Introduction
Since development of Mac-2 binding protein
glycosylation isomer (M2BPGi) as a new serum biomarker for liver
fibrosis by Kuno et al (1),
it is now recognized as the most precise predictor of liver
fibrosis in chronic hepatitis as well as liver cirrhosis compared
with conventional fibrotic markers, such as the FIB-4 index and
hyaluronic acid (1,2). The presence of M2BPGi also may predict
the presence of hepatocellular carcinoma in patients with cirrhosis
(3,4). Since many studies using M2BPGi have
been conducted targeting liver diseases, the marker may be misled
as a specific for liver disease. However, recent studies have
indicated that M2BPGi is positively correlated with biliary
abnormalities (increased biliary enzymes and bile duct damage) in
patients with primary biliary cirrhosis (PBC) and primary
sclerosing cholangitis (PSC) (5,6). These
results implied a potential non-specific elevation of serum M2BPGi
level in patients with biliary disease.
In the current study, the aim was to clarify changes
in serum M2BPGi levels in non-cirrhotic patients with biliary
diseases.
Materials and methods
Patients and sample collection
Between April 2015 and December 2017, serum was
prospectively collected from 78 patients with pancreaticobiliary
diseases. Additionally, stored serum of 30 healthy volunteers was
used as control. All cancer cases were pathologically confirmed.
Patients with a history of chronic liver disease were excluded as
such diseases can affect the serum level of M2BPGi, as previously
described (1–4). Written, informed consent was obtained
from all patients and healthy volunteers. The study protocol
conformed to the ethical guidelines of the 1975 Declaration of
Helsinki and was approved by the Institutional Review Committee of
Fukushima Medical University (Fukushima, Japan).
Patient clinical data, including age, sex, serum
M2BPGi level and other serum laboratory data [aspartate
aminotransferase (AST), alanine aminotransferase (ALT),
γ-glutamyltranspeptidase (γ-GTP), alkaline phosphatase (ALP), total
bilirubin (TB), direct bilirubin (DB), c-reactive protein (CRP),
carcinoembryonic antigen (CEA) and carbohydrate antigen 19-9
(CA19-9)] were obtained from electronic medical records. Blood
samples were collected after obtaining informed consent, then
immediately processed to separate the serum and stored at
−20°C.
Measurement of M2BPGi levels
Serum (0.4 ml) was sent to a company (LSI Medience
Corporation, Tokyo, Japan) and the levels of M2BPGi in serum were
measured with a sandwich immunoassay as previously described
(1,2). Briefly, glycosylated M2BP was captured
by Wisteria floribunda agglutinin (WFA) that was immobilized
on magnetic beads. The bound product was assayed with an anti-human
M2BP monoclonal antibody linked to alkaline phosphatase. Two
reagent packs (M2BP-WFA detection pack and a chemiluminescence
substrate pack, Sysmex, Kobe, Japan) were loaded into an HISCL-5000
automated immunoassay machine (Sysmex, Kobe, Japan). The detection
pack comprised three reagents: A reaction buffer solution (R1), a
WFA-coated magnetic bead solution (R2) and an ALP-aM2BP solution
(R3). The chemiluminescence substrate reagent pack contained a
CDP-Star substrate solution (R4) and a stopping solution (R5).
Typically, serum (10 ml) was diluted to 60 ml with R1 and then
mixed with R2 (30 ml). Following the binding reaction, R3 (100 ml)
was added to the reaction solution. The resultant conjugates were
magnetically separated from unbound components, and mixed well with
R4 (50 ml) and R5 (100 ml) prior to reading of the fluorescence.
The chemiluminescent intensity was acquired within 17 min of the
aforementioned procedure. All counts were standardized and
converted to a cut-off index (COI) for M2BPGi (1).
Statistical analysis
Continuous variables (age and serum CEA, CA 19-9,
M2BPGi, AST, ALT, ALP, γ-GTP, TB and DB) are reported as the median
(interquartile range) values, and were compared with the
Mann-Whitney U test. Sex and the presence of biliary stricture were
compared with Fisher's exact probability tests. Correlations
between laboratory data and M2BPGi were analyzed with Spearman's
correlation analyses. Data are presented as the median and
interquartile range. All statistical analyses were performed with
GraphPad Prism 6.0 (GraphPad Software, Inc., La Jolla, CA, USA).
P<0.05 was considered to indicate a statistically significant
difference.
Results
We included 32 with biliary cancer (median age: 72.5
year-old, 22 male and 10 female) and 46 with benign diseases
(median age: 69.5 year-old, 35 male and 11 female) in this study
(Table I). While there were no
significant differences in age and sex (P=0.05 and P=0.13,
respectively), all laboratory data values were increased in
patients with a biliary tumor compared with the benign controls
(Table II).
 | Table I.Clinical characteristics of
patients. |
Table I.
Clinical characteristics of
patients.
| Characteristic | Benign disease
(n=46) | Biliary cancer
(n=32) | P-value |
|---|
| Age (years), median
(IQR) | 69.5 (64.0–79.0) | 72.5
(66.7–80.5) | 0.05 |
| Sex (M/F) | 35/11 |
24/10 | 0.13 |
| Background disease,
(n) | Bile stone (21) | Cholangiocarcinoma
(32) | NA |
|
| Chronic pancreatitis
(18) |
|
|
|
| Autoimmune
pancreatitis (4) |
|
|
|
| Not specified
(3) |
|
|
| Biliary stricture, n
(%) | 15 (32) | 32
(100) | <0.001 |
| Presence of liver
metastasis, n (%) | NA | 5
(14.7) | NA |
 | Table II.Comparison of laboratory data between
the benign disease and biliary cancer groups. |
Table II.
Comparison of laboratory data between
the benign disease and biliary cancer groups.
| Laboratory data | Benign disease
(n=46) | Biliary cancer
(n=32) | P-value |
|---|
| AST (U/L) | 24.0 (18.7–56.3) | 91.5
(50.0–131.0) | <0.0001 |
| ALT (U/L) | 22.5 (14.7–61.8) | 108 (50.7–194.5) | <0.0001 |
| γ-GTP (U/L) | 87.0
(29.0–313.0) | 544
(230.8–1066.0) | <0.0001 |
| ALP (U/L) | 293
(192.8–602.8) | 918.5
(521.8–1789) | <0.0001 |
| TB (mg/dL) | 0.9 (0.65–1.45) | 2.3 (0.97–10.4) | 0.0003 |
| DB (mg/dL) | 0.1 (0.1–0.4) | 0.9 (0.1–7.3) | 0.0019 |
| CRP (mg/dL) | 0.28 (0.05–1.54) | 0.89 (0.29–3.29) | 0.01 |
| CEA (ng/ml) | 2.0 (1.4–2.9) | 2.9 (1.67–4.92) | 0.03 |
| CA 19-9 (U/L) | 8.1 (4.0–25.7) | 66.9
(30.6–767.5) | <0.0001 |
| M2BPGi (COI) | 0.73 (0.41–1.1) | 1.91 (1.0–2.7) | <0.0001 |
Among all 78 patients, the serum M2BPGi level was
positively correlated with all variables (AST, ALT, ALP, TB and DB
levels, and γ-GTP, CRP, CEA and CA 19-9 levels; Table III). On the other hand, among 32
cases of biliary cancer, serum M2BPGi level was not correlated with
ALT, γ-GTP, CRP, CEA or CA 19-9 levels. Additionally, among 46
cases of benign disease, serum M2BPGi level was not correlated with
TB, CEA or CA 19-9 levels.
 | Table III.Correlation between serum Mac-2
binding protein glycosylation isomer levels and laboratory data in
78 patients with biliary diseases. |
Table III.
Correlation between serum Mac-2
binding protein glycosylation isomer levels and laboratory data in
78 patients with biliary diseases.
|
| All cases (n=78) | Biliary cancer
(n=32) | Benign disease
(n=46) |
|---|
|
|
|
|
|
|---|
| Serum laboratory
data | rho | P-value | rho | P-value | rho | P-value |
|---|
| AST | 0.53 | <0.0001 | 0.36 | 0.03 | 0.48 | 0.0008 |
| ALT | 0.5 | <0.0001 | 0.18 | 0.30 | 0.39 | 0.007 |
| γ-GTP | 0.52 | <0.0001 | 0.13 | 0.46 | 0.47 | 0.002 |
| ALP | 0.66 | <0.0001 | 0.36 | 0.03 | 0.60 | <0.0001 |
| TB | 0.47 | <0.0001 | 0.43 | 0.01 | 0.28 | 0.060 |
| DB | 0.48 | <0.0001 | 0.41 | 0.01 | 0.35 | 0.020 |
| CRP | 0.43 | 0.0001 | 0.28 | 0.11 | 0.41 | 0.006 |
| CEA | 0.25 | 0.030 | 0.27 | 0.11 | −0.04 | 0.78 |
| CA19-9 | 0.36 | 0.002 | 0.26 | 0.13 | 0.036 | 0.83 |
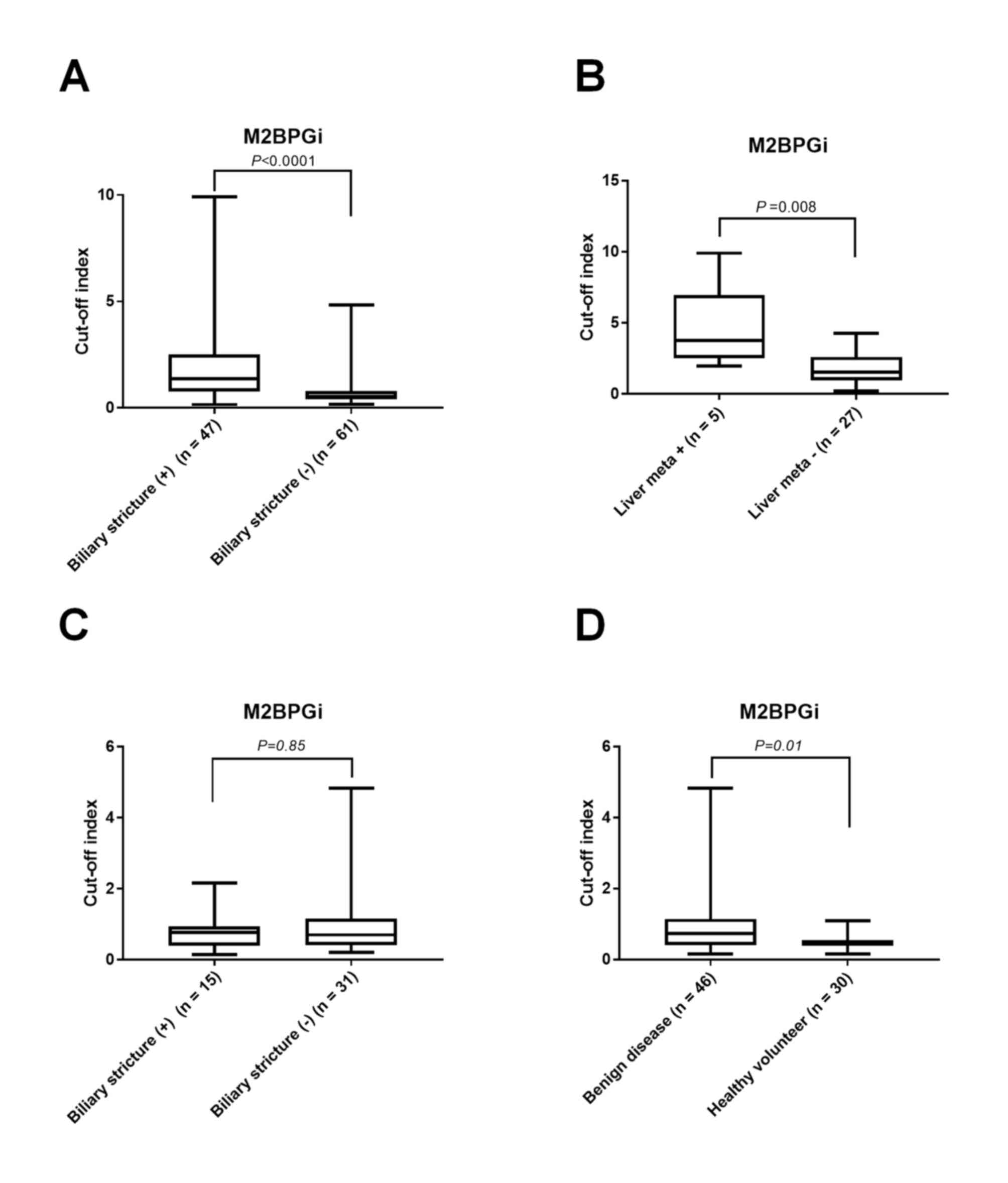
M2BPGi was increased in patients with biliary
strictures (COI, 1.36 vs. 0.53; P<0.0001; Fig. 1A) and liver metastases (COI, 3.75 vs.
1.53; P=0.008; Fig. 1B) compared
with cases without those findings. In benign disease, no
significant difference was identified in the serum level of M2BPGi
between 15 patients with biliary stricture and 31 patients without
biliary stricture (COI, 0.77 vs. 0.70; P=0.85; Fig. 1C), while the levels were higher in
cases of benign disease compared with healthy volunteer controls
(COI, 0.46; n=30; P=0.01; Fig.
1D).
Discussion
In the current study, changes in serum M2BPGi levels
in biliary diseases were investigated and identified to be
increased, along with the levels of abnormal hepatobiliary enzymes,
in both biliary tumor and benign biliary disease cases.
Additionally, the proportion of patients with extrahepatic biliary
stricture/obstruction was higher in patients with biliary tumors
compared with patients without biliary strictures (100 vs. 32%;
P<0.001). To the best of our knowledge, this was the first study
to report a non-specific elevation of serum M2BPGi levels in
non-cirrhotic patients.
The source of M2BPGi had been uncertain until Bekki
et al (7) first demonstrated
that hepatic stellate cells (HSCs) may be a source of M2BPGi in
liver cirrhosis. To clarify which liver cell subpopulation secreted
M2BPGi, the group measured M2BPGi levels in the cell culture
supernatant of primary HSCs, Kupffer cells, hepatocytes, biliary
epithelial cells and endothelial cells, and identified that HSCs
secreted M2BPGi. The group also identified that M2BPGi secreted
from HSCs induced expression of Mac-2 in Kupffer cells, which in
turn activated HSCs to be fibrogenic. These results could also
explain the elevated M2BPGi levels in other chronic hepatobiliary
diseases, including PSC and PBC, in which activation of HSCs has
been observed (8,9). Activation of HSCs is also induced
during acute liver injury and biliary obstruction (10–12).
This could explain why M2BPGi levels are increased in patients with
biliary diseases.
Furthermore, M2BPGi levels could be elevated in
fibrosis of other organs, including the heart (13), lung (14) and pancreas (15). Pancreatic ductal adenocarcinoma also
exhibited elevated M2BPGi levels compared with other
pancreaticobiliary diseases, which may reflect the desmoplastic
reaction in pancreatic ductal adenocarcinoma (16). This might be a reason why the serum
levels of M2BPGi in benign disease controls which included several
pancreatitis patients were higher than healthy controls.
The present study was limited by the relatively
small number of samples that were collected at a single
institution. Further studies with a larger number of patients are
required to consolidate the results of this preliminary study.
In conclusion, M2BPGi levels may be increased by
biliary obstruction. Therefore, elevated M2BPGi levels should be
interpreted carefully if patients with cirrhosis present with
concomitant diseases that may have an effect on M2BPGi
elevation.
Acknowledgements
The authors would like to thank Ms. Chikako Saito
and Ms. Rie Hikichi (Department of Gastroenterology, Fukushima
Medical University School of Medicine, Fukushima, Japan) for their
assistance during the experiments.
Funding
No funding was received.
Availability of data and materials
All data generated or analyzed during the present
study are included in this published article.
Authors' contributions
TT, RS designed the experiment. TT, RS, MS, NK, YS,
HI, KW, JN, MT, TH and HO performed the experiments. TT and RS
wrote the manuscript and analyzed the data.
Ethics approval and consent to
participate
The present study was approved by the Institutional
Review Committee of Fukushima Medical University School of Medicine
(Fukushima, Japan; IRB no. 2387) and patients provided written
informed consent.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Kuno A, Ikehara Y, Tanaka Y, Ito K,
Matsuda A, Sekiya S, Hige S, Sakamoto M, Kage M, Mizokami M, et al:
A serum ‘sweet-doughnut’ protein facilitates fibrosis evaluation
and therapy assessment in patients with viral hepatitis. Sci Rep.
3:10652013. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Narimatsu H: Development of M2BPGi: A
novel fibrosis serum glyco-biomarker for chronic
hepatitis/cirrhosis diagnostics. Expert Rev Proteomics. 12:683–693.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Toyoda H, Kumada T, Tada T, Kaneoka Y,
Maeda A, Korenaga M, Mizokami M and Narimatsu H: Serum
WFA+ -M2BP levels as a prognostic factor in patients
with early hepatocellular carcinoma undergoing curative resection.
Liver Int. 36:293–301. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Yamasaki K, Tateyama M, Abiru S, Komori A,
Nagaoka S, Saeki A, Hashimoto S, Sasaki R, Bekki S, Kugiyama Y, et
al: Elevated serum levels of Wisteria floribunda
agglutinin-positive human Mac-2 binding protein predict the
development of hepatocellular carcinoma in hepatitis C patients.
Hepatology. 60:1563–1570. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Umemura T, Joshita S, Sekiguchi T, Usami
Y, Shibata S, Kimura T, Komatsu M, Matsumoto A, Ota M and Tanaka E:
Serum Wisteria floribunda agglutinin-positive Mac-2-binding protein
level predicts liver fibrosis and prognosis in primary biliary
cirrhosis. Am J Gastroenterol. 110:857–864. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Umetsu S, Inui A, Sogo T, Komatsu H and
Fujisawa T: Usefulness of serum Wisteria floribunda
agglutinin-positive Mac-2 binding protein in children with primary
sclerosing cholangitis. Hepatol Res. 48:355–363. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Bekki Y, Yoshizumi T, Shimoda S, Itoh S,
Harimoto N, Ikegami T, Kuno A, Narimatsu H, Shirabe K and Maehara
Y: Hepatic stellate cells secreting WFA+ -M2BP: Its role
in biological interactions with Kupffer cells. J Gastroenterol
Hepatol. 32:1387–1393. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Tahashi Y, Matsuzaki K, Date M, Yoshida K,
Furukawa F, Sugano Y, Matsushita M, Himeno Y, Inagaki Y and Inoue
K: Differential regulation of TGF-β signal in hepatic stellate
cells between acute and chronic rat liver injury. Hepatology.
35:49–61. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Williams EJ, Gaça MD, Brigstock DR, Arthur
MJ and Benyon RC: Increased expression of connective tissue growth
factor in fibrotic human liver and in activated hepatic stellate
cells. J Hepatol. 32:754–761. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Iredale JP: Hepatic stellate cell behavior
during resolution of liver injury. Semin Liver Dis. 21:427–436.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Koeppel TA, Trauner M, Baas JC, Thies JC,
Schlosser SF, Post S, Gebhard MM, Herfarth C, Boyer JL and Otto G:
Extrahepatic biliary obstruction impairs microvascular perfusion
and increases leukocyte adhesion in rat liver. Hepatology.
26:1085–1091. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Morio K, Imamura M, Daijo K, Teraoka Y,
Honda F, Nakamura Y, Kobayashi T, Nakahara T, Nagaoki Y, Kawaoka T,
et al: Wisteria floribunda agglutinin positive Mac-2-binding
protein level increases in patients with acute liver injury. J
Gastroenterol. 52:1252–1257. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Okada A, Kanzaki H, Hamatani Y, Takashio
S, Takahama H, Amaki M, Hasegawa T, Sugano Y, Yasuda S and Anzai T:
Increased serum Wisteria floribunda agglutinin positive Mac-2
binding protein (Mac-2 binding protein glycosylation isomer) in
chronic heart failure: A pilot study. Heart Vessels. 33:385–392.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Kono M, Nakamura Y, Oyama Y, Mori K,
Hozumi H, Karayama M, Hashimoto D, Enomoto N, Fujisawa T, Inui N,
et al: Increased levels of serum Wisteria floribunda
agglutinin-positive Mac-2 binding protein in idiopathic pulmonary
fibrosis. Respir Med. 115:46–52. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Fujiyama T, Ito T, Ueda K, Tachibana Y,
Yasunaga K, Miki M, Takaoka T, Lee L, Kawabe K and Ogawa Y: Serum
levels of Wisteria floribunda agglutinin-positive Mac-2 binding
protein reflect the severity of chronic pancreatitis. J Dig Dis.
18:302–308. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Waragai Y, Suzuki R, Takagi T, Sugimoto M,
Asama H, Watanabe K, Kikuchi H, Hikichi T, Masamune A, Kang Y, et
al: Clinical significance of serum Wisteria floribunda
agglutinin-positive Mac-2 binding protein in pancreatic ductal
adenocarcinoma. Pancreatology. 16:1044–1050. 2016. View Article : Google Scholar : PubMed/NCBI
|