nasal cavity
arising in patients with GPA (32,33), and
there is evidence supporting a high risk of cancer in these patient
due to immunosuppression, as recorded by the long-term post-trial
follow-up of the WGET cohort (34).
Whether radiotherapy may also exert an effect remains
controversial. The role of radiotherapy and chemoradiotherapy on
the local control, survival and organ sparing (35,36) in
laryngeal cancer is well-known. A diagnosis of collagen vascular
disease as GPA may predispose to high-grade radiation-induced
toxicity, although it has been demonstrated by a case control study
that radiotherapy is generally well-tolerated, but bears a higher
risk of severe late toxicity when delivered to the pelvis, as in
the setting of systemic lupus erythematosus or scleroderma
(37). It may be argued that
radiotherapy was not effective in this patient, but some studies
reported its efficacy in selected patients with solitary WG lesions
refractory to systemic therapy or involving critical organs. For
example, subglottic stenosis due to fibrosis in a WG patient who
was treated with effective low-dose radiation was described in a
previous case report (38).
Moreover, solitary granulomatosis lesions have successfully
resolved with low-dose radiotherapy, as reported in another case
report (39). In our patient, 60 Gy
delivered radiation dose was effective in reducing the
cancer-related glottic stenosis, as shown by the MRI sequences and
confirmed by biopsy, confirming its therapeutic effect (Fig. 2).
In conclusion, we herein described a case of glottic
cancer in a WG patient treated with radiotherapy alone. The patient
developed a fatal flare of the autoimmune disease soon after RTX
therapy interruption. The main finding was a c-ANCA-negative
relapse with lower cranial nerve palsies, affecting the swallowing
ability and leading to a marked weight loss during the radiation
treatment. Therefore, radiation treatment should be administered
with caution to patients affected by AAV who are in remission after
immunosuppressive therapy, as the swallowing function may be
compromised by acute radiation-related injury and neurological
complications may appear as a consequence of the relapse of this
collagen vascular disease.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
GL drafted the manuscript and administered
radiotherapy to this patient. ABV provided and revised the MRI
images. MDC conducted the swallowing tests and fibroscopic
examination. GB managed the medical therapy to this patient. GS
contributed to the critical review and supervised the entire work.
All authors have read and approved the final version of this
manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Written informed consent was obtained from the
patient for publication of this case report and any accompanying
images.
Competing interests
The authors declare that they have no competing
interests to disclose.
References
|
1
|
Bacon PA: The spectrum of Wegener's
granulomatosis and disease relapse. N Engl J Med. 352:330–332.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Kim SH, Park J, Bae JH, Cho MS, Park KD
and Jeong JH: ANCA-negative Wegener's granulomatosis with multiple
lower cranial nerve palsies. J Korean Med Sci. 28:1690–1696. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Armani M, Spinazzi M, Andrigo C, Fassina
A, Mantovan M and Tavolato B: Severe dysphagia in lower cranial
nerve involvement as the initial symptom of Wegener's
granulomatosis. J Neurol Sci. 263:187–190. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Fauci AS and Wolff SM: Wegener's
granulomatosis: Studies in eighteen patients and a review of the
literature. Medicine (Baltimore). 52:535–561. 1973. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Smith RM, Jones RB and Jayne DR: Progress
in treatment of ANCA-associated vasculitis. Arthritis Res Ther.
14:2102012. View
Article : Google Scholar : PubMed/NCBI
|
|
6
|
Venhoff N, Effelsberg NM, Salzer U,
Warnatz K, Peter HH, Lebrecht D, Schlesier M, Voll RE and Thiel J:
Impact of rituximab on immunoglobulin concentrations and B cell
numbers after cyclophosphamide treatment in patients with
ANCA-associated vasculitides. PLoS One. 7:e376262012. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Pankhurst T, Savage CO, Gordon C and
Harper L: Malignancy is increased in ANCA-associated vasculitis.
Rheumatology (Oxford). 43:1532–1535. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Faurschou M, Sorensen IJ, Mellemkjaer L,
Loft AG, Thomsen BS, Tvede N and Baslund B: Malignancies in
Wegener's granulomatosis: Incidence and relation to
cyclophosphamide therapy in a cohort of 293 patients. J Rheumatol.
35:100–105. 2008.PubMed/NCBI
|
|
9
|
Russell KA, Fass DN and Speks U: Clinical
and prognostic value of antineutrophil cytoplasmic antibodies in
Wegener's granulomatosis and microscopic polyangiitis : Comment on
the article by Russell et al. Arthritis and Rheumatology.
46:278–280. 2002. View Article : Google Scholar
|
|
10
|
Fujimoto S, Watts RA, Kobayashi S, Suzuki
K, Jayne DR, Scott DG, Hashimoto H and Nunoi H: Comparison of the
epidemiology of anti-neutrophil cytoplasmic antibody-associated
vasculitis between Japan and U.K. Rheumatology (Oxford).
50:1916–1920. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Mohammad AJ, Jacobsson LT, Westman KW,
Sturfelt G and Segelmark M: Incidence and survival rates in
Wegener's granulomatosis, microscopic polyangiitis, Churg-Strauss
syndrome and polyarteritis nodosa. Rheumatology (Oxford).
48:1560–1565. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Jennette JC, Falk RJ, Bacon PA, Basu N,
Cid MC, Ferrario F, Flores-Suarez LF, Gross WL, Guillevin L and
Hagen EC: 2012 revised International Chepel Hill Consensus
Conference Nomenclature of Vasculitides. Arthritis Rheum. 65:1–11.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Pagnoux C: Updates in ANCA-associated
vasculitis. Eur J Rheumatol. 3:122–133. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Capuano G, Grosso A, Gentile PC, Battista
M, Bianciardi F, Di Palma A, Pavese I, Satta F, Tosti M, Palladino
A, et al: Influence of weight loss on outcomes in patients with
head and neck cancer undergoing concomitant chemoradiotherapy. Head
Neck. 30:503–508. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Paccagnella A, Morello M, Da Mosto MC,
Baruffi C, Marcon ML, Gava A, Baggio V, Lamon S, Babare R, Rosti G,
et al: Early nutritional intervention improves treatment tolerance
and outcomes in head and neck cancer patients undergoing concurrent
chemoradiotherapy. Support Care Cancer. 18:837–845. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Luk YS, Shum JSF, Sze HCK, Chan LL, Ng WT
and Lee AW: Predictive factors and radiological features of
radiation-induced cranial nerve palsy in patients with
nasopharyngeal carcinoma following radical radiotherapy. Oral
Oncol. 49:49–54. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Cattaneo L, Chierici E, Pavone L,
Grasselli C, Manganelli P, Buzio C and Pavesi G: Peripheral
neuropathy in Wegener's granulomatosis, Churg-Strauss syndrome and
microscopic polyangiitis. J Neurol Neurosurg Psychiatry.
78:1119–1123. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Chiarugi G and Bucciante L: Istituzioni di
Anatomia dell'Uomo. 10th. Vallardi, Milan: pp. 719–743. 1968-1972,
(In Italian).
|
|
19
|
Nishino H, Rubino FA, DeRemee RA, Swanson
JW and Parisi JE: Neurological involvement in Wegener's
granulomatosis: An analysis of 324 consecutive patients at the Mayo
Clinic. Ann Neurol. 33:4–9. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Kashiyama T, Suzuki A and Mizuguchi K:
Wegener's granulomatosis with multiple cranial nerve involvements
as the initial clinical manifestations. Intern Med. 34:1110–1113.
1995. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Nölle B, Specks U, Lüdemann J, Rohrbach
MS, DeRemee RA and Gross WL: Anticytoplasmic autoantibodies: Their
immunodiagnostic value in Wegener granulomatosis. Ann Intern Med.
111:28–40. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Finkielman JD, Lee AS, Hummel AM, Viss MA,
Jacob GL, Homburger HA, Peikert T, Hoffman GS, Merkel PA, Spiera R,
et al: WGET Research Group: ANCA are detectable in nearly all
patients with active severe Wegener's granulomatosis. Am J Med.
120:643.e9–643.e14. 2007. View Article : Google Scholar
|
|
23
|
Aries PM, Hellmich B, Voswinkel J, Both M,
Nölle B, Holl-Ulrich K, Lamprecht P and Gross WL: Lack of efficacy
of rituximab in Wegener's granulomatosis with refractory
granulomatous manifestations. Ann Rheum Dis. 65:853–858. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Omdal R, Wildhagen K, Hansen T, Gunnarsson
R and Kristoffersen G: Anti-CD20 therapy of treatment-resistant
Wegener's granulomatosis: Favourable but temporary response. Scand
J Rheumatol. 34:229–232. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Hebert LA, Ardoin S and Shim RL: Rituximab
or cyclophosphamide in ANCA-associated renal vasculitis. N Engl J
Med. 363:2073–2074. 2010.PubMed/NCBI
|
|
26
|
Besada E, Koldingsnes W and Nossent JC:
Serum immunoglobulin levels and risk factors for
hypogammaglobulinaemia during long-term maintenance therapy with
rituximab in patients with granulomatosis with polyangiitis.
Rheumatology (Oxford). 53:1818–1824. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Jones RB, Ferraro AJ, Chaudhry AN, Brogan
P, Salama AD, Smith KGC, Savage CO and Jayne DR: A multicenter
survey of rituximab therapy for refractory antineutrophil
cytoplasmic antibody-associated vasculitis. Arthritis Rheum.
60:2156–2168. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Jones RB, Tervaert JW, Hauser T, Luqmani
R, Morgan MD, Peh CA, Savage CO, Segelmark M, Tesar V, van Paassen
P, et al; European vasculitis study group, . Rituximab versus
cyclophosphamide in ANCA-associated renal vasculitis. N Engl J Med.
363:211–220. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Stone JH, Merkel PA, Spiera R, Seo P,
Langford CA, Hoffman GS, Kallenberg CG, St Clair EW, Turkiewicz A,
Tchao NK, et al; RAVE-ITN Research Group, . Rituximab versus
cyclophosphamide for ANCA-associated vasculitis. N Engl J Med.
363:221–232. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Looney RJ: B cells as a therapeutic target
in autoimmune diseases other than rheumatoid arthritis.
Rheumatology (Oxford). 44 Suppl 2:ii13–ii17. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Thiel J, Rizzi M, Engesser M, Dufner AK,
Troilo A, Lorenzetti R, Voll RE and Venhoff N: B cell repopulation
kinetics after rituximab treatment in ANCA-associated vasculitides
compared to rheumatoid arthritis, and connective tissue diseases: A
longitudinal observational study on 120 patients. Arthritis Res
Ther. 19:1012017. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Stein J, Sridharan ST, Eliachar I, Niv A,
Wood B and Hoffman GS: Nasal cavity squamous cell carcinoma in
Wegener's granulomatosis. Arch Otolaryngol Head Neck Surg.
127:709–713. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Kuan EC, Peng KA, Gonzales LO and Sercarz
JA: A case of squamous cell carcinoma of the nasal cavity in a
patient with granulomatosis with polyangiitis (Wegener
granulomatosis). Ear Nose Throat J. 97:E37–E41. 2018.PubMed/NCBI
|
|
34
|
Silva F, Seo P, Schroeder D, Stone JH,
Merkel PA, Hoffman GS, Spiera R, Sebastian JK, Davis JC Jr, St
Clair EW, et al: Solid malignancies among patients with Wegener's
granulomatosis treated with Etarnercept: Long-term follow-up of a
multicenter longitudinal cohort. Arthritis Rheum. 63:2495–2503.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Pignon JP, le Maître A, Maillard E and
Bourhis J; MACH-NC Collaborative Group, . Meta-analysis of
chemotherapy in head and neck cancer (MACH-NC): An update on 93
randomised trials and 17,346 patients. Radiother Oncol. 92:4–14.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Forastiere AA, Zhang Q, Weber RS, Maor MH,
Goepfert H, Pajak TF, Morrison W, Glisson B, Trotti A, Ridge JA, et
al: Long-term results of RTOG 91-11: A comparison of three
nonsurgical treatment strategies to preserve the larynx in patients
with locally advanced larynx cancer. J Clin Oncol. 31:845–852.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Lin A, Abu-Isa E, Griffith KA and
Ben-Josef E: Toxicity of radiotherapy in patients with collagen
vascular disease. Cancer. 113:648–653. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Neviani CB, Carvalho HA, Hossamu C, Aisen
S and Nadalin W: Radiation therapy as an option for upper airway
obstruction due to Wegener's granulomatosis. Otolaryngol Head Neck
Surg. 126:195–196. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Wygoda A, Rutkowski T, Składowski K and
Hejduk B: Low dose radiotherapy as an effective treatment in a
patient with solitary Wegener's granulomatosis resistant to
systemic treatment - case report. Contemp Oncol (Pozn). 17:107–111.
2013.PubMed/NCBI
|