Introduction
Gastric cancer is a common malignant disease with
high incidence and mortality rates worldwide (1). Scirrhous gastric cancer accounts for 10
20% of all gastric cancers and is characterized by an abundant
stroma in comparison with medullary carcinomas, which are
characterized by scant stroma (2).
Despite recent advances in the diagnosis and treatment of gastric
cancer, the prognosis of the affected population remains poor. The
clinicopathological characteristics of this population include
poorly differentiated and diffusely infiltrating tumor cells that
disseminate to the peritoneum and retroperitoneum, where they
induce fibrosis (3,4). This type of dissemination accompanied
by fibrosis frequently causes bowel obstruction, obstructive
jaundice, and retroperitoneal fibrosis (RPF), which lead to
ureteral obstruction and renal failure (5). Organ dysfunction progresses and the
performance status of patients in this population worsens rapidly
because of the rapid progression of this cancer; it then becomes
difficult to administer chemotherapy, and thus these patients have
a poor prognosis (6,7). Therefore, earlier diagnosis and
treatment may be especially important for this population. Although
it appears that the clinical course of scirrhous gastric cancer is
not well understood by general physicians; despite the lack of
clear evidence and reports on systemic chemotherapy in these
patients, experienced gastrointestinal oncologists, who are already
experienced can share information about the effectiveness of the
early administration of systemic chemotherapy in these cases.
Experienced oncologists seldom hesitate to initiate systemic
chemotherapy before disease progression in these patients.
Here, we report a patient who suffered from organ
dysfunction, including acute renal dysfunction with RPF due to
scirrhous gastric cancer, and who previously underwent hemodialysis
(HD). During the time the patient underwent HD, 5-fluorouracil/l
leucovorin (5-FU/l LV) was administered and was efficacious; this
contributed to his withdrawal from HD and an improvement in his
performance status, despite his poor condition. We suggest that it
will be better for physicians to consider the administration of
systemic chemotherapy before supportive HD was introduced and
before his general condition worsened.
Case report
A 68-year-old Japanese male who was diagnosed with
scirrhous gastric cancer with peritoneal and retroperitoneal
dissemination was referred to our hospital for further treatment.
His medical history was unremarkable. Scirrhous gastric cancer was
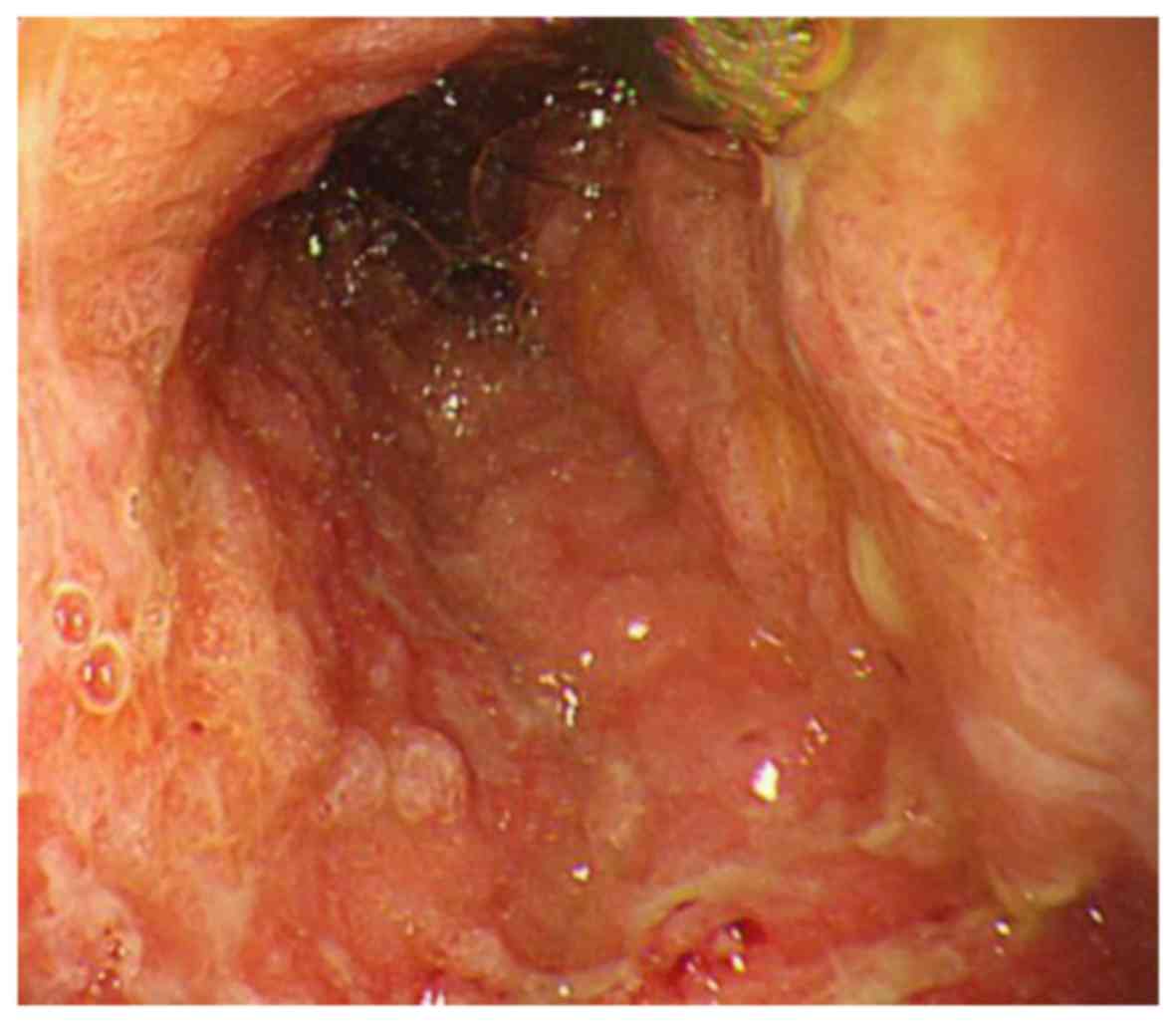
identified by esophagogastroduodenoscopy as shown in Fig. 1. The histological diagnosis was
poorly differentiated adenocarcinoma, which was HER2 negative by
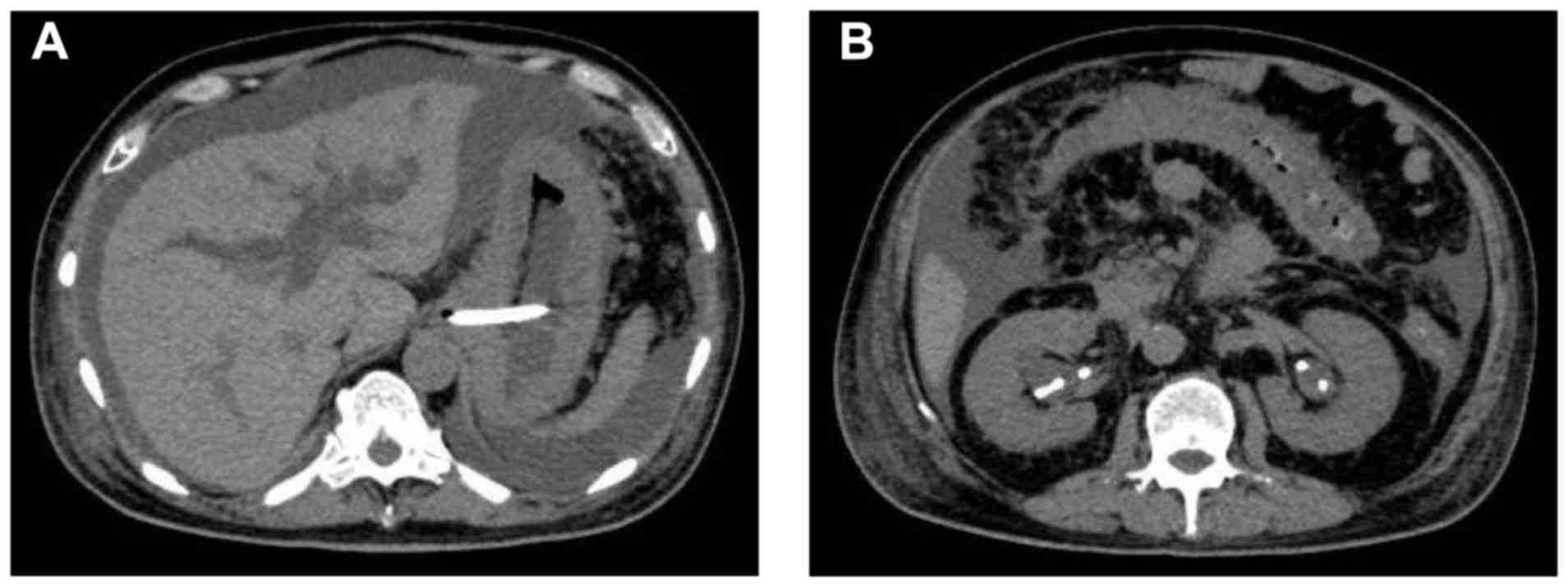
immunohistochemistry. Abdominal computed tomography (CT) showed
gastric wall thickening, moderate ascites, bilateral
hydronephrosis, and intrahepatic bile duct dilatation (Fig. 2). When he was previously treated at
another hospital, the renal and hepatic function were initially
normal but were elevated as time progressed; he then became anuric
and exhibited obstructive jaundice. He became anuric after stenting
for ureteral obstruction, and thus HD was initiated. Furthermore,
he was unable to receive food or medication orally because of bowel
obstruction by peritoneal metastasis. He was then transferred to
our hospital, and the laboratory data obtained upon admission are
listed in Table I. The serum
creatinine and total bilirubin levels were high at 11.1 and 15.5
mg/dl, respectively. In Keio University Hospital (Tokyo, Japan), we
performed percutaneous transhepatic biliary drainage, and
consequently, the patient's jaundice improved gradually. Although
his performance status was poor because of the placement of several
medically necessary tubes, there were no contraindications, such as
active infection, for systemic chemotherapy. We shared this medical
information with the patient and his family and recommended the
best supportive care. However, they were eager to learn about any
possible treatments that might be recommended, even those with
risks. After several days of careful planning, we proposed weekly
5-FU/l-LV and modified to 80% dose of 5-FU(5-FU 500
mg/m2, l-LV 250 mg/m2 on days 1, 8, 15, 22,
29 and 36; 8 weeks/cycle), which has shown non-inferiority to oral
fluoropyrimidines against metastatic gastric cancer, as the
recommended treatment. This regimen would be useful with a small
fluid volume compared to other 5-FU regimens (8).
 | Table I.Laboratory data on admission. |
Table I.
Laboratory data on admission.
| Category | Value | Unit |
|---|
| WBCs |
13.5×103 | /µl |
| RBCs |
376×104 | /µl |
| Hb | 10.4 | g/dl |
| Hct | 29.7 | % |
| Plt |
59.3×104 | /µl |
| TP | 5.9 | g/dl |
| Alb | 1.7 | g/dl |
| BUN | 50.9 | mg/dl |
| Cr | 11.1 | mg/dl |
| Na | 133.6 | mEq/l |
| K | 4.7 | mEq/l |
| Cl | 96 | mEq/l |
| T.bil | 15.5 | mg/dl |
| D.bil | 12.9 | mg/dl |
| AST | 718 | IU/l |
| ALT | 341 | IU/l |
| LDH | 374 | IU/l |
| ALP | 5232 | IU/l |
| γ-GTP | 630 | IU/l |
| CRP | 5.7 | mg/dl |
| CEA | 5.9 | ng/ml |
| CA19-9 | 38.1 | ng/ml |
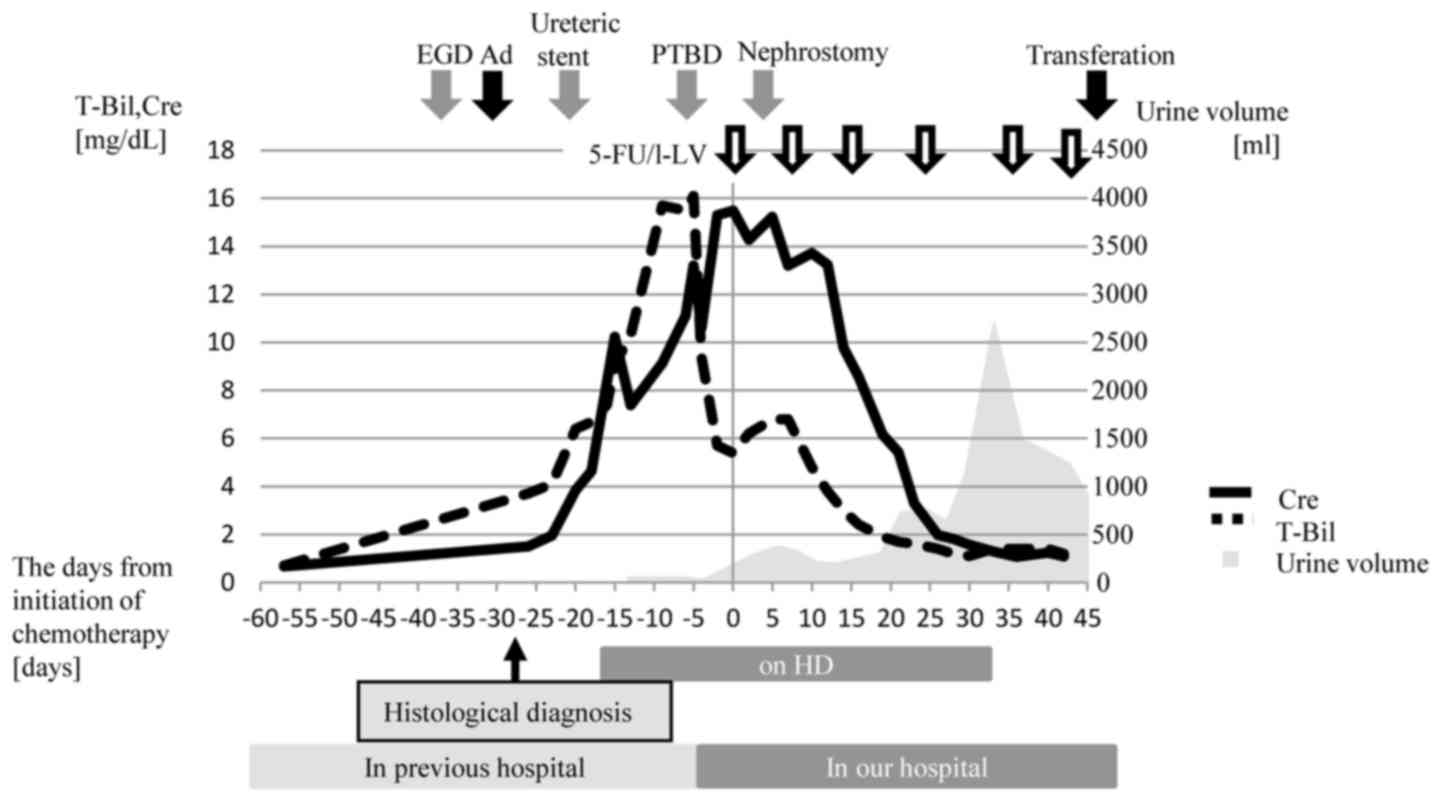
We then initiated 5-FU/l LV treatment with
supportive care. Despite the placement of a nephrostomy tube on day
3 after the initiation of chemotherapy, the patient's renal
function did not improve, and we continued HD. This suggested that
his renal dysfunction was not only by postrenal but also prerenal
factor, intravenous dehydration due to malignant ascites and the
relative lack of fluid volume. Then we increased the amount of
fluid volume, the urine volume from the nephrostomy tube and also
transurethral volume had gradually increased, from day 23 after
chemotherapy. It appeared that both chemotherapy and supportive
care were effective for his prerenal and postrenal dysfunction due
to improve of ascites and RPF. Finally, he ended treatment with HD
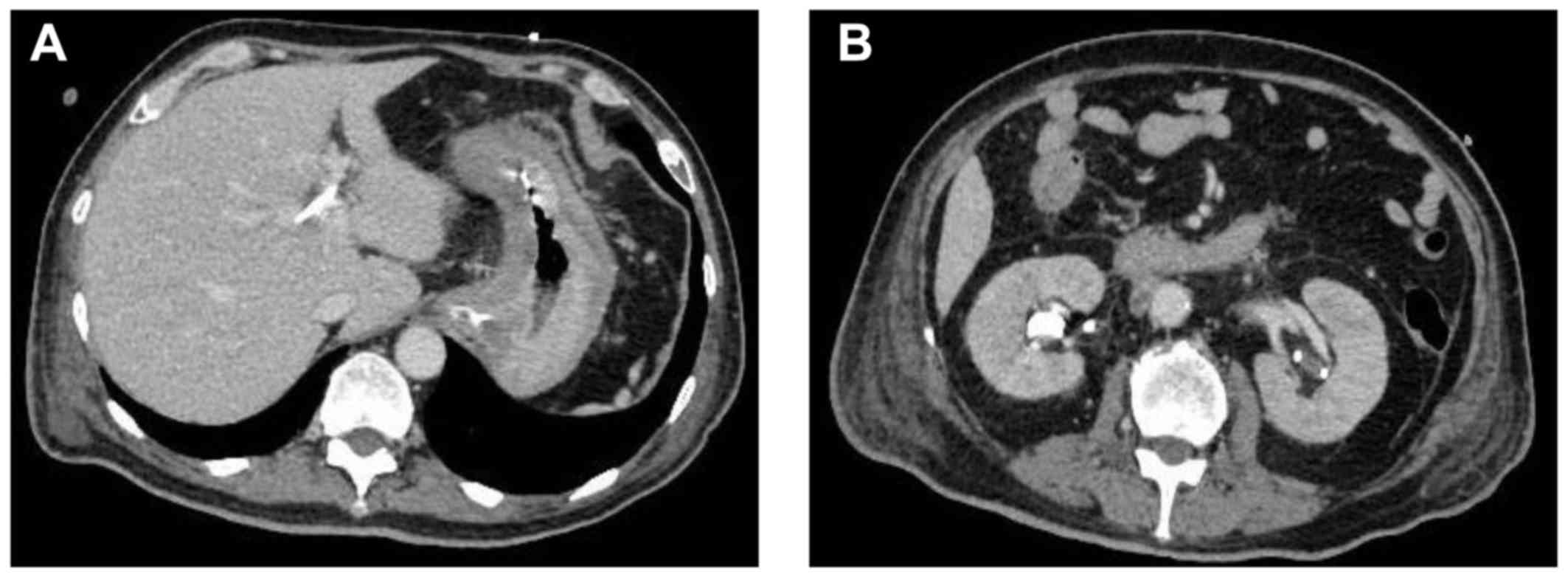
on day 28. On day 30, abdominal CT revealed the disappearance of
ascites, shrinkage of a swollen lymph node, and improvement of
hydronephrosis (Fig. 3). The effect
of 5-FU/l LV was evaluated as non-complete response/non-progressive
disease (non-CR/non-PD) in accordance with the Response Evaluation
Criteria in Solid Tumors version 1.1 (9). No severe adverse event was observed
according to the Common Terminology Criteria for Adverse Events
(CTCAE) version 4 (10). He was then
able to consume food orally, which resulted in an improvement in
his general condition. As a result, he returned to the previous
hospital on day 45 (Fig. 4). He
continued 5-FU/l LV therapy for several months, and at the first
progression at 3 months, subsequent systemic treatment was given.
Finally, 11 months later, the patient died of gastric cancer after
the cessation of HD.
Discussion
In the present study, we report a patient who was
diagnosed with scirrhous gastric cancer with RPF. Although his
performance status was poor because of the placement of several
medical tubes, he was essentially healthy and had finally improved
sufficiently as a result of effective chemotherapy to prompt his
withdrawal from HD treatment.
In the present case, we initiated chemotherapy on
day 28 after pathological diagnosis. It seems possible that an
earlier intervention with systemic chemotherapy might prevent the
progression of organ dysfunction. It is of vital interest to
consider that scirrhous gastric cancer and hydronephrosis secondary
to RPF could be rescued by an early administration of systemic
chemotherapy. It is important to predict specific cases that are
prone to develop this type of typical complicated course that
includes ascites, bowel obstruction, hydronephrosis, and
obstructive jaundice (11). It is
also important to select the most feasible treatment regimen for
these cases. Intravenous drug administration is better than oral
medicine because these patients often develop a difficulty in the
ability to consume medication orally.
RPF is a rare disease characterized by the presence
of retroperitoneal tissue that features chronic inflammation and
marked fibrosis, which often entraps the ureters or other abdominal
organs (12). Approximately 70% of
RPF is idiopathic, whereas the remaining 30% is secondary to other
causes, including malignancy (13).
RPF secondary to malignancy is well-known, but it is still a
relatively uncommon clinical condition that is characterized by the
presence of a fibroinflammatory soft tissue mass surrounding the
blood vessels, nerves, and ureters (14,15). The
diagnosis of RPF is often made through imaging studies, although a
definitive diagnosis may require a biopsy (16). However, a dilemma in the diagnosis
and management of this disease may arise because even when negative
biopsies are obtained, some cases of scirrhous gastric cancer with
RPF do not show masses on imaging (5,6,17,18).
Actually, in the present case, there were no definitive imaging
findings, and the diagnosis of RPF was not confirmed by
pathological means. Therefore, other causes of hydronephrosis
should be considered in the differential diagnosis. Several studies
have reported the ureteral metastasis of gastric cancer and
demonstrated that the true metastasis of gastric cancer to the
ureter is extremely rare (19,20).
Peritoneal metastasis can also obstruct the ureter, but serious,
life threatening conditions sometimes do not permit further
examinations. Tahara et al (21) reported that 14% of advanced gastric
cancer cases with peritoneal metastasis are complicated by
hydronephrosis. Similarly, Hamamoto (11) reported that 10% of advanced gastric
cancer cases with peritoneal metastasis developed hydronephrosis.
Hydronephrosis associated with peritoneal metastasis or RPF is
often readily detected by experienced medical oncologists,
especially those with an expertise in gastric cancer.
Peritoneal metastasis is reported to be a common
reason for the unresectability of gastric cancer (22). Clinical trials for patients who are
unable to consume oral medications because of peritoneal
dissemination of gastric cancer have demonstrated that 5-FU based
therapy or paclitaxel monotherapy is useful (21,23,24). In
general, the prognosis of those who undergo systemic chemotherapy
is dependent on the patient's status (25). The patient, whose case we presented,
would typically not be recommended to undergo systemic chemotherapy
considering his poor performance status. However, his status was
limited by several medical treatments for organ dysfunction, and
thus it was believed that the patient would tolerate a modest
regimen with intensive supportive treatments. Organ function
improved after supportive treatments, and no active infection or
other contraindication for anticancer treatments was observed.
Importantly, the patient was unaware of the available anticancer
treatments, and along with his family, he was compelled to combat
cancer with active treatments. We predicted that his life
expectancy would be longer than two months. Several reviews that
focus on chemotherapy for inadequate organ function have been
published (26). Although 5-FU is
primarily metabolized by the enzyme dihydropyrimidine dehydrogenase
(DPD), which functions predominantly in the liver, a clinical trial
demonstrated that patients with renal and hepatic dysfunction could
be safely treated with 5-FU without any modifications (27). Reports on hemodialyzed patients have
indicated that 5-FU can be used without dosage adjustment in
patients with renal dysfunction who undergo HD, and it is
recommended that 5-FU be administered after an HD session because
the drug may be removed by the procedure (28).
We would like to emphasize that advanced scirrhous
gastric cancer is prone to the development of a unique clinical
course, including the occurrence of RPF, with rapid progression.
Delayed systemic treatment results in a poor outcome, and thus an
appropriate and prompt clinical diagnosis is important. Several
pitfalls exist in the management of scirrhous gastric cancer, such
as the following: i) CEA is seldom increased (29); ii) helical CT and FDG-PET are not
useful for the detection of metastasis even for advanced stage
cancers (30); iii) the cancer
involves multiple segments of the digestive tract without the
presence of a mass (31); iv)
various non specific symptoms appear with rapid onset; v) this type
of cancer lacks a standard definition (32); and vi) no clear treatment proposal is
described in the guidelines (33,34).
In our experience, palliative chemotherapy is
efficacious for malignancy-associated RPF in patients with organ
dysfunction and poor general condition. It would be important to
select the appropriate chemotherapy regimen in combination with
supportive care according to the patient's condition. An earlier
administration of systemic chemotherapy would be helpful for
patients with advanced scirrhous gastric cancer who are predicted
to experience rapid disease progression such as those with RPF.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
YA, YH and YS collected data and wrote manuscript.
TK and HT made substantial contributions to the study conception
and design. AU, KT, TS, KK, KH and AK contributed to data
collection and interpretation, and critically reviewed the
manuscript. All authors approved the final version of the
manuscript.
Ethics approval and consent to
participate
Written informed consent was obtained from the
patient's next-of-kin for publication of this case report and any
accompanying images.
Patient consent for publication
Written informed consent was obtained from the
patient's next-of-kin for publication of this case report and any
accompanying images. A copy of the written consent is available for
review by the Editor of this journal.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Torre LA, Bray F, Siegel RL, Ferlay J,
Tieulent Lortet J and Jemal A: Global cancer statistics, 2012. CA
Cancer J Clin. 65:87–108. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Japanese Gastric Cancer Association:
Japanese classification of gastric carcinoma: 3rd English edition.
Gastric Cancer. 14:101–112. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Yashiro M, Chung YS, Nishimura S, Inoue T
and Sowa M: Fibrosis in the peritoneum induced by scirrhous gastric
cancer cells may act as ‘soil’ for peritoneal dissemination.
Cancer. 77:S1668–S1675. 1996. View Article : Google Scholar
|
|
4
|
Kitamura K, Beppu R, Anai H, Ikejiri K,
Yakabe S, Sugimachi K and Saku M: Clinicopathologic study of
patients with Borrmann type IV gastric carcinoma. J Surg Oncol.
58:112–117. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Dohmen K, Mizukami Y, Tanaka K, Nakamura
H, Arase K, Yokogawa Y, Asayama R, Kato A, Kato M, Nakagaki M, et
al: Retroperitoneal fibrosis associated with scirrhous gastric
cancer. Gastroenterol Jpn. 28:699–705. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Peixoto RD, Al Barrak J, Lim H and Renouf
D: Gastroesophageal cancer and retroperitoneal fibrosis: Two case
reports and review of the literature. World J Gastrointest Oncol.
5:68–70. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Sasaki T, Koizumi W, Higuchi K, Ishido K,
Ae T, Nakatani K, Katada C, Tanabe S and Saigenji K: Therapeutic
strategy for type 4 gastric cancer from the clinical oncologist
standpoint. Gan To Kagaku Ryoho. 34:988–992. 2007.(In Japanese).
PubMed/NCBI
|
|
8
|
Sawaki A, Yamaguchi K, Nabeya Y, Sakai Y,
Osanai H, Denda T, Furue H and Kurihara M: 5 FU/l LV(RPMI) versus S
1 as first line therapy in patients with advanced gastric cancer: A
randomized phase III non inferiority trial (ISO 5FU10 study group
trial). Eur J Cancer, Suppl. 7:3632009. View Article : Google Scholar
|
|
9
|
Eisenhauer EA, Therasse P, Bogaerts J,
Schwartz LH, Sargent D, Ford R, Dancey J, Arbuck S, Gwyther S,
Mooney M, et al: New response evaluation criteria in solid tumours:
Revised RECIST guideline (version 1.1). Eur J Cancer. 45:228–247.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Common Terminology Criteria for Adverse
Events (CTCAE). Version 4.0. https://evs.nci.nih.gov/ftp1/CTCAE/CTCAE_4.03/Archive/CTCAE_4.0_2009
05 29_QuickReference_8.5×11.pdfSeptember 5–2018
|
|
11
|
Hamamoto Y: Complications in advanced or
recurrent gastric cancer patients with peritoneal metastasis during
and after palliative systemic chemotherapy. Mol Clin Oncol.
3:539–542. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Koep L and Zuidema GD: The clinical
significance of retroperitoneal fibrosis. Surgery. 81:250–257.
1977.PubMed/NCBI
|
|
13
|
Vaglio A, Salvarani C and Buzio C:
Retroperitoneal fibrosis. Lancet. 367:241–251. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Webb AJ and Edwards Dawson P: Malignant
retroperitoneal fibrosis. Br J Surg. 54:505–508. 1967. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Thomas MH and Chisholm GD: Retroperitoneal
fibrosis associated with malignant disease. Br J Cancer.
28:453–458. 1973. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Cronin CG, Lohan DG, Blake MA, Roche C,
McCarthy P and Murphy JM: Retroperitoneal fibrosis: A review of
clinical features and imaging findings. AJR Am J Roentgenol.
191:423–431. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Usher SM, Brendler H and Ciavarra VA:
Retroperitoneal fibrosis secondary to metastatic neoplasm. Urology.
9:191–194. 1977. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Yokoyama R, Tazaki R, Morita H, Nishitani
H, Ariumi S, Osuga S, Sohmiya K, Kono T, Narumi Y, Tsuji M, et al:
Retroperitoneal fibrosis in a patient with gastric cancer
manifested by lower extremity edema and hydrocele. Intern Med.
51:2157–2160. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Bisof V, Juretic A, Pasini J, Coric M,
Grgic M, Gamulin M, Rakusic Z, Krajina Z, Koretic Basic M, Misir A,
et al: Ureteral metastasis as the first and sole manifestation of
gastric cancer dissemination. Radiol Oncol. 44:262–264. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Shimoyama Y, Ohashi M, Hashiguchi N,
Ishihara M, Sakata M, Tamura A, Asato Y, Saitoh K and Mukai M:
Gastric cancer recognized by metastasis to the ureter. Gastric
Cancer. 3:102–105. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Tahara M, Ohtsu A, Boku N, Nagashima F,
Muto M, Sano Y, Yoshida M, Mera K, Hironaka S, Tajiri H, et al:
Sequential methotrexate and 5 fluorouracil therapy for gastric
cancer patients with peritoneal dissemination: A retrospective
study. Gastric Cancer. 4:212–218. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Dupont JB Jr, Lee JR, Burton GR and Cohn I
Jr: Adenocarcinoma of the stomach: Review of 1,497 cases. Cancer.
41:941–947. 1978. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Shirao K, Boku N, Yamada Y, Yamaguchi K,
Doi T, Goto M, Nasu J, Denda T, Hamamoto Y, Takashima A, et al:
Gastrointestinal Oncology Study Group of the Japan Clinical
Oncology Group: Randomized Phase III study of 5 fluorouracil
continuous infusion vs. sequential methotrexate and 5 fluorouracil
therapy in far advanced gastric cancer with peritoneal metastasis
(JCOG0106). Jpn J Clin Oncol. 43:972–980. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Nishina T, Boku N, Gotoh M, Shimada Y,
Hamamoto Y, Yasui H, Yamaguchi K, Kawai H, Nakayama N, Amagai K, et
al: Gastrointestinal Oncology Study Group of the Japan Clinical
Oncology Group: Randomized phase II study of second line
chemotherapy with the best available 5 fluorouracil regimen versus
weekly administration of paclitaxel in far advanced gastric cancer
with severe peritoneal metastases refractory to 5 fluorouracil
containing regimens (JCOG0407). Gastric Cancer. 19:902–910. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Iwasa S, Nakajima TE, Nakamura K,
Takashima A, Kato K, Hamaguchi T, Yamada Y and Shimada Y: Systemic
chemotherapy for peritoneal disseminated gastric cancer with
inadequate oral intake: A retrospective study. Int J Clin Oncol.
16:57–62. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Superfin D, Iannucci AA and Davies AM:
Commentary: Oncologic drugs in patients with organ dysfunction: a
summary. Oncologist. 12:1070–1083. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Field KM, Michael M and Part II: Part II:
Liver function in oncology: Towards safer chemotherapy use. Lancet
Oncol. 9:1181–1190. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Janus N, Thariat J, Boulanger H, Deray G
and Vacher Launay V: Proposal for dosage adjustment and timing of
chemotherapy in hemodialyzed patients. Ann Oncol. 21:1395–1403.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Shimada H, Noie T, Ohashi M, Oba K and
Takahashi Y: Clinical significance of serum tumor markers for
gastric cancer: A systematic review of literature by the Task Force
of the Japanese Gastric Cancer Association. Gastric Cancer.
17:26–33. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Stahl A, Ott K, Weber WA, Becker K, Link
T, Siewert JR, Schwaiger M and Fink U: FDG PET imaging of locally
advanced gastric carcinomas: Correlation with endoscopic and
histopathological findings. Eur J Nucl Med Mol Imaging. 30:288–295.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Burgain C, Germain A, Bastien C, Orry X,
Choné L, Claudon M and Laurent V: Computed tomography features of
gastrointestinal linitis plastica: Spectrum of findings in early
and delayed phase imaging. Abdom Radiol (NY). 41:1370–1377. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Agnes A, Estrella JS and Badgwell B: The
significance of a nineteenth century definition in the era of
genomics: Linitis plastica. World J Surg Oncol. 15:1232017.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Japanese gastric cancer association.
Japanese gastric cancer treatment guidelines 2014 (ver.4). Gastric
Cancer. 20:1–19. 2017. View Article : Google Scholar
|
|
34
|
NCCN Clinical Practice Guideline in
Oncology (NCCN Guidelines®). version 5.2017. https://ctep.cancer.gov/protocolDevelopment/electronic_applications/docs/CTCAE_v5_Quick_Reference_8.5×11.pdfDecember
7–2017
|