Introduction
Sympathetic paragangliomas (PGL) secreting
catecholamines are relatively rare neuroendocrine tumors arising
from sympathetic paraganglia and the adrenal medulla (known as
pheochromocytoma, PCC) (1,2). These tumors comprise specialized
neuroendocrine cells of neural crest origin (chromaffin cells),
which form multiple nodular structures surrounded by sustentacular
cells called ‘zellballen’. This organoid structure is lost during
malignant transformation.
One-third of PCC/PGL cases are associated with
inherited tumor syndromes, in which mutations have been identified
in more than 15 genes, including fumarate hydratase
(FH), MEN1, neurofibromatosis type-1 (NF1), RET,
succinate dehydrogenase (SDH)A, SDHB, SDHC, and SDHD
(3). These mutations occur in the
genes of germline and/or somatic cells (3–5). In
nonfamilial PCC/PGL tumors, 41% harbored mutations and one-fifth
were in germline genes, including multiple mutations (6). The major genetic abnormalities of
PCC/PGL have been divided into two major categories, such as the
pseudohypoxia-driven tumor and the kinase signaling subgroup.
Furthermore, a recent paper added two other subgroups, Wnt-altered
and cortical admixture subtypes (5).
Although the malignant phenotype with metastasis was reported to
account for 14 to 50% of all PCC/PGL cases, it remains difficult to
identify (2,7).
Gastrointestinal stromal tumor (GIST) is a
relatively rare tumor. The pathogenesis of most GISTs is a mutation
in one of two receptor tyrosine kinase genes, kit and
platelet-derived growth factor receptor α (PDGFRα)
(8). Although PCC/PGL and GIST are
uncommon neoplasms that occur mostly in a sporadic and isolated
form, they could be components of syndromes, such as
neurofibromatosis type 1 and the Carney triad (9).
A case of a rare and aggressive tumor in the
posterior mediastinum, which was initially thought to be GIST
arising from the esophagus, but was finally diagnosed as malignant
PGL by immunohistochemistry using a battery of different markers,
followed by genomic investigations using semiconductor-based
next-generation sequencing, is reported.
Materials and methods
DNA preparation
This study was approved by the Institutional Review
Board of Sapporo Shirakaba-dai Hospital. Formalin-fixed,
paraffin-embedded (FFPE) blocks were used for targeted sequencing.
To enrich the tumor content, FFPE tumor tissues were subjected to
macrodissection. DNA was isolated using the QIAamp DNA FFPE Tissue
kit (Qiagen, Hilden, Germany). Normal liver tissue from this case
was used as matched non-tumor control. The TaqMan RNase P Detection
Reagents kit (Thermo Fisher Scientific, Waltham, MA, USA) was used
to quantify purified DNA (10).
Semiconductor-based next-generation
sequencing
DNA (40 ng) was used for multiplex PCR amplification
with an Ion Ampliseq Comprehensive Cancer Panel, enabling the
targeted coverage of all exons of 409 cancer-related genes (Thermo
Fisher Scientific). The 15,992 amplicons obtained represented more
than 1.69 megabases of target sequence. Library preparation and
sequencing with the Ion Torrent sequencer were performed as
previously described (10). Somatic
mutations (single nucleotide mutations, insertions, and deletions)
and copy number variations (CNVs) were detected using statistical
approaches in tumor and normal samples from the Ion Reporter
software 5.0 tumor-normal workflow (Thermo Fisher Scientific).
Recurrent genomic regions with CNVs were identified using copy
numbers greater than 3 and less than 1 for gains and losses,
respectively. Germline DNA mutations were identified using the
Torrent Suite variant caller plugin 5.0 with high-stringency
germline parameters and annotated using Ion Reporter.
Case report
A 44-year-old man was admitted to Sapporo Medical
University Hospital with back pain, numbness of the legs, and a
gait disturbance. He had a three-year history of hypertension. He
was diagnosed with myeloparalysis due to spinal cord compression by
an invasive tumor of the posterior mediastinum. After he underwent
laminectomy (Th4-6) and posterior interbody fusion (Th4-9), he was
treated with steroid pulse therapy. The laboratory findings on
admission showed leukocytosis (1,380×106/l) with
granulocytosis (1,303×106/l) and hypoalbuminemia (3.6
mg/dl). Although only two tumor markers were elevated, Cypra at 3.1
ng/ml and neuron specific enolase (NSE) at 17.3 ng/ml, others were
normal, such as carcinoembryonic antigen (CEA) at 0.8 ng/ml, CA19-9
at 5.7 U/ml, α fetoprotein at 4 ng/ml, and interleukin-2 receptor
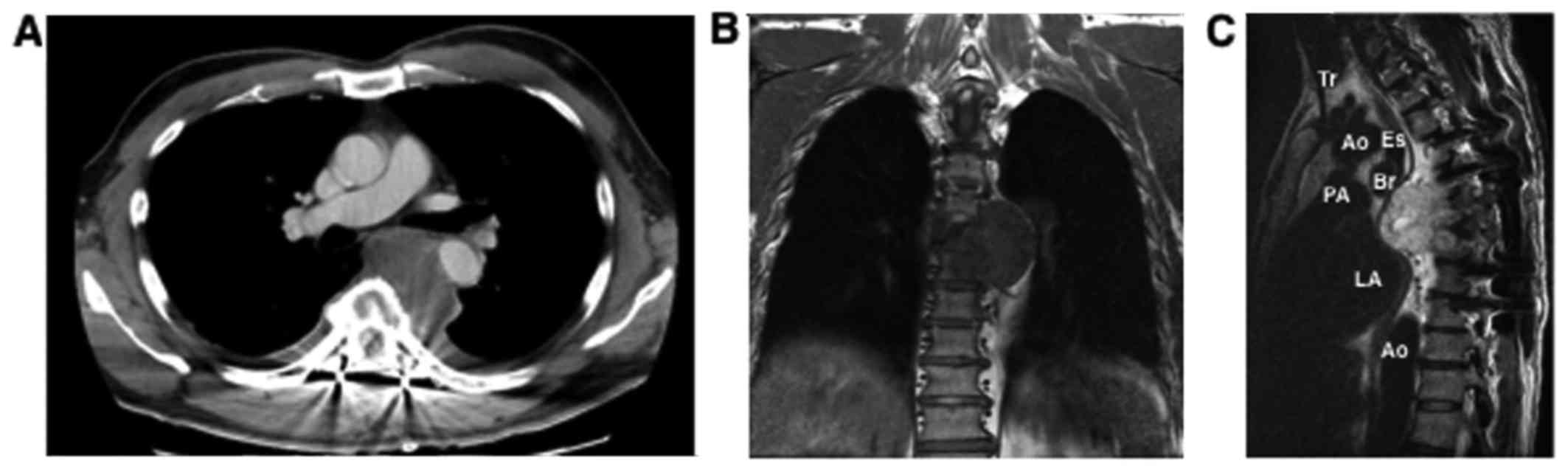
at 259 U/ml. Computed tomography (CT) showed a faintly enhanced
solid mass, 7 cm in diameter, that was surrounded by the trachea,
aorta, esophagus and thoracic vertebrae (Fig. 1). Magnetic resonance imaging (MRI)
showed heterogeneous T2-hyperintense signal tumors invading into
6th and 7th thoracic vertebral column. Since the tumor was located
on the left main bronchus, endobronchial ultrasound fine-needle
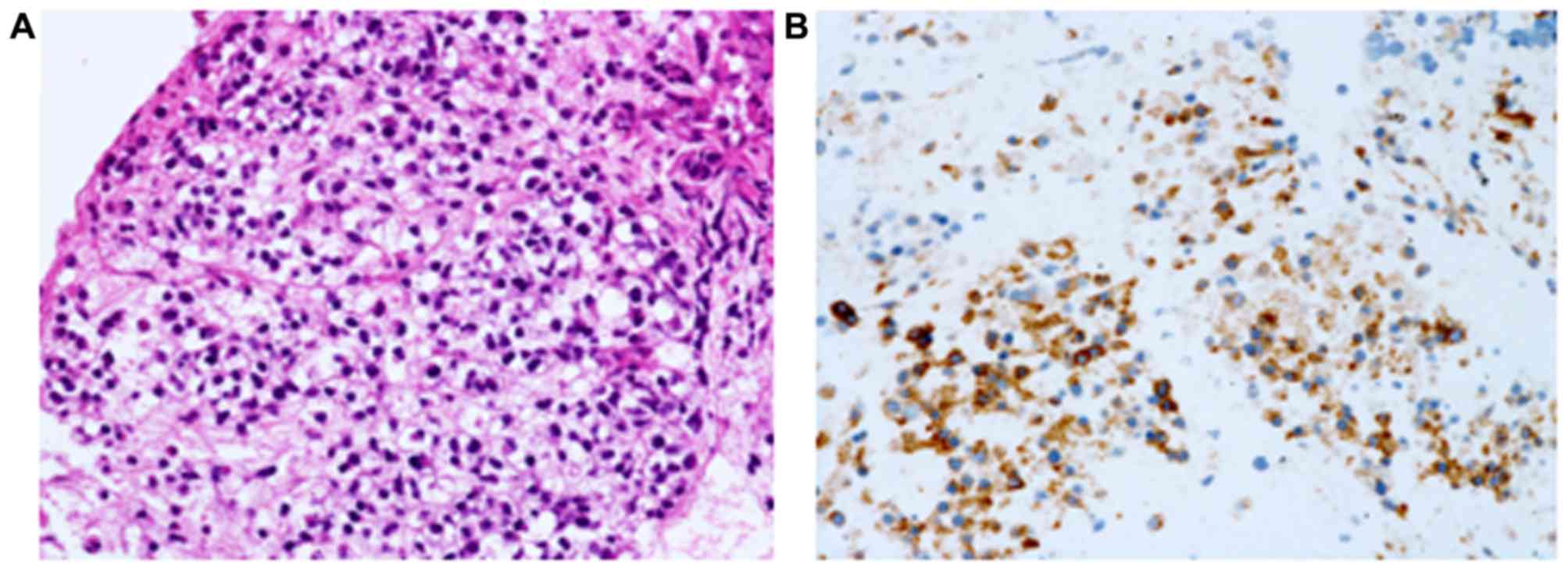
aspiration was performed. Epithelial and spindle-shaped tumor cells
showed alveolar growth and were positive for Ki67 (30%), c-kit, and
vimentin, but not for α-smooth muscle actin or S100 (Fig. 2, Table
I). Thus, he was diagnosed with GIST that probably originated
from the esophagus. After receiving radiation (40 Gy/16 Fr) for
pain relief, the size of the primary tumor shrank slightly, but
multiple lung metastases were detected. Thus, cT3N0M1, stage IV was
diagnosed according to UICC 7th. He was treated with 50 mg of
sunitinib and then he transferred to Sapporo Shirakaba-dai
Hospital. Although the primary tumor was unchanged after 2 courses
of sunitinib, the lung metastases were enlarged and there was a
bloody pleural effusion that increased (Fig. 3). He was then treated with 400 mg of
imatinib, followed by 160 mg of regorabfenib. The tumor, however,
progressed clinically and radiologically. The patient died after 8
months, and an autopsy was performed.
 | Table I.Antibodies and immunohistchemical
staining. |
Table I.
Antibodies and immunohistchemical
staining.
| Autopsy | Biopsy | Antibody | Clone | Vender | Dilution |
|---|
| ++ | ++ | Ki67 | MIB-1 | D | 1:100 |
| ++ | N/A | p53 | DO-7 | D | 1:50 |
| ++ | N/A | Neuron-specific
enolase (NSE) | Polyclonal | N | 1:1 |
| + | ++ | Vimentin | V9 | D | 1:1 |
| + | ++ | c-kit (CD117) | Polyclonal | D | 1:50 |
| + | − | Synaptophysin
(SP) | 27G12 | L | 1:50 |
| + | N/A | Neurofilament protein
(NFP) | 2F11 | D | 1:50 |
| + | N/A | Calretinin | DAK-Calret1 | D | 1:25 |
| + | N/A | WT1 | WT49 | L | 1:40 |
| − | + | CD34 | QBEnd10 | D | 1:50 |
| − | − | α smooth muscle
actin (αSMA) | 1A4 | D | 1:50 |
| − | − | chromogranin A
(CGA) | DAK-A3 | D | 1:100 |
| − | − | Cytokeratin
AE1/AE3 | AE1/AE3 | D | 1:1 |
| − | − | Discovered on
GIST-1 (DOG1) | K9 | L | 1:100 |
| − | − | Melanoma-associated
antigen, HMB45 | HMB45 | D | 1:50 |
| − | − | S100 | S100 | D | 1:500 |
| − | − | SOX10 | Polyclonal | C | 1:20 |
| − | − | Thyroid
transcription factor-1 (TTF1) | 8G7G3/1 | D | 1:50 |
| − | − | CD5 | CD5/54/F6 | D | 1:50 |
| − | − | CD10 | 56C6 | L | 1:40 |
| − | − | CD45 | 2B11+PD7/26 | D | 1:1 |
| − | − | CD56 | 123C3 | M | 1:10 |
| − | N/A | α phetoprotein
(AFP) | Polyclonal | D | 1:1,000 |
| − | N/A | Carcinoembryonic
antigen (CEA) | II-7 | D | 1:1,000 |
| − | N/A | Desmin (Des) | D33 | D | 1:50 |
| − | N/A | Low molecular
weight keratin (LMWK) | CAM5.2 | B | 1:1 |
| − | N/A | MDM2 | IF2 | I | 1:100 |
| − | N/A | Myogenic
differentiation 1 (myoD1) | 5.8A | D | 1:100 |
| − | N/A | OCT3 | C-10 | S | 1:100 |
| − | N/A | Podoplanin | D2-40 | D | 1:50 |
| − | N/A | Prostate-specific
antigen (PSA) | ER-PR8 | D | 1:500 |
| − | N/A | SALL4 | M03 | AN | 1:400 |
| − | N/A | Succinate
dehydrogenase-B (SDHB) | Polyclonal | AA | 1:700 |
| − | N/A | CD3 | F7.2.38 | D | 1:25 |
| − | N/A | CD20 | L26 | D | 1:200 |
| − | N/A | CD30 | Ber-H2 | D | 1:25 |
| − | N/A | CD68 | KP1 | L | 1:200 |
| − | N/A | CD163 | AM-3K | T | 1:25 |
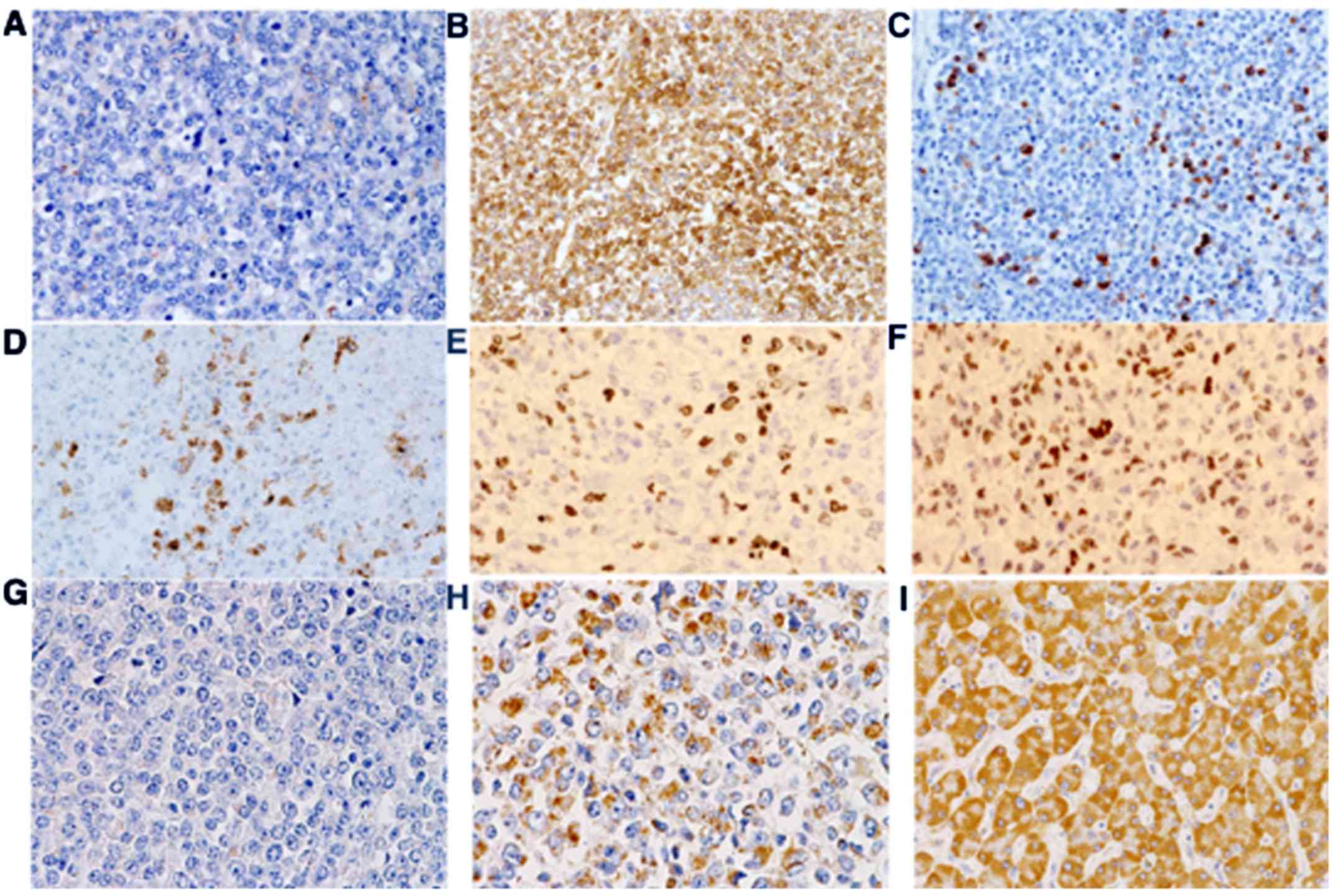
The primary mediastinal tumor had shrunk and was not
in contact with the esophagus. Necrosis and granulation tissue were
seen in the tumor. Multiple metastasized nodules were detected in
both lungs. The majority of tumor cells showed rounded, mid-size
cells (Fig. 4A-C). There were small
amounts of large and pleomorphic cells or mucous cells.
Immunohistochemical examination showed that the tumor cells were
positive for Ki67 and p53 (Fig. 5,
Table I). c-kit was positive in less
than 1% of tumor cells, with no staining for either DOG1 or CD34.
NSE was positive, and both synaptophysin and neurofilament were
positive in 10% of the tumor cells, but chromogranin-A was
negative. Thus, the diagnosis was malignant paraganglioma with
anaplasia. Although tumor cells in low-density nests expressed
succinate dehydrogenase (SDH)-B, those in high-density areas did
not show much expression (Fig. 5).
In addition, histological examination showed bilateral
adrenomedullary hyperplasia, which diffusely expanded adrenal
medulla and invaded partially into the adrenal cortex (Fig. 4D and E).
Semiconductor-based next-generation sequencing of
409 cancer-related genes showed both germline and somatic mutations
and CNVs of genes. Table II shows
that germline heterozygous variants in NF1 (p.Gln27His;
c.81G>T) and RET (p.Asn437Lys: c.1311C>G) were found.
Moreover, somatic mutations in NF1 (p.Arg1306Gln;
c.3917G>A) and FH (p.Ala104Thr; c.310G>A) were
identified with frequencies of 29.4 and 28.6%, respectively. CNV
analysis using sequencing data detected copy number gains
encompassing c-kit, NF1, and RET and copy number
losses encompassing FH and SDHB.
 | Table II.Summary of gene mutations. |
Table II.
Summary of gene mutations.
| Cell type | Gene | Locus | Mutation type | Protein change | Coding | SIFT | PolyPhen-2 | Grantham |
|---|
| Germline | RET | chr10:43606702 | Missense | p.Asn437Lys | c.1311C>G | 0.02 | 0.171 | 94 |
|
| NF1 | chr17:29483021 | Missense | p.Gln27His | c.81G>T | 0.27 | 0 | 24 |
| Somatic | NF1 | chr17:29562982 | Missense | p.Arg1306Gln | c.3917G>A | 0.15 | 0.323 | 43 |
|
| FH | chr1:241676971 | Missense | p.Ala104Thr | c.310G>A | 0 | 1 | 58 |
Discussion
First, in the present case it had to be determined
whether the neoplasm in question was malignant PGL or GIST. Genetic
mutation profiling of this tumor demonstrated that the tumor had
either germline or somatic mutations of NF1, RET, and
FH, but not of SDHs, kit or PDGFRa, suggesting
the tumor to be PGL, but not of GIST. The reason why the first
biopsied tumor material expressed c-kit might be sampling bias,
because PGL could express c-kit in some cases, and the biopsy could
have been taken from the highly c-kit expressed area of the tumor.
Alternatively, tyrosine kinase inhibitors might eliminate
c-kit-expressing cells, resulting in the very low expression of
c-kit in the residual neoplastic lesions at the time of autopsy,
despite the increased copy number of kit gene in these lesions. The
pulmonary metastasis showed a tiny area of c-kit-positive tumor
cells, which occupied less than 1% of the total tumor areas
examined. In addition, although both CD34 and DOG1 have been
reported to be expressed highly in GIST (8), but not in PGL, both molecules were not
expressed in both biopsied and autopsied tumor samples. This result
might support a diagnosis of PGL, not GIST, for this neoplasm.
Furthermore, the tumor cells highly expressed NSE and partially
expressed synaptophysin and neurofilament protein, supporting the
neuroendocrine origin of the tumor.
The primary mediastinal tumor was 7 cm in diameter
and might have had histological heterogeneity. Since fine-needle
aspiration could take only a limited part of the tumor, sampling
bias might have occurred and led to misdiagnosis. Larger biopsied
samples by surgical biopsy or multiple aspiration biopsies might be
necessary to make the true diagnosis. In addition, if larger
samples had been taken, genetic analysis by next-generation
sequencing might have been an option for making the diagnosis.
Highly expressed SDHB in the patient's hepatocytes
might indicate that there is no germline mutation of SDH group
genes. In addition, genetic studies demonstrated neither germline
nor somatic mutations of SDHs in this case. In addition to
the reduced number of mitochondria in the tumor cells as compared
with the hepatocytes, the reason why the pulmonary metastatic
lesions showed reduced expression of SDHB could be due to the
micro-environment surrounding the tumor cells, because a high cell
density area of the tumor showed reduced SDHB expression but the
low cell density area showed high expression in the tumor cells. In
addition, the tumor cells around the ischemic coagulation necrosis
also showed reduced SDHB expression. It might be possible to assume
from these observations that the SDHB expression in the tumor cells
would be dependent on the local oxygen content around the tumor
cells, since the SDH group enzymes are oxygen-dependent and located
within the mitochondria. With the observed reduced copy number of
SDHB gene in the tumor cells it is difficult to explain such
an uneven expression of SDHB in the tumor areas.
In order to identify causal gene mutations for
PCC/PGL without a syndromic presentation, next-generation
sequencing might be a promising strategy. Among mutated driver
genes for the induction of PGL in the present case, one must
consider the possible role of the receptor tyrosine-kinase
RET, which showed germline mutation and copy number gain in
the tumor cells. Extreme bilateral adrenomedullary hyperplasia,
which expanded diffusely in the adrenal medulla and partly invaded
into the adrenal cortex, was noted. In this connection, it is
noteworthy that the patient had hypertension. These conditions,
together with the occurrence of posterior mediastinal PGL, could
have been related to the RET abnormalities described.
Alternatively, it might be possible to presume that the PGL widely
metastasized into lungs might produce some neural growth factors
resulting in the bilateral adrenomedullary hyperplasia.
Malignant transformation of this PGL with a poor
outcome is intriguing (2). The tumor
cells showed high Ki67 labeling and p53 expression and metastasized
widely into lungs. The tumor lacked S100-positive sustentacular
cells, as consistently observed in benign PGL. Accumulation of
multiple gene alterations including NF1, RET, and FH
might be responsible for the malignant transformation of PGL in
this case. This might be supported by a report that metastatic
phenotype and multiple tumors were significantly more frequent in
PGL with FH mutations than in those without (11).
Neurogenic tumors account for around 20% of all
primary neoplasms in the mediastinum. In the mediastinum, PCC/PGL
occurs in around 1% of all tumors (12,13).
Similar to the present case, spinal cord compression was caused by
PCC/PGL (14,15).
Surgery is a standard therapy for PCC/PGL, and
either radiation or combination chemotherapy with cyclophosphamide,
vincristine, and dacarbazine is optional (16,17).
Tyrosine kinase inhibitors, such as sunitinib, are an additional
option (18,19). In this case, radiation might be
effective for the primary mediastinal tumor, but the effect of
targeted therapies for c-kit was limited.
In cases of histological examination of a small
piece of tumor, one should remember the possibility of sampling
bias and misdiagnosis. In order to detect causal gene alterations
for PCC/PGL without a syndromic presentation, next-generation
sequencing might be a promising approach. In the present case,
there were several gene alterations with CNV, such as ‘two hits’ in
the NF1, in both germline and somatic cells, a germline
variant in RET, and a somatic mutation in FH. They
might affect the pathogenesis of this malignant phenotype of PGL.
Thus, assessment for these precise effects of each gene alteration
will be needed.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Authors' contributions
YA designed the present study and wrote the
manuscript mainly. HM analyzed the clinical and published data. YS
analyzed genetic data and critically revised the manuscript. RH,
MN, TK and YY were part of the gastrointestinal treatment team that
treated the present case in Sapporo Shirakaba-dai Hospital. KO and
KY were part of the gastrointestinal treatment team that treated
the present case in Sapporo Medical University Hospital. YI
analyzed pathological data and critically revised the manuscript.
TE designed the present study and critically revised the
manuscript.
Ethics approval and consent to
participate
This study was approved by the Institutional Review
Board of Sapporo Shirakaba-dai Hospital.
Patient consent for publication
The patient's family provided consent for
publication of the data.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
CEA
|
carcinoembryonic antigen
|
|
CNV
|
copy number variation
|
|
CT
|
computed tomography
|
|
FFPE
|
formalin-fixed, paraffin-embedded
|
|
FH
|
fumarate hydratase
|
|
GIST
|
gastrointestinal stromal tumor
|
|
MRI
|
magnetic resonance imaging
|
|
NF1
|
neurofibromatosis type-1
|
|
NSE
|
neuron specific enolase
|
|
PCC
|
pheochromocytoma
|
|
PDGFRα
|
platelet-derived growth factor
receptor α
|
|
PGL
|
paraganglioma
|
|
SDH
|
succinate dehydrogenase
|
References
|
1
|
Jochmanova I and Pacak K:
Pheochromocytoma: The first metabolic endocrine cancer. Clin Cancer
Res. 22:5001–5011. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Hamidi O, Young WF Jr, Gruber L, Smestad
J, Yan Q, Ponce OJ, Prokop L, Murad MH and Bancos I: Outcomes of
patients with metastatic phaeochromocytoma and paraganglioma: A
systematic review and meta-analysis. Clin Endocrinol (Oxf).
87:440–450. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Neumann HP, Bausch B, McWhinney SR, Bender
BU, Gimm O, Franke G, Schipper J, Klisch J, Altehoefer C, Zerres K,
et al: Germ-line mutations in nonsyndromic pheochromocytoma. N Engl
J Med. 346:1459–1466. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Currás-Freixes M, Inglada-Pérez L,
Mancikova V, Montero-Conde C, Letón R, Comino-Méndez I,
Apellániz-Ruiz M, Sánchez-Barroso L, Aguirre Sánchez-Covisa M,
Alcázar V, et al: Recommendations for somatic and germline genetic
testing of single pheochromocytoma and paraganglioma based on
findings from a series of 329 patients. J Med Genet. 52:647–656.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Fishbein L, Leshchiner I, Walter V,
Danilova L, Robertson AG, Johnson AR, Lichtenberg TM, Murray BA,
Ghayee HK, Else T, et al: Comprehensive molecular characterization
of pheochromocytoma and paraganglioma. Cancer Cell. 31:181–193.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Welander J, Andreasson A, Juhlin CC,
Wiseman RW, Backdahl M, Höög A, Larsson C, Gimm O and Söderkvist P:
Rare germline mutations identified by targeted next-generation
sequencing of susceptibility genes in pheochromocytoma and
paraganglioma. J Clin Endocrinol Metab. 99:E1352–E1360. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Turchini J, Cheung VKY, Tischler AS, De
Krijger RR and Gill AJ: Pathology and genetics of phaeochromocytoma
and paraganglioma. Histopathology. 72:97–105. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Wong NA: Gastrointestinal stromal
tumours-an update for histopathologists. Histopathology.
59:807–821. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Perry CG, Young WF Jr, McWhinney SR, Bei
T, Stergiopoulos S, Knudson RA, Ketterling RP, Eng C, Stratakis CA
and Carney JA: Functioning paraganglioma and gastrointestinal
stromal tumor of the jejunum in three women: Syndrome or
coincidence. Am J Surg Pathol. 30:42–49. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Nakagaki T, Tamura M, Kobashi K, Koyama R,
Fukushima H, Ohashi T, Idogawa M, Ogi K, Hiratsuka H, Tokino T and
Sasaki Y: Profiling cancer-related gene mutations in oral squamous
cell carcinoma from Japanese patients by targeted amplicon
sequencing. Oncotarget. 8:59113–59122. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Castro-Vega LJ, Buffet A, De Cubas AA,
Cascón A, Menara M, Khalifa E, Amar L, Azriel S, Bourdeau I, Chabre
O, et al: Germline mutations in FH confer predisposition to
malignant pheochromocytomas and paragangliomas. Hum Mol Genet.
23:2440–2446. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Azarow KS, Pearl RH, Zurcher R, Edwards FH
and Cohen AJ: Primary mediastinal masses. A comparison of adult and
pediatric populations. J Thorac Cardiovasc Surg. 106:67–72.
1993.PubMed/NCBI
|
|
13
|
Takeda S, Miyoshi S, Minami M and Matsuda
H: Intrathoracic neurogenic tumors-50 years' experience in a
Japanese institution. Eur J Cardiothorac Surg. 26:807–812. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Reyes MG, Fresco R and Bruetman ME:
Mediastinal paraganglioma causing spinal cord compression. J Neurol
Neurosurg Psychiatry. 40:276–279. 1977. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Moran CA, Suster S, Fishback N and Koss
MN: Mediastinal paragangliomas. A clinicopathologic and
immunohistochemical study of 16 cases. Cancer. 72:2358–2364. 1993.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Garibaldi E, Bresciani S, Panaia R,
Delmastro E, Malinverni G and Gabriele P: Hereditary paraganglioma
syndrome associated with SDHD gene mutations: A patient with
multicentric presentation treated with radiotherapy. Case report.
Tumori. 97:214–220. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Baudin E, Habra MA, Deschamps F, Cote G,
Dumont F, Cabanillas M, Arfi-Roufe J, Berdelou A, Moon B, Al
Ghuzlan A, et al: Therapy of endocrine disease: Treatment of
malignant pheochromocytoma and paraganglioma. Eur J Endocrinol.
171:R111–R122. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Ayala-Ramirez M, Chougnet CN, Habra MA,
Palmer JL, Leboulleux S, Cabanillas ME, Caramella C, Anderson P, Al
Ghuzlan A, Waguespack SG, et al: Treatment with sunitinib for
patients with progressive metastatic pheochromocytomas and
sympathetic paragangliomas. J Clin Endocrinol Metab. 97:4040–4050.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Cassol CA, Winer D, Liu W, Guo M, Ezzat S
and Asa SL: Tyrosine kinase receptors as molecular targets in
pheochromocytomas and paragangliomas. Mod Pathol. 27:1050–1062.
2014. View Article : Google Scholar : PubMed/NCBI
|