Introduction
According to the American Joint Committee on Cancer
(AJCC) TNM staging system, pathological lymph node status 3 (pN3a)
for breast cancer is defined as 10 or more metastatic lymph node
involvement in pathologic evaluation (1). Nodal (N) status is a strong and
independent negative prognostic factor and pN3a patients have the
worst prognosis among breast cancer patients without distant
metastases (2).
Older chemotherapy regimens resulted in poor
clinical outcome 22–41% 5-year disease-free survival (DFS) and
39–41% 5-year overall survival (OS) rates in patients with N3a
disease (3–5); however, the outcome of N3a patients has
been favorably improved over years with recent treatment modalities
and 5-year DFS rate and OS probability have been reported to reach
66 and 81% respectively (6).
Because of the limitations of traditional prognostic
factors (such as tumor size, N involvement, nuclear grade,
histologic type, molecular markers, and surgical margins), recent
studies have focused on defining biological characteristics of
disease to provide better risk stratification (7,8). Breast
cancer is classified into distinct biological subtypes as a
consequence of global gene expression profiling studies (9,10). These
subtypes have subsequently been shown to correlate with prognosis,
locoregional recurrence and response to systemic therapy. The
Her2-positive and basal-like subtypes had the poorest prognosis.
The luminal subtypes are the most heterogeneous group as the
Luminal A subtype had better prognosis compared with the other
subtypes, and the Luminal B subtype had an intermediate outcome
(11). However, it remains unclear
whether distinct molecular subtypes have different prognosis in
pN3a patients who are already at high risk for recurrence because
of extensive N involvement.
In the present study, we aimed to evaluate
prognostic value of molecular subtypes in breast cancer patients
with pN3a N involvement without distant metastasis, in the modern
therapeutic era.
Materials and methods
Patients
We retrospectively evaluated 521 breast cancer
patients who had 10 or more metastatic lymph nodes and received
adjuvant systemic therapy at the oncology department of four
centers from Turkey between 2000 and 2015. Patient (age,
menopause), tumor (size, grade, lymphatic invasion, vascular
invasion, hormone receptors, Her2 expression) and treatment related
(surgery, adjuvant chemotherapy, radiotherapy, endocrine therapy
and trastuzumab) characteristics were recorded from patients
files.
Patients who received neoadjuvant chemotherapy were
excluded from the study. All patients intended to receive
anthracycline based regimen followed by a taxane therapy and then
radiotherapy. Patients with Her2 expression score 3(+) with
immunohistochemistry (IHC) or 2(+) with IHC and fluorescent in
situ hybridization (FISH) positive, also received adjuvant
trastuzumab therapy if, they were diagnosed after June 2007 with
the approval of adjuvant use of trastuzumab by Turkish Ministry of
Health. Ki-67 level <14% considered as low expression while
values ≥14% is considered high expression. Tumors were regarded as
positive for estrogen receptor (ER) and progesterone receptor (PR)
when ≥1% of the tumor cells showed nuclear staining and patients
with hormone positive tumors also received endocrine therapy. The
patients were divided into four groups: Luminal A subtype (ER+
and/or PR+, Her2- and Ki-67<14%), Luminal B subtype (ER+ and/or
PR+ and Her2+ or Ki-67>14%), Her2 positive subtype (ER- and PR-,
Her2+), and triple negative subtype (ER-, PR-, Her2-). The present
study was approved by Dicle University Ethics Committee
(Diyarbakir, Turkey).
Statistical analysis
DFS was estimated as the duration of time from
surgery date until first relapse (local, regional or distant) or
death from any cause. OS was defined from the date of surgery to
the date of death from any cause; surviving patients were censored
at the date of last follow-up. The DFS and OS rates were calculated
by using Kaplan-Meier method. Cox regression analysis was used to
analyze the impact of clinical/pathological characteristics on
survival. P<0.05 was considered to indicate a statistically
significant difference. Statistical analysis was performed with
SPSS for MacOS version 24.0 (IBM Corp., Armonk, NY, USA).
Results
Patient characteristics
The clinical and histopathological features of the
patients are shown in Table I.
Median age at diagnosis was 50 years and a total of 58 patients
(11.0%) were younger than 35 years. A total of 226 (43.4%) patients
were premenopausal. Most tumors were at stage T1-2 (59%). The
median number of metastatic lymph node was 15 (range, 10–95). The
majority of the patients (72.5%) had hormone receptor-positive
tumors. The most common subtype was Luminal A (47.4%), followed by
Luminal B (26.7%), Her2 enriched (15.5%), and triple negative
(10.4%) subtypes. Patients received anthracycline and taxane-based
regimen after surgery. A total of 81.7% of Her2 positive patients
received trastuzumab therapy. A total of 93.4% patients received
radiotherapy.
 | Table I.Patient characteristics. |
Table I.
Patient characteristics.
| Characteristics | Total no. (%) |
|---|
| Age, years |
|
| ≤35 | 58 (11.1) |
|
>35 | 463 (88.9) |
| Menopause |
|
| Pre | 226 (43.4) |
| Post | 276 (53.0) |
|
Unknown | 19 (3.6) |
| Tumor location |
|
|
Right | 266 (51.1) |
| Left | 255 (48.9) |
| ER |
|
|
Positive | 349 (67) |
|
Negative | 172 (33) |
| PR |
|
|
Positive | 330 (63.3) |
|
Negative | 191 (36.7) |
| Her2 |
|
|
Positive | 218 (41.8) |
|
Negative | 303 (58.2) |
| Subtype |
|
| Luminal
A | 247 (47.4) |
| Luminal
B | 139 (26.7) |
| Her2
positive | 81 (15.5) |
| Triple
negative | 54 (10.4) |
| Metastatic lymph
node |
|
|
10–20 | 410 (78.7) |
|
21–30 | 72 (13.8) |
|
>31 | 39 (7.5) |
| Tumor |
|
|
T1-T2 | 308 (59) |
|
T3-T4 | 191 (36.6) |
|
Unknown | 22 (4.4) |
| Operation |
|
|
Mastectomy | 447 (85.8) |
| BCS | 74 (14.2) |
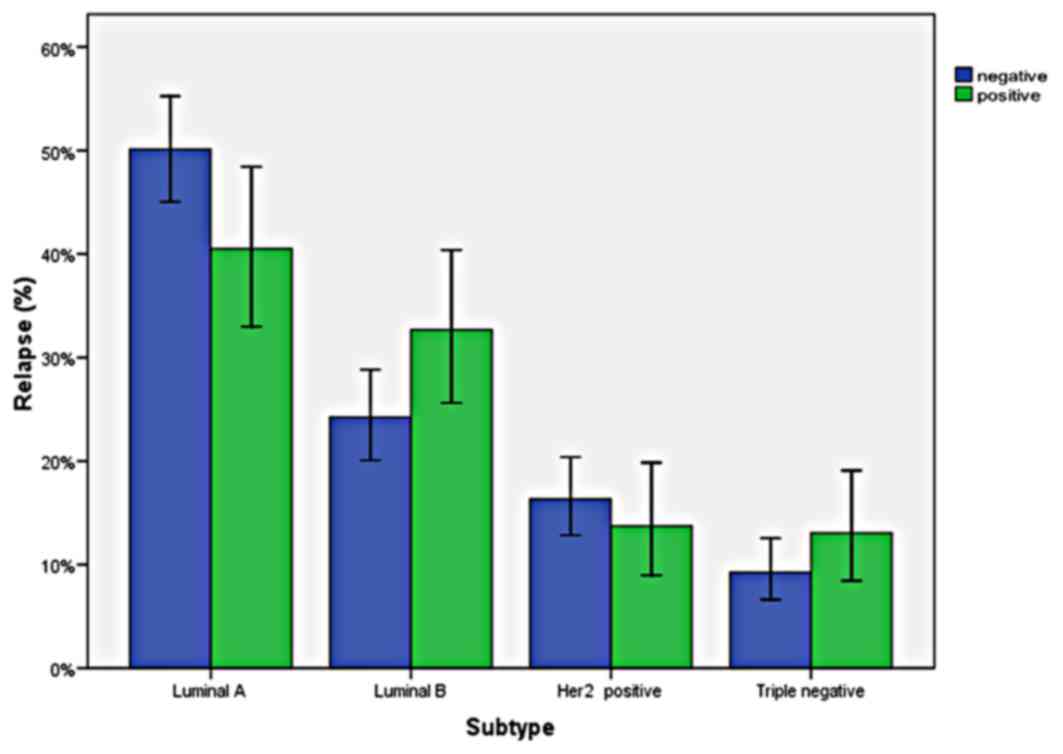
Effect of breast cancer subtypes on
relapse
There were not any significant differences in
relapse rates according to molecular subtypes (P=0.07). Distant
relapse rates of subgroups were as follows: 25.2% for Luminal A,
36% for Luminal B, 25.9% for Her2-enriched and 37% for triple
negative subtype. Relapse rates by breast cancer subtype, are shown
in Fig. 1.
DFS according to tumor subtypes
The five year DFS rate was 62% for the whole study
population, 67% for Luminal A tumors, 53% for Luminal B tumors, 64%
for Her2 positive tumors and 56% for triple negative tumors.
Luminal A patients had better PFS when compared with Luminal B
(P=0.026) and triple negative (P=0.07) patients (Fig. 2).
Factors related with DFS
As shown in Table
II, pT stage (P<0.001), operation type (P=0.04) and
biological subtypes were predictors for DFS in univariate analysis.
Lymphovascular invasion was prognostic for Her2 enriched
tumors.
 | Table II.Factors associated with disease free
survival. |
Table II.
Factors associated with disease free
survival.
|
| Univariate | Multivariate |
|---|
|
|
|
|
|---|
| Variables | HR | P-value | HR | P-value |
|---|
| Age, years |
| 0.80 |
|
|
| ≤35 | 1 |
|
|
|
|
>35 | 0.93 |
|
|
|
| Menopause | 1 | 0.17 | 1 | 0.67 |
| Pre | 0.79 |
| 0.91 |
|
| Post |
|
|
|
|
| Surgery | 1 | 0.04a | 1 | 0.46 |
| BCS | 0.54 |
| 0.77 |
|
| TM |
|
|
|
|
| Grade |
|
|
|
|
| 1 | 1 |
|
|
|
| 2 | 0.97 | 0.95 |
|
|
| 3 | 1.04 | 0.90 |
|
|
| Hormone receptor |
| 0.16 |
|
|
|
Negative | 1 |
|
|
|
|
Positive | 0.78 |
|
|
|
| Her2 |
| 0.12 |
|
|
|
Negative | 1 |
|
|
|
|
Positive | 1.29 |
|
|
|
| Lymphatic
invasion |
| 0.16 |
| 0.182 |
|
Negative | 1 |
| 1 |
|
|
Positive | 1.34 |
| 1.33 |
|
| T stage |
|
|
|
|
| 1 | 1 |
| 1 |
|
| 2 | 1.10 | 0.71 | 1.13 | 0.70 |
| 3 | 1.13 | 0.66 | 1.06 | 0.85 |
| 4 | 2.97 | 0.001c | 2.77 | 0.009b |
| Subtype |
|
|
|
|
| Luminal
A | 1 |
| 1 |
|
| Luminal
B | 1.53 | 0.02a | 1.5 | 0.04a |
| Her2
(+) | 1.33 | 0.27 | 1.2 | 0.57 |
| Triple
(−) | 1.56 | 0.08 | 1.9 | 0.04 |
When these variables were analyzed with Cox
proportional hazard model, pT stage (P<0.001), and breast cancer
subtype (P<0.001), remained significant independent factors for
DFS. T4 tumor stage, was a negative prognostic factor for DFS.
Patients with Luminal A tumors significantly had longer DFS when
compared with Luminal B and triple negative subtypes.
Discussion
In the present study, we evaluated prognostic value
of molecular subtypes for pN3 breast cancer patients in a large
patient population. We showed that, Luminal A subtype was
associated with better prognosis as compared to Luminal B and
triple negative subtype for pN3a positive breast cancer
patients.
Several studies showed that increasing number of N
metastasis is associated with poor prognosis and predicts early
relapse after adjuvant chemotherapy (12). However, patients with extensive N
metastasis represent heterogeneous clinical outcomes and
identification of prognostic factors is important to detect
patients who might require more intensive or less therapy.
It has been shown that, molecular subtypes have
impact on response to therapy and survival in breast cancer
patients. Early-stage Luminal A breast cancer patients have a
better prognosis with a significantly lower relapse rate of 27.8%
as compared with other biological subtypes. In addition, survival
from the time of relapse was also longer (13). However, prognostic value of molecular
subtypes in patients with extensive N metastasis is uncertain. A
small number of trials evaluated this question. Yang et al
(14), found that there was a
significant survival difference among patients with pN0-pN2 disease
according to breast cancer subtypes in terms of Luminal A breast
cancer patients had the best survival outcome when compared with
other subtypes. However, molecular subtypes had no prognostic
effect on survival for patients with pN3 disease (14). Kim et al (15), evaluated N3 breast cancer patients
for prognostic factors and identified young age, high serum
neutrophil/lymphocyte ratio (>3.0), high N ratio and molecular
subgroups as important prognostic factors. They found that patients
with the HR+ Her2- subtype had longest DFS while triple negative
subtype showed the worst outcome compatible with our findings,
however the patient population was relatively small and they did
not take into consideration Ki-67 levels when they define molecular
subtypes (15).
In our patient population, 41.8% of patients were
Her2 positive. The most commonly diagnosed molecular subtype was
Luminal A, while triple negative subtype was rare which consisted
of 10.4% of the patients. There is still controversy regarding the
association between breast cancer subtype and lymph node status.
Liu et al (16), showed that
both TNBC and Luminal A breast cancer subtypes were related with a
lower risk of pN3 stage when compared with Luminal B and Her2
overexpression breast cancer subtypes. Proportion of triple
negative patients in pN3 group seems to be low as compared to whole
breast cancer population. Although TNBC is more aggressive, it may
be related with less frequent involvement of lymph nodes and it is
not frequently associated with a pN3 stage disease.
It has been shown that N3 patients have improved
prognosis with novel therapies as compared with old chemotherapy
regimens. Taxanes have proven as effective agents in the adjuvant
treatment of breast cancer. Adjuvant use of taxanes were evaluated
in several randomized clinical trials, and results provided a
strong evidence for node positive breast cancer. Three
meta-analyses showed that adjuvant anthracycline-based chemotherapy
followed by taxane chemotherapy significantly improved DFS and OS
rates with an absolute 5-year risk reduction of DFS and OS, 5% and
3% respectively (17–19). Emerging data show that the addition
of trastuzumab to adjuvant chemotherapy results in durable survival
benefits for patients with Her2-positive breast cancer. With a
median on-study time of 8.4 years, the addition of trastuzumab
resulted in a 37% improvement in OS and a 40% improvement in DFS
(20). Our patients received
anthracycline based chemotherapy followed by taxane treatment.
Trastuzumab therapy added to chemotherapy regimen if the patient
diagnosed after 2007 with the approval of the drug. This therapy
resulted with a 5 year DFS rate 67% in Luminal A, 53% in Luminal B,
64% Her2 positive group and 56% in triple negative group.
The present study has some limitations. First,
surgery was not uniform, as they are performed by different
surgeons. Secondly, patients who admitted to hospital from 2003 to
2010 are included in this study and some of the adjuvant therapies
do not reflect current clinical practice (e.g., some of the
patients with Her2-positive disease did not receive trastuzumab
therapy) Therefore, a prospective and randomized controlled trial
will be important to validate our findings in this study.
Acknowledgements
The authors would like to thank Ferhat Yildiz
(Department of Public Health, Adnan Menderes University, Aydin,
Turkey) for statistical analysis.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
ÖY conceived and designed the study, acquired the
patients' data and was a major contributor in writing the
manuscript. MAK, AI, NÖ and MA interpreted the patient data on
breast cancer treatment and survival. SB revised the manuscript
critically for important intellectual content and was a major
contributor in writing the manuscript. HİE performed the
histological examination of the breast. NM interpreted the
molecular characteristics of the breast cancer patients. All
authors read and approved the final manuscript.
Ethics approval and consent to
participate
The present study was approved by Dicle University
Ethics Committee (Diyarbakir, Turkey).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Amin MB, Edge S, Greene F, Byrd DR,
Brookland RK, Washington MK, Gershenwald JE, Compton CC, Hess KR,
Sullivan DC, et al: AJCC Cancer Staging Manual. 8th edition.
Springer; New York, NY: 2017, View Article : Google Scholar
|
|
2
|
Nemoto T, Vana J, Bedwani RN, Baker HW,
McGregor FH and Murphy GP: Management and survival of female breast
cancer: Results of a national survey by the American College of
Surgeons. Cancer. 45:2917–2924. 1980. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Walker MJ, Osborne MD, Young DC,
Schneebaum S, La Valle GJ and Farrar WB: The natural history of
breast cancer with more than 10 positive nodes. Am J Surg.
169:575–579. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Buzdar AU, Kau SW, Hortobagyi GN, Ames FC,
Holmes FA, Fraschini G, Hug V, Theriault RL, McNeese MD and
Singletary SE: Clinical course of patients with breast cancer with
ten or more positive nodes who were treated with
doxorubicin-containing adjuvant therapy. Cancer. 69:448–452. 1992.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Schmoor C, Sauerbrei W, Bastert G, Bojar H
and Schumacher M: German Breast Cancer Study Group: Long-term
prognosis of breast cancer patients with 10 or more positive lymph
nodes treated with CMF. Eur J Cancer. 37:1123–1131. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Basaran G, Devrim C, Caglar HB, Gulluoglu
B, Kaya H, Seber S, Korkmaz T, Telli F, Kocak M, Dane F, et al:
Clinical outcome of breast cancer patients with N3a (≥10 positive
lymph nodes) disease: Has it changed over years? Med Oncol.
28:726–732. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Perou CM, Sørlie T, Eisen MB, van de Rijn
M, Jeffrey SS, Rees CA, Pollack JR, Ross DT, Johnsen H, Akslen LA,
et al: Molecular portraits of human breast tumours. Nature.
406:747–752. 2000. View
Article : Google Scholar : PubMed/NCBI
|
|
8
|
Sørlie T, Perou CM, Tibshirani R, Aas T,
Geisler S, Johnsen H, Hastie T, Eisen MB, van de Rijn M, Jeffrey
SS, et al: Gene expression patterns of breast carcinomas
distinguish tumor subclasses with clinical implications. Proc Natl
Acad Sci USA. 98:10869–10874. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Sotiriou C, Neo SY, McShane LM, Korn EL,
Long PM, Jazaeri A, Martiat P, Fox SB, Harris AL and Liu ET: Breast
cancer classification and prognosis based on gene expression
profiles from a population-based study. Proc Natl Acad Sci USA.
100:10393–10398. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Abd El-Rehim DM, Ball G, Pinder SE, Rakha
E, Paish C, Robertson JF, Macmillan D, Blamey RW and Ellis IO:
High-throughput protein expression analysis using tissue microarray
technology of a large well-characterised series identifies
biologically distinct classes of breast cancer confirming recent
cDNA expression analyses. Int J Cancer. 116:340–350. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Yersal O and Barutca S: Biological
subtypes of breast cancer: Prognostic and therapeutic implications.
World J Clin Oncol. 5:412–424. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Cianfrocca M and Goldstein LJ: Prognostic
and predictive factors in early-stage breast cancer. Oncologist.
9:606–616. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Kennecke H, Yerushalmi R, Woods R, Cheang
MC, Voduc D, Speers CH, Nielsen TO and Gelmon K: Metastatic
behavior of breast cancer subtypes. J Clin Oncol. 28:3271–3277.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Yang ZJ, Yu Y, Hou XW, Chi JR, Ge J, Wang
X and Cao XC: The prognostic value of node status in different
breast cancer subtypes. Oncotarget. 8:4563–4571. 2017.PubMed/NCBI
|
|
15
|
Kim YY, Park HK, Lee KH, Kim KI and Chun
YS: Prognostically distinctive subgroup in pathologic N3 breast
cancer. J Breast Cancer. 19:163–168. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Liu N, Yang Z, Liu X and Niu Y: Lymph node
status in different molecular subtype of breast cancer: Triple
negative tumours are more likely lymph node negative. Oncotarget.
8:55534–55543. 2017.PubMed/NCBI
|
|
17
|
Bria E, Nistico C, Cuppone F, Carlini P,
Ciccarese M, Milella M, Natoli G, Terzoli E, Cognetti F and
Giannarelli D: Benefit of taxanes as adjuvant chemotherapy for
early breast cancer: Pooled analysis of 15,500 patients. Cancer.
106:2337–2344. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
De Laurentiis M, Cancello G, D'Agostino D,
Giuliano M, Giordano A, Montagna E, Lauria R, Forestieri V,
Esposito A, Silvestro L, et al: Taxane-based combinations as
adjuvant chemotherapy of early breast cancer: A meta-analysis of
randomized trials. J Clin Oncol. 26:44–53. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Ferguson T, Wilcken N, Vagg R, Ghersi D
and Nowak AK: Taxanes for adjuvant treatment of early breast
cancer. Cochrane Database Syst Rev. 4:CD0044212007.
|
|
20
|
Perez EA, Romond EH, Suman VJ, Jeong JH,
Sledge G, Geyer CE Jr, Martino S, Rastogi P, Gralow J, Swain SM, et
al: Trastuzumab plus adjuvant chemotherapy for human epidermal
growth factor receptor 2-positive breast cancer: Planned joint
analysis of overall survival from NSABP B-31 and NCCTG N9831. J
Clin Oncol. 32:3744–3752. 2014. View Article : Google Scholar : PubMed/NCBI
|