Introduction
Collagenous fibroma is a rare benign fibrous soft
tissue tumor. This tumor was first described by Evans (1) as desmoplastic fibroblastoma, and
subsequently renamed collagenous fibroma by Nielsen et al
(2). It is a mass with slow growth
located in subcutaneous tissue or skeletal muscle, and the most
common sites are the upper extremities (1,3–5).
Diagnostic imaging data of collagenous fibroma have
not been clearly described. Although magnetic resonance imaging
(MRI) data for collagenous fibroma have been described in previous
studies (3–12), the tumors did not exhibit a common
pattern, and often produced similar results compared with fibrous
tumors, including desmoid tumors (13,14). It
is important to achieve the correct diagnosis and offer management
including surgical treatment for collagenous fibroma, as neither
local recurrence nor metastasis following surgical resection have
been identified.
The present case study describes an unusual case of
collagenous fibroma arising from the subacromial region in a
patient with osteosarcoma who was observed for follow-up of local
recurrence and metastasis. Lesions of the subacromial region have
rarely been described. To the best of our knowledge, this case was
the second to occur in the subacromial region, following a case
described by Milnes et al (8). In addition, multimodal radiological
data using not only MRI but also thallium-201 scintigraphy, bone
scintigraphy and positron emission tomography (PET) are presented,
and the distinguishing features of collagenous fibroma from desmoid
tumors are also discussed.
Case report
The patient was 42-year-old man with a 6-month
history of dull pain in his left shoulder. He had no tenderness, no
mass and no limitation in the range of motion in his shoulder. He
had no history of trauma. Laboratory examination revealed that his
white blood cell count was 5,500 µl (normal range, 4,000–9,000/µl),
C-reactive protein levels were 0.13 mg/dl (normal range, <0.25
mg/dl) and alkaline phosphatase levels were 214 U/l (normal range,
1–340 U/l). Plasma electrolytes, liver and kidney function tests
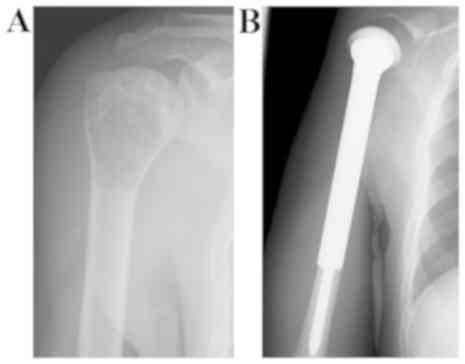
were within the normal ranges. His history described osteosarcoma
in his right proximal humerus with pain in his right shoulder 3
years previously (Fig. 1A). He
received chemotherapy and underwent wide tumor excision and
endoprosthetic replacement (Fig.
1B). Although thallium-201 scintigraphy and bone scintigraphy
had been performed to check postoperative recurrence and metastasis
1 year previously, these investigations indicated that there was no
uptake in his left shoulder (Fig.
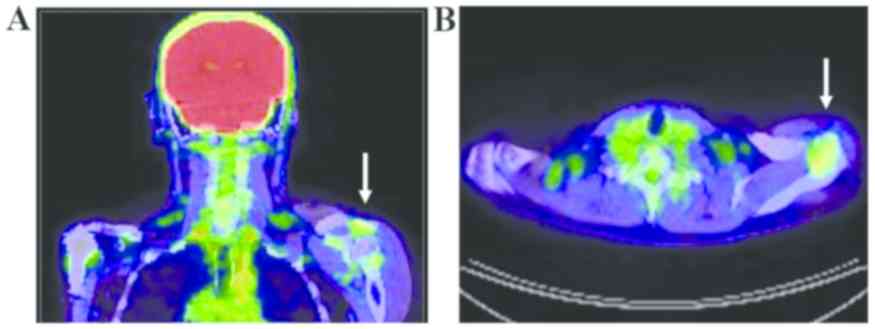
2). There was also no evidence of recurrence or metastasis. PET
scans were conducted to assess for metastatic lesions, and
demonstrated uptake in his left shoulder (Fig. 3). The maximum standardized uptake
value (SUVmax) was 2.4. MRI scans demonstrated a mass
with clear margins, measuring 46×22×14 mm in size in the
subacromial region. The margins between the mass and the
supraspinatus or infraspinatus muscle were clear. The lesion was
iso-intense to muscle on T1-weighted images (Fig. 4A) and iso-intense with a slightly
high intensity area on T2-weighted images (Fig. 4B). Post-contrast fat-suppressed MRI
scans indicated slightly heterogeneous enhancement of the lesion
(Fig. 4C). There were no data from
the X-ray scans performed.
An open biopsy was planned, although metastasis of
osteosarcoma was not suspected from the clinical imaging data.
During the procedure, the deltoid muscle was split, and a whitish
mass was identified in the subacromial space. When a part of the
mass was pinched, the white and elastic hard mass was easily
excised, as it was not adhered to adjacent tissues (Fig. 5A). Macroscopically, it appeared as a
white, elastic, hard and well-circumscribed mass (Fig. 5B). The resected tissue was fixed with
10% formalin for 24 h at room temperature. Paraffin embedding was
performed as follows: 70% ethanol (4 h), 80% and 90% ethanol (1 h
each), 100% ethanol (4 h), xylene (4 h), and paraffin wax (60°C; 3
h). The paraffin embedded specimens (2 µm) were examined via
hematoxylin and eosin staining (at room temperature for 50 min) and
analyzed under an optical microscope (magnification, ×40 and ×100).
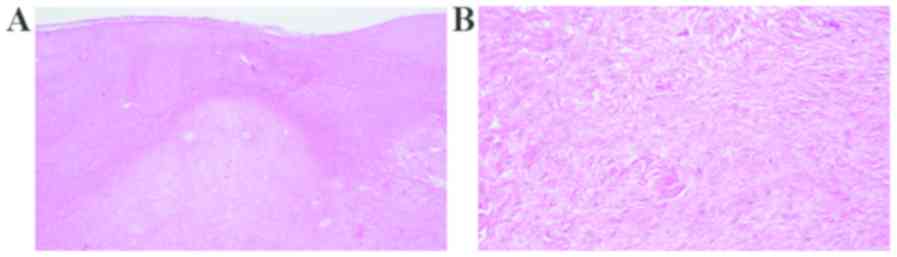
The tumor demonstrated fibrous and myxomatous matrix areas. Few
vascular spaces were observed (Fig.
6A). Histological examination revealed a hypocellular tumor
consisting of spindle- or stellate-shaped fibroblasts with oval
nuclei in abundant collagen fiber and myxocollagenous areas. The
tumor exhibited low vascularity. No mitosis or necrosis was
observed (Fig. 6B). Histological
results were consistent with a collagenous fibroma. Excision
resulted in immediate pain relief. He has had no recurrence during
the 4 years since the surgery.
Discussion
Collagenous fibroma is a rare benign fibrous soft
tissue tumor with a slow growth rate and clear margins, located in
subcutaneous and skeletal muscle. The tumor is more common in males
aged 50–70 years old. Miettinen and Fetsch (3) suggested that these types of tumors
range in size from 1–20 cm (median 3 cm), and common locations
include the arms (24%), shoulders (19%), posterior neck and upper
back, in particular the scapular areas (14%), feet and ankles
(14%), legs (14%), hands (8%), and abdominal wall and hip regions
(6%). Although the shoulder girdle is the second most common
location (5–7), involvement of the subacromial region is
rare. The present case report is the second case occurring in the
subacromial region, following a case described by Milnes et
al (8). The majority of previous
studies described tumors as being painless (1,3–5,7). In the
present case, the patient experienced dull pain in his shoulder.
The cause was considered to be the location of the tumor,
contributing to pain by compressing the subacromial space.
Histologically, collagenous fibroma is characterized
by spindle- to stellate-shaped fibroblasts and myofibroblasts
embedded in a prominent collagenous matrix (1). The cellularity is low, mitotic figures
are rare or absent and tumor necrosis is usually not observed
(1,3,4,9–12). The
differential diagnosis for collagenous fibroma includes fibroma of
the tendon sheath, nodular fasciitis, neurofibroma, desmoid tumors
and low-grade malignant tumors (1–3). In
particular, it is important to distinguish collagenous fibroma from
desmoid tumors, as the strategies for treatment and incidence of
recurrence are different, despite similar imaging and cytological
features of the two tumors (1,12).
Although the treatment for collagenous fibroma is local resection,
with no demonstrated incidence of local recurrence or metastases,
wide excision may be performed with wide margins including
surrounding muscle to prevent local recurrence of desmoid tumors
(9,12). It is therefore important to
accurately diagnose tumors as collagenous fibroma using
preoperative diagnostic imaging, to prevent overtreatment that may
result in a loss of function (7,12).
However, the radiological features of collagenous fibroma are not
widely recognized as there are so few published studies.
MRI data for collagenous fibroma have been described
previously (3–12). All the cases predominantly described
iso-intensity to muscle, including several areas of low intensity
on T1-weighted images. T2-weighted images demonstrated mixed signal
intensity. The range of area and degree of high signal intensity
were different in each case. Post-contrast T1-weighted images
with/without fat suppression revealed inhomogeneous enhancement of
the lesion.
A typical desmoid tumor exhibits iso-intensity on
T1-weighted images, high intensity on T2-weighted images, and
marked heterogeneous enhancement on enhanced T1-weighted images
(12–14). Although slight differentiation in
results from T2-weighted images may be observed, it is difficult to
distinguish collagenous fibroma from desmoid tumors. These
decreased signal areas on T1- and T2- weighted images probably
reflect the abundant collagen content (12,15,16).
These results are a common characteristic for tumors with fibrous
components. The low intensity areas correspond to areas of dense
collagen, while the high intensity areas may represent areas of
increased cellularity or fibromyxoid matrices within the tumor
(10). Therefore, it is unlikely
that collagenous fibroma may be distinguished from desmoid tumors
based on MRI analysis alone.
The usefulness of PET scans for the differentiation
between soft tissue sarcomas and benign lesions is well known.
However, aggressive tumors, including desmoid tumors, often exhibit
results similar to sarcoma (14,17–22).
Certain studies have included data from PET scans of desmoid tumors
(14,17–19). In
a study by Xu et al (14),
the median value of SUVmax on PET scans was 3.1 (range,
2.0–7.3), and Kasper et al (18) described a median SUVmax
value 4.1 (range, 1.0–8.1). In addition, Xu et al (14) suggested that desmoid tumors usually
appear moderately hypermetabolic on PET scans even in large masses,
while smaller tumors tend to appear hypometabolic. To the best of
our knowledge, the present case report is the first description of
the appearance of collagenous fibroma on PET scans. In the present
study, uptake by the tumor in the subacromial region was relatively
low compared with desmoid tumors (14,18). As
aforementioned, this tumor exhibited a pattern on the MRI scans
similar to desmoid tumors, but low cellularity and rare or absent
mitosis on histological examination. This feature may affect the
aggressiveness of the tumor, similar to previous case reports of
desmoid tumors (19–21).
Thallium-201 scintigraphy also is useful to
differentiate between soft tissue sarcomas and benign lesions.
Relatively aggressive tumors, including giant cell tumors of the
tendon sheath, pigmented villonodular synovitis, neurofibroma and
desmoid tumor, demonstrate high uptake on thallium-201
scintigraphy, compared with the majority of other benign tumors
(23–26). In the present case report, no
accumulation of thallium-201 scintigraphy was observed in the area
of the tumor, which poses the question of whether or not the tumor
was present 1 year ago. However, this cannot be confirmed as MRI
and PET scans were not performed at that time point, due to the
lack of uptake of thallium-201 scintigraphy. However, it is
difficult to conclusively state that this tumor, which was >5
cm, was absent 1 year ago, as growth of collagenous fibroma is not
rapid. Therefore, using the results of the present study,
thalium-201 scintigraphy may be able to differentiate desmoid
tumors from collagenous fibroma, although the usefulness of
thallium-201 scintigraphy in collagenous fibroma has not been
previously described.
In conclusion, the present case study has described
multimodal data, including MRI scans, thallium-201 scintigraphy and
PET scans of a collagenous fibroma arising from the subacromial
region. Regarding thallium-201 scintigraphy and PET scans, these
are not gold standard imaging techniques for soft tissue tumors. As
aforementioned, the majority of tumors, including desmoid tumors,
exhibit similar results for PET scans and thallium-201
scintigraphy. However, the present case exhibited distinct results
between PET scans and thallium-201 scintigraphy. Although the
detailed mechanism is unclear, different results between PET scans
and thallium-201 scintigraphy may be a characteristic of
collagenous fibroma. The multimodal radiological data of this case
may be useful as an additional tool to differentiate between
collagenous fibroma and desmoid tumors for future investigations.
Methods to correctly diagnose this tumor are important in order to
select the appropriate management strategies, including surgical
treatment.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Authors' contribution
HM designed the study, contributed to analysis of
data, and wrote the manuscript. All authors, including KI, KN and
YN, have contributed to the clinical management of the patient. All
authors critically reviewed the manuscript and approved the final
manuscript.
Ethics approval and consent to
participate
Ethical approval for this study was obtained from
Matsushita Memorial Hospital Ethics Committee (approval no. 18008).
Written informed consent was obtained from the patient.
Patient consent for publication
Written informed consent was obtained from the
patient.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
MRI
|
magnetic resonance imaging
|
|
PET
|
positron emission tomography
|
|
SUVmax
|
maximum standardized uptake value
|
References
|
1
|
Evans HL: Desmoplastic fibroblastoma. A
report of seven cases. Am J Surg Pathol. 19:1077–1081. 1995.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Nielsen GP, O'Connell JX, Dickersin GR and
Rosenberg AE: Collagenous fibroma (desmoplastic fibroblastoma): A
report of seven cases. Mod Pathol. 9:781–785. 1996.PubMed/NCBI
|
|
3
|
Miettinen M and Fetsch JF: Collagenous
fibroma (desmoplastic fibroblastoma): A clinicopathologic analysis
of 63 cases of a distinctive soft tissue lesion with
stellate-shaped fibroblasts. Hum Pathol. 29:676–682. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Yamamoto A, Abe S, Imamura T, Takada K,
Enomoto Y, Harasawa A, Matsushita T and Furui S: Three cases of
collagenous fibroma with rim enhancement on postcontrast
T1-weighted images with fat suppression. Skeletal Radiol.
42:141–146. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Walker KR, Bui-Mansfield LT, Gering SA and
Ranlett RD: Collagenous fibroma (desmoplastic fibroblastoma) of the
shoulder. AJR Am J Roentgenol. 183:17662004. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Marinelli M, Lupetti E, Gigante A,
Mandolesi A, Bearzi I and de Palma L: Collagenous fibroma of the
deltoid muscle: Clinical, surgical and histopathological aspects. J
Orthop Traumatol. 8:91–94. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Bonardi M, Zaffarana VG and Precerutti M:
US and MRI appearance of a collagenous fibroma (desmoplastic
fibroblastoma) of the shoulder. J Ultrasound. 17:53–56. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Milnes LK, Tennent TD and Pearse EO: An
unusual cause of subacromial impingement: A collagenous fibroma in
the bursa. J Shoulder Elbow Surg. 19:e15–e17. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Kamata Y, Anazawa U, Morioka H, Morii T,
Miura K, Mukai M, Yabe H and Toyama Y: Natural evolution of
desmoplastic fibroblastoma on magnetic resonance imaging: A case
report. J Med Case Reports. 5:1392011. View Article : Google Scholar
|
|
10
|
Shuto R, Kiyosue H, Hori Y, Miyake H,
Kawano K and Mori H: CT and MR imaging of desmoplastic
fibroblastoma. Eur Radiol. 12:2474–2476. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Beggs I, Salter DS and Dorfman HD:
Synovial desmoplastic fibroblastoma of hip joint with bone erosion.
Skeletal Radiol. 28:402–406. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Ogose A, Hotta T, Emura I, Higuchi T,
Kusano N and Saito H: Collagenous fibroma of the arm: A report of
two cases. Skeletal Radiol. 29:417–420. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Hartman TE, Berquist TH and Fetsch JF: MR
imaging of extraabdominal desmoids: Differentiation from other
neoplasms. AJR Am J Roentgenol. 158:581–585. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Xu H, Koo HJ, Lim S, Lee JW, Lee HN, Kim
DK, Song JS and Kim MY: Desmoid-type fibromatosis of the thorax:
CT, MRI, and FDG PET characteristics in a large series from a
tertiary referral center. Medicine (Baltimore). 94:e15472015.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Sundaram M, McGuire MH and Schajowicz F:
Soft-tissue masses: Histologic basis for decreased signal (short
T2) on T2-weighted MR images. AJR Am J Roentgenol. 148:1247–1250.
1987. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Kransdorf MJ, Jelinek JS, Moser RP Jr, Utz
JA, Hudson TM, Neal J and Berrey BH: Magnetic resonance appearance
of fibromatosis. A report of 14 cases and review of the literature.
Skeletal Radiol. 19:495–499. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Souza FF, Fennessy FM, Yang Q and van den
Abbeele AD: Case report. PET/CT appearance of desmoid tumour of the
chest wall. Br J Radiol. 83:e39–e42. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Kasper B, Dimitrakopoulou-Strauss A,
Strauss LG and Hohenberger P: Positron emission tomography in
patients with aggressive fibromatosis/desmoid tumours undergoing
therapy with imatinib. Eur J Nucl Med Mol Imaging. 37:1876–1882.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Nishio J, Aoki M, Nabeshima K, Iwasaki H
and Naito M: Imaging features of desmoid-type fibromatosis in the
teres major muscle. In Vivo. 27:555–559. 2013.PubMed/NCBI
|
|
20
|
Hourani R, Taslakian B, Shabb NS, Nassar
L, Hourani MH, Moukarbel R, Sabri A and Rizk T: Fibroblastic and
myofibroblastic tumors of the head and neck: Comprehensive
imaging-based review with pathologic correlation. Eur J Radiol.
84:250–260. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Ebrahim L, Parry J and Taylor DB:
Fibromatosis of the breast: A pictorial review of the imaging and
histopathology findings. Clin Radiol. 69:1077–1083. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Janssen ML, van Broekhoven DL, Cates JM,
Bramer WM, Nuyttens JJ, Gronchi A, Salas S, Bonvalot S, Grünhagen
DJ and Verhoef C: Meta-analysis of the influence of surgical margin
and adjuvant radiotherapy on local recurrence after resection of
sporadic desmoid-type fibromatosis. Br J Surg. 104:347–357. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Terui S, Terauchi T, Abe H, Fukuma H,
Beppu Y, Chuman K and Yokoyama R: On clinical usefulness of Tl-201
scintigraphy for the management of malignant soft tissue tumors.
Ann Nucl Med. 8:55–64. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Sato O, Kawai A, Ozaki T, Kunisada T,
Danura T and Inoue H: Value of thallium-201 scintigraphy in bone
and soft tissue tumors. J Orthop Sci. 3:297–303. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Murata H, Kusuzaki K, Hirata M, Hashiguchi
S and Hirasawa Y: Extraabdominal desmoid tumor with dissemination
detected by thallium-201 scintigraphy. Anticancer Res.
20:3963–3966. 2000.PubMed/NCBI
|
|
26
|
Kawakami N, Kunisada T, Sato S, Morimoto
Y, Tanaka M, Sasaki T, Sugihara S, Yanai H, Kanazawa S and Ozaki T:
Thallium-201 scintigraphy is an effective diagnostic modality to
distinguish malignant from benign soft-tissue tumors. Clin Nucl
Med. 36:982–986. 2011. View Article : Google Scholar : PubMed/NCBI
|