Introduction
An estimated 1,095,000 men worldwide were diagnosed
with prostate cancer in 2012, resulting in 307,000 deaths (1). In Japan, the incidence of prostate
cancer was estimated of 86,100 cases in 2017 (2), and in 2015, 11,326 patients died of
prostate cancer; the proportion of death from prostate cancer was
larger in elderly patients (age 70 and over) (3).
There are several options to treat prostate cancer,
such as surgery, external beam radiation therapy (EBRT),
brachytherapy, and androgen-deprivation therapy (ADT), excluding
active surveillance and watchful waiting. Patients may receive one
or a combination of these treatments (4). Among them, EBRT is less invasive than
surgery or brachytherapy and has curability. Intensity-modulated
radiation therapy (IMRT), a technique of EBRT, is especially
effective and has lower rates of gastrointestinal (GI) adverse
events than conventional EBRT (5–9). The
safety of IMRT also allows dose escalation, leading to better tumor
control (10). However, elderly
patients were more likely to receive ADT alone (11). A recent study with elderly patients
reported that the conservative management with ADT alone does not
improve the overall survival rate (OS) (12).
According to data collected in 2016, the life
expectancy of 75- and 80-year-old Japanese men is 12.14 and 8.92
years, respectively (13).
This study aimed to evaluate the efficacy and safety
of IMRT for elderly prostate cancer patients (age more than or
equal to 75 years) compared with younger patients (age less than 75
years).
Materials and methods
Patients
From March 2006 to July 2014, 1,252 prostate cancer
patients were treated with IMRT at our hospital. Exclusion criteria
included presence of lymph node metastases or distant metastases;
furthermore, patients whose prostate-specific antigen (PSA) levels
were not measured at least once after IMRT were excluded.
Histologically, all tumors were found to be adenocarcinomas. Among
the 1,091 patients, 238 patients were aged 75 years or older, the
younger group comprised 853 patients. Patient characteristics are
listed in Table I. This study was
approved by the Ethics Committee of Kizawa Memorial Hospital. All
patients provided written informed consent before receiving
radiotherapy.
 | Table I.Patient characteristics. |
Table I.
Patient characteristics.
| Characteristics | Age ≥75 years
(n=238) | Age <75 years
(n=853) | P-value |
|---|
| Median age (range),
years | 77 (75–87) | 69 (39–74) | – |
| Median initial PSA
(range), ng/ml | 9.59
(4.09–356.00) | 8.27
(2.65–370.00) | 0.1520 |
| Initial PSA ng/ml, n
(%) |
|
<10 | 125 (52.5) | 505 (59.2) |
|
| ≥10,
≤20 | 66 (27.7) | 214 (25.1) |
|
|
>20 | 47 (19.7) | 133 (15.6) |
|
| T Stage, n (%) |
|
| 0.0017 |
| T1c | 76 (31.9) | 351 (41.1) |
|
| T2a | 69 (29.0) | 262 (30.7) |
|
| T2b | 12 (9.7) | 26 (3.0) |
|
| T2c | 33 (13.9) | 92 (10.8) |
|
| T3a | 41 (17.2) | 81 (9.5) |
|
| T3b | 4 (1.7) | 35 (4.1) |
|
| T4 | 3 (1.3) | 6 (0.7) |
|
| Gleasons score, n
(%) |
|
| 0.0815 |
| ≤6 | 56 (23.5) | 260 (30.5) |
|
| 7 | 111 (46.6) | 371 (43.5) |
|
| 8 | 36 (15.1) | 139 (16.3) |
|
| 9 | 5 (2.1) | 74 (8.7) |
|
| 10 | 14 (1.3) | 9 (1.1) |
|
| D'Amico
classification, n (%) |
|
| 0.0055 |
| Low
risk | 31 (13.0) | 174 (20.4) |
|
|
Intermediate risk | 78 (32.8) | 338 (39.6) |
|
| High
risk | 129 (54.5) | 341 (40.0) |
|
| ADT use, n (%) |
|
| <0.0001 |
| Yes | 170 (71.4) | 476 (55.8) |
|
| No | 68 (28.6) | 377 (44.2) |
|
According to the classification of D'Amico et
al (14), patients were
stratified into three risk groups. More than half of the patients
in the elder group were high-risk, and the total risk of the elder
group was higher than that of the younger group. The rate of ADT
use administration was higher in the elder group than in the
younger group. The median follow-up periods of the elder and
younger groups were 42 (range, 2–108) and 49 (range, 2–120) months,
respectively.
Treatment planning
One hour after urine collection, each patients was
positioned supine with the immobilization devices and computed
tomography (CT) scanning was performed. Axial CT scans of 2.5-mm
thick sections were obtained from the superior border of the
sacroiliac joint to 5 cm below the ischial tuberosity. TomoTherapy
Treatment Planning System (TomoTherapy Inc., Madison, WI, USA) is a
radiation delivery system that combines dynamic IMRT with an
image-guided radiation therapy system. IMRT involving helical
tomotherapy (HT) was planned using an inverse-planning approach.
For T1-T3a cancer patients, the clinical target volume (CTV) was
defined as the prostate and proximal portions of the seminal
vesicles. For T3b cancer patients, the CTV included the prostate
and entire seminal vesicles. The planning target volume (PTV) was
set by expanding the CTV by 5 mm in all directions. Patients at
low- and intermediate-risk with biopsy-positive core rate ≤50% were
irradiated with 74 Gy in 37 fractions. Patients at
intermediate-risk with biopsy-positive core rate >50% were
irradiated with 76 Gy in 38 fractions. For high-risk patients, the
prescribed dose was 78 Gy in 39 fractions. Radiation therapy was
administered five times a week. The dose limits for PTV were as
follows: the volume of PTV receiving 95% of the prescribed dose
(V95) was >90% (preferably >95%); the volume of the PTV
receiving at least 90% of the prescribed dose (V90) was >96%
(preferably >98%); and maximum dose to the PTV was <110% of
the prescribed dose. The rectum was delineated from 15 mm superior
to 15 mm inferior to the PTV. Rectal wall thickness and bladder
wall thickness, both of 3 mm, were created. The dose constrains for
the rectum were V40<60%, V60<30%, V70<20% and V78<1%.
The dose constrains for the bladder were V40<60% and V70<30%.
Vx was defined as the percentage of structure volume receiving at
least one dose of ‘x’ Gy. Megavoltage CT image-guided verification
was carried out every day prior to each treatment. A 6-MV photon
beam was used for treatment.
ADT was started before IMRT and was continued during
IMRT for patients in the intermediate-, and high-risk groups.
Neoadjuvant ADT was initiated 3–6 months before IMRT. For high-risk
patients, adjuvant ADT was continued for up to 2 years.
Evaluation
Biochemical failure was defined as PSA nadir plus 2
ng/ml according to the Phoenix criteria (15). In patients suspected of having local
recurrence, magnetic resonance imaging (MRI) was performed and any
MRI positive lesions were diagnosed using. A clinical failure was
defined as the presence of local recurrence or metastases, and was
confirmed by imaging, such as CT, MRI, or bone scintigraphy, in
addition to checking PSA elevation. The examination was repeatedly
conducted in patients with biochemical failure at appropriate
timing, such as upon further elevation of PSA or appearance of
symptom. Failure-free rates and survival rates were calculated from
the completion date of IMRT to the occurrence of any event. We
calculated the biochemical failure-free rate (BFFR), clinical
failure-free rate (CFFR), and OS rates using the Kaplan-Meier
method. Comparisons between the groups were conducted using the
log-rank test. Using the Kaplan-Meier method, the cumulative
incidence rate of grade ≥2 GI and genitourinary (GU) toxicities
were calculated according to the Common Terminology Criteria for
Adverse Events (v4.0) (16). At our
hospital, a radiation oncologist and urologist interviewed the
patient regarding presence or absence of rectal bleeding upon each
visit after IMRT. Moreover, a urologist performed digital rectal
examination at every visit. When rectal bleeding is present,
patients are required to undergo colonoscopy.
Comparison between clinical factors of the two
groups was performed using the chi-square test and R 2.13.0
software (www.r-project.org/). P<0.05 was
considered to indicate a statistically significant difference.
Results
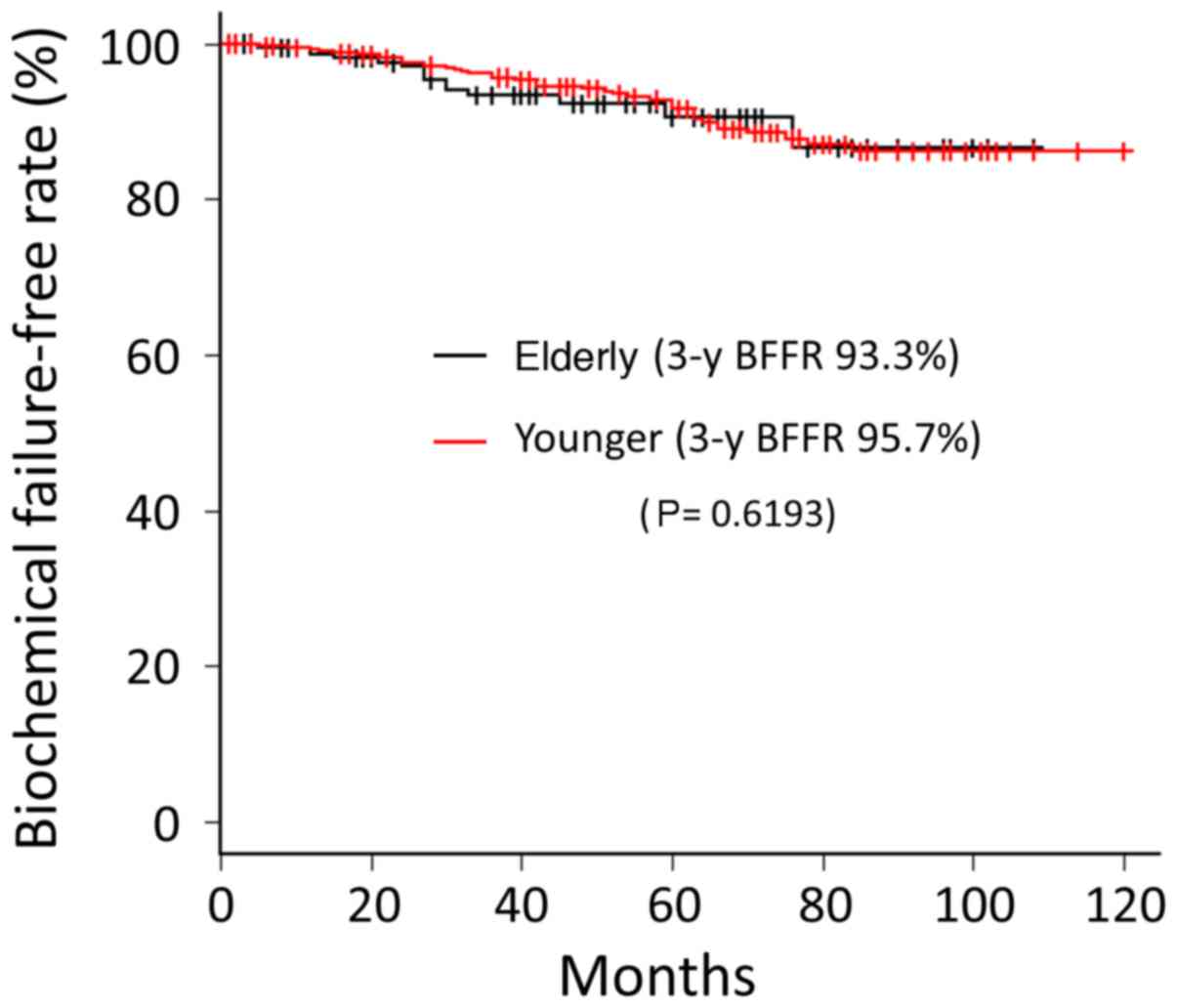
Biochemical failure was observed in 15 (6.3%)
patients in the elder group. The BFFRs at 3-year follow-up for
elder and younger groups were 93.3 and 95.7%, respectively. There
was no significant difference between the two groups in BFFR
(P=0.6193) (Fig. 1).
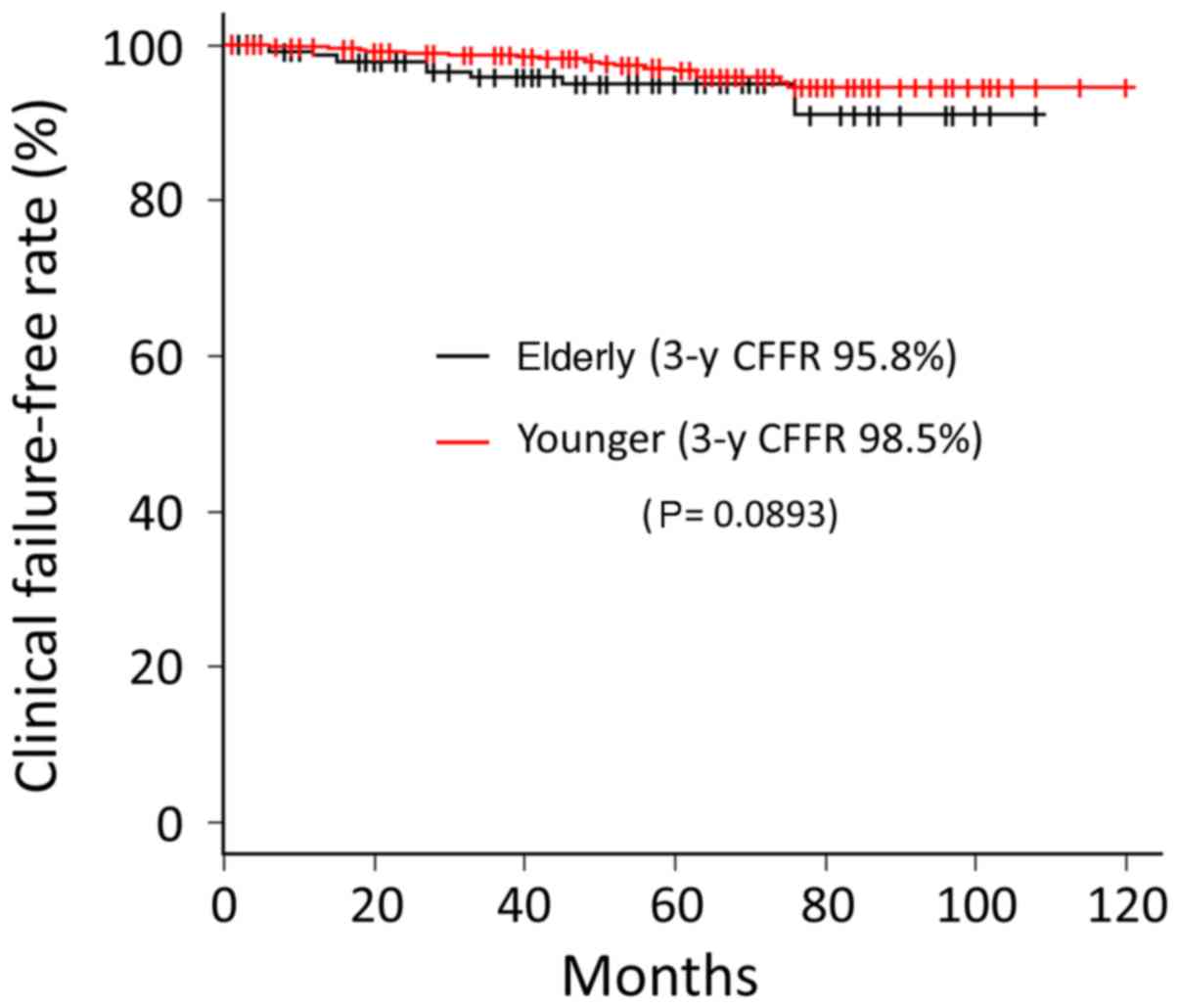
Ten (4.2%) cases of clinical failure were observed
in the elder group. The first failure sites were pelvic node, bone,
and lungs in five, four, and one patient, respectively. Local
failure was not observed in the elder group during the follow-up
period. Conversely, the sites of first failure in the younger group
were local (prostate), pelvic node, para-aortic node, and bone in
eight, five, one, and nine patients, respectively. The CFFR at
3-year follow-up for elder and younger groups were 95.8 and 98.5%,
respectively. No significant difference was observed between the
two groups (P=0.0893) (Fig. 2).
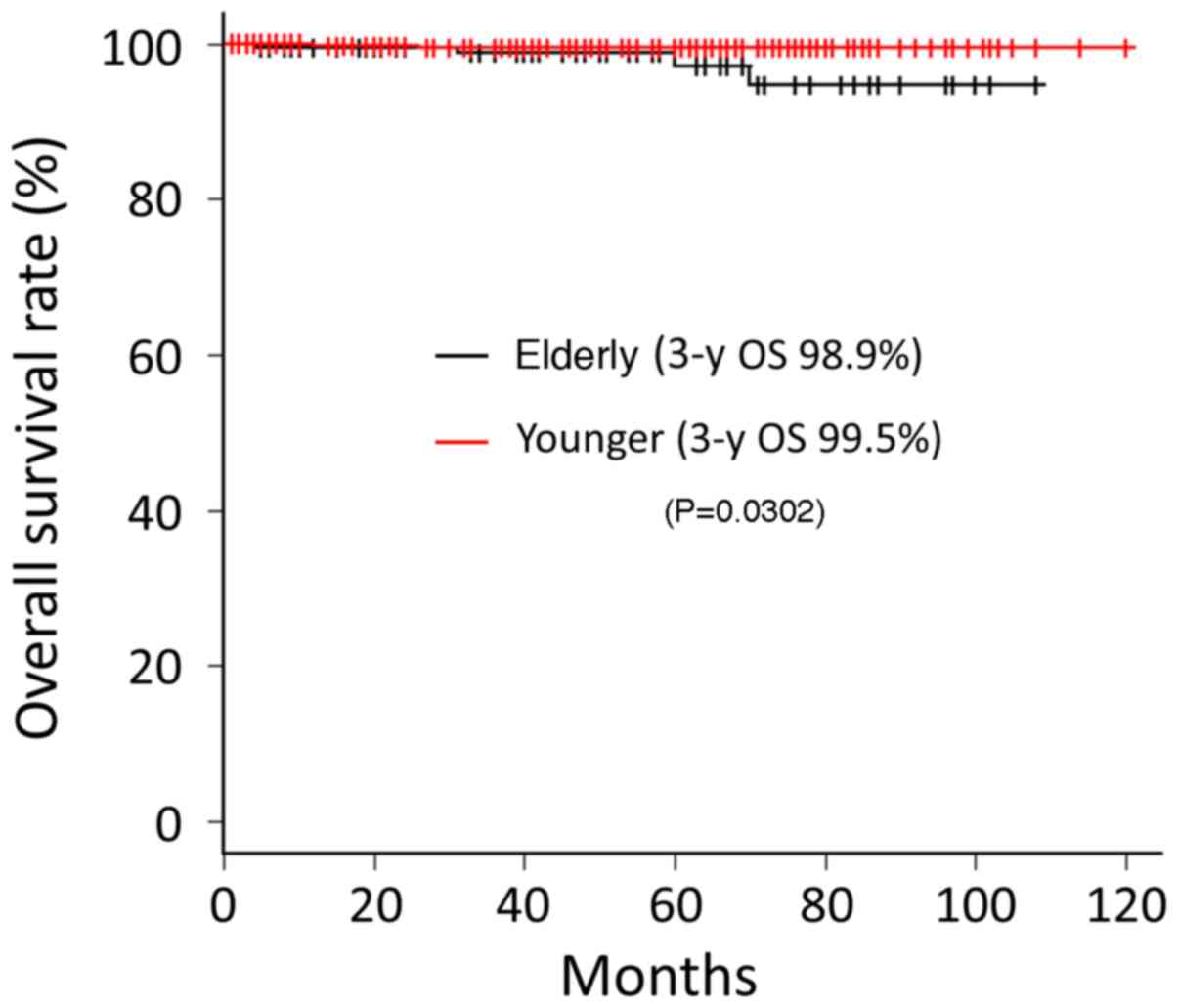
Five (2.1%) patients in the elder group died during
the follow-up period. Among them, two died due to prostate cancer,
two died due to another cancer, and one died due to pneumonia. The
OS rates at 3-year follow-up for elder and younger groups were 98.9
and 99.5%, respectively; the difference between the two groups was
statistically significant (P=0.0302) (Fig. 3). We compared the OS rates of elderly
and younger patients in each group. There were no significant
differences between elderly and younger patients in the
intermediate-, high-, and very high-risk groups (P=0.236, 0.841,
and 0.215, respectively). In the low-risk group, the OS rate of
elderly group was significantly lower than that of younger group
(P=0.00169). Meanwhile, there was no significant difference between
elderly and young patients in CFFR, even in the low-risk group
(P=0.443).
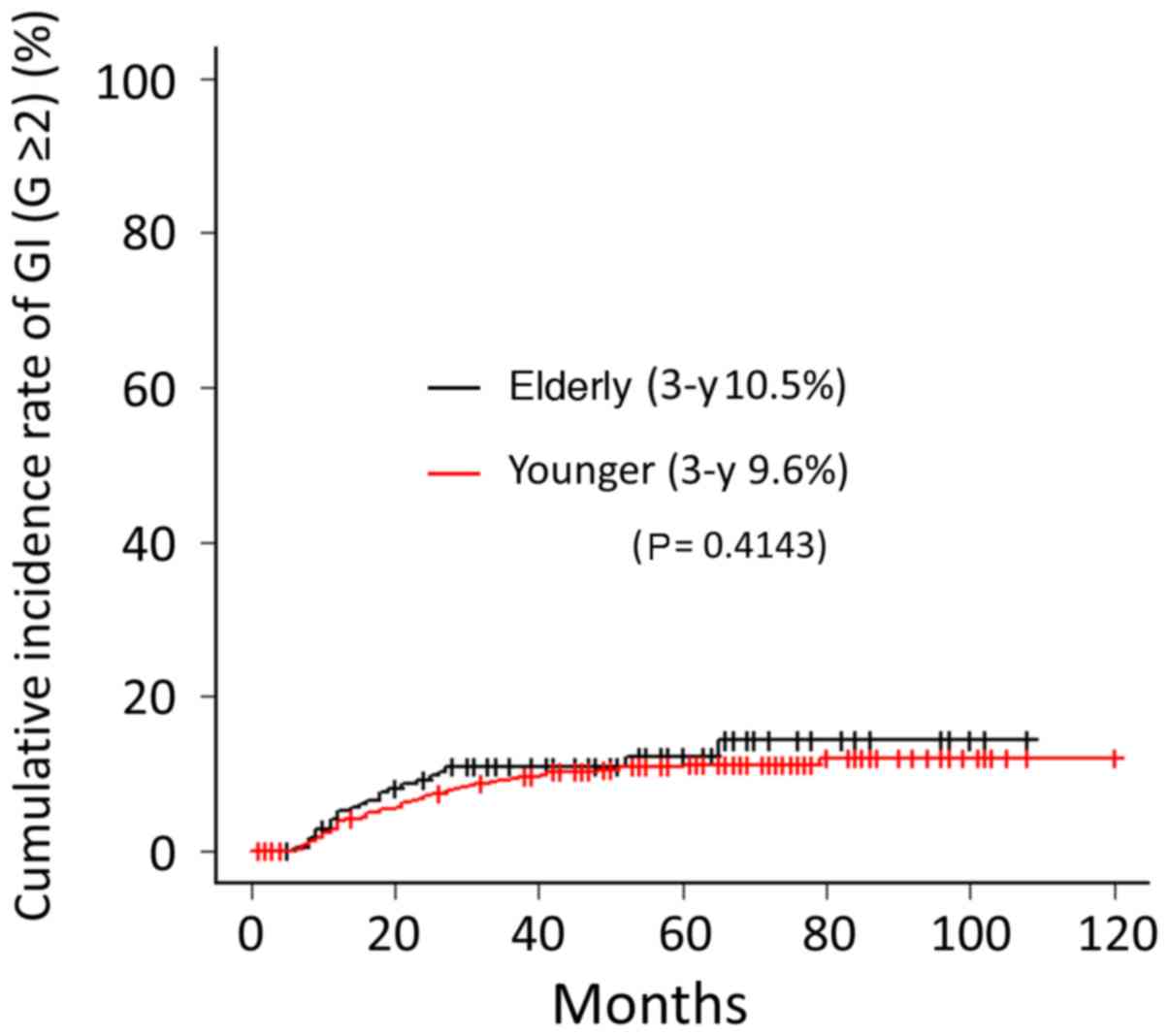
Grade ≥2 GI toxicity was observed in 23 (9.7%) cases
in the elder group. Among them, six (2.5%) cases were grade 3. No
cases of grade 4 GI toxicity were observed. The cumulative
incidence rates of GI toxicity (grade ≥2) at 3-year follow-up were
10.5 and 9.6% for elder and younger groups, respectively; no
significant difference between the two groups was founded
(P=0.4143) (Fig. 4). Grade ≥2 GU
toxicity was observed in nine (3.8%) cases in the elder group. No
cases with grade ≥3 GU toxicity were noted. The cumulative
incidence rates of GU toxicity (grade ≥2) at 3-year follow-up were
1.3 and 0.6% in the elder and younger groups, respectively; no
significant difference was observed between the two groups
(P=0.0769) (Fig. 5). There were no
significant differences in the incidence rates of GI or GU ≥ grade
2 overall, or in elderly patients only, due to differences in
prescription dose or risk classification.
Discussion
IMRT has been widely used for EBRT of localized
prostate cancer. The outcome of IMRT is comparable to other
treatment modalities, such as prostatectomy or brachytherapy
(17). Although each modality has
advantages and disadvantages, the incidence rate of adverse events
in every modality is within acceptable range (18). However, IMRT is the least invasive
choice among curable treatment options. To treat cancer in the
elderly, the treatment is chosen with consideration of age, life
expectancy, general condition, and comorbidity. Elderly patients
with prostate cancer were more likely to receive ADT alone
(11); however, a study in elderly
patients reported that conservative management with ADT alone does
not improve the OS rate (12). The
life expectancy of the elderly is increasing, and a curable
approach should be considered for patients in good general
condition independent of age. IMRT would be the most promising
modality among curable treatment choices due to its lower
invasiveness. During treatment of elderly cancer patients, a higher
frequency and degree of adverse events may be observed (19). It is worthwhile to note that in our
study, the IMRT for prostate cancer in the elderly was comparable
to that in younger patients. Although no significant difference
between elderly and the young patient groups was founded in regard
to GU rates, it is necessary to attend to the fact that the
incidence rate of GU was higher in the elder group (1.3% compared
to 0.6% in elder and younger groups, respectively).
In our study, the elder group had a higher overall
risk than the younger group. This result is in agreement with the
report of Shao et al showing increased proportion of higher
risk prostate cancer with increased age at the time of diagnosis
(20). Despite the large proportion
of high-risk patients, the BFFR and CFFR of the elder group did not
significantly differ from those of the younger group. This result
may be partly due to the high ADT usage in the elder group;
however, the reason for the high usage of ADT was the high
proportion of intermediate- and high-risk patients in the elder
group. Even regarding adverse events, no significant difference in
the incidence rates of both GI and GU could be found between elder
and younger groups. The incidence rates of GU tended to increase
after five years in both groups; this tendency was particularly
strong in the elder group. Therefore, we think that long-term
follow-up is necessary even for elderly patients treated with
IMRT.
Our study had one major limitation. Since we
retrospectively investigated the paper-based medical chart, we
could not accurately evaluate any acute adverse events including
skin toxicity. The presence or absence of complications was of
course confirmed prior to treatment, but we could not accurately
record this from the paper-based medical charts.
In conclusion, IMRT has an equivalent treatment
effect for prostate cancer in patients aged 75 years or older as in
patients younger than 75 years, and the incidence rates for adverse
events are also comparable between the two groups. In elderly
prostate cancer patients who have good general condition and long
life expectancy, IMRT should be considered as a treatment
option.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
HT, SI, SG, SO and MM conceived and designed the
experiments. HT, YN, MI, TY, KE, SK, SI, MH and MM analyzed the
data. HT, YN, MI, TY, KE, SK and SG wrote the first draft of the
manuscript. HT, YN, MI, TY, KE, SK, SI, MH, SO, SG and MM
contributed to the writing of the manuscript, and discussed the
results and conclusions. HT, YN, MI, TY, KE, SK, SI, MH, SO, SG and
MM jointly developed the structure and arguments for the paper,
critically revised the manuscript for important intellectual
content, and reviewed and approved the final manuscript.
Ethics approval and consent to
participate
The present study was approved by the Ethics
Committee of Kizawa Memorial Hospital. Written informed consents
were obtained from the patients.
Patients consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
GLOBOCAN 2012, . Estimated cancer
incidence, mortality and prevalence worldwide in 2012. Leyon: World
Health Organization; http://globocan.iarc.fr/Pages/fact_sheets_cancer.aspxOctober
31–2018
|
|
2
|
Projected cancer statistics, 2017. Tokyo,
. Center for Cancer Control and Information Services, National
Cancer Center. https://ganjoho.jp/en/public/statistics/short_pred.htmlOctober
31–2018
|
|
3
|
Cancer statistics in Japan 2016. Tokyo, .
Center for Cancer Control and Information Services, National Cancer
Center. https://ganjoho.jp/en/professional/statistics/brochure/2016_en.htmlOctober
31–2018
|
|
4
|
NCCN Guidelines for treatment of cancer by
site, . Prostate Cancer. Fort Washington: National Comprehensive
Cancer Network. https://www.nccn.org/professionals/physician_gls/pdf/prostate.pdfOctober
31–2018
|
|
5
|
Someya M, Hori M, Tateoka K, Nakata K,
Takagi M, Saito M, Hirokawa N, Hareyama M and Sakata K: Results and
DVH analysis of late rectal bleeding in patients treated with
3D-CRT or IMRT for localized prostate cancer. J Radiat Res (Tokyo).
56:122–127. 2015. View Article : Google Scholar
|
|
6
|
Cahlon O, Zelefsky MJ, Shippy A, Chan H,
Fuks Z, Yamada Y, Hunt M, Greenstein S and Amols H: Ultra-high dose
(86.4 Gy) IMRT for localized prostate cancer: Toxicity and
biochemical outcomes. Int J Radiat Oncol Biol Phys. 71:330–337.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Yamazaki H, Nakamura S, Nishimura T,
Yoshida K, Yoshioka Y, Koizumi M and Ogawa K: Transitioning from
conventional radiotherapy to intensity-modulated radiotherapy for
localized prostate cancer: Changing focus from rectal bleeding to
detailed quality of life analysis. J Radiat Res (Tokyo).
55:1033–1047. 2014. View Article : Google Scholar
|
|
8
|
Dolezel M, Odrazka K, Zouhar M, Vaculikova
M, Sefrova J, Jansa J, Paluska P, Kohlova T, Vanasek J and Kovarik
J: Comparing morbidity and cancer control after 3D-conformal (70/74
Gy) and intensity modulated radiotherapy (78/82 Gy) for prostate
cancer. Strahlenther Onkol. 191:338–346. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Tanaka H, Yamaguchi T, Hachiya K, Kamei S,
Ishihara S, Hayashi M, Ogawa S, Nishibori H, Goshima S and Matsuo
M: Treatment outcomes and late toxicities of intensity-modulated
radiation therapy for 1091 Japanese patients with localized
prostate cancer. Rep Pract Oncol Radiother. 23:28–33. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Alicikus ZA, Yamada Y, Zhang Z, Pei X,
Hunt M, Kollmeier M, Cox B and Zelefsky MJ: Ten-year outcomes of
high-dose, intensity-modulated radiotherapy for localized prostate
cancer. Cancer. 117:1429–1437. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Bechis SK, Carroll PR and Cooperberg MR:
Impact of age at diagnosis on prostate cancer treatment and
survival. J Clin Oncol. 29:235–241. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Lu-Yao GL, Albertsen PC, Moore DF, Shih W,
Lin Y, DiPaola RS and Yao SL: Survival following primary androgen
deprivation therapy among men with localized prostate cancer. JAMA.
300:173–181. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Japan ALTF: Tokyo. Ministry of Health,
Labour and Welfare, Japan, . 2016, http://www.mhlw.go.jp/english/database/db-hw/lifetb16/index.html
|
|
14
|
D'Amico AV, Whittington R, Malkowicz SB,
Cote K, Loffredo M, Schultz D, Chen MH, Tomaszewski JE, Renshaw AA,
Wein A, et al: Biochemical outcome after radical prostatectomy or
external beam radiation therapy for patients with clinically
localized prostate carcinoma in the prostate specific antigen era.
Cancer. 95:281–286. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Roach M III, Hanks G, Thames H Jr,
Schellhammer P, Shipley WU, Sokol GH and Sandler H: Defining
biochemical failure following radiotherapy with or without hormonal
therapy in men with clinically localized prostate cancer:
Recommendations of the RTOG-ASTRO Phoenix Consensus Conference. Int
J Radiat Oncol Biol Phys. 65:965–974. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Common Terminology Criteria for Adverse
Events (CTCAE) Version 4.0. Bethesda, . National Institutes of
Healths, Department of Health and Human Services. https://www.eortc.be/services/doc/ctc/CTCAE_4.03_2010-06-14_QuickReference_5x7.pdfOctober
31–2018
|
|
17
|
Wilcox SW, Aherne NJ, McLachlan CS, McKay
MJ, Last AJ and Shakespeare TP: Is modern external beam
radiotherapy with androgen deprivation therapy still a viable
alternative for prostate cancer in an era of robotic surgery and
brachytherapy: A comparison of Australian series. J Med Imaging
Radiat Oncol. 59:125–133. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Zelefsky MJ, Poon BY, Eastham J, Vickers
A, Pei X and Scardino PT: Longitudinal assessment of quality of
life after surgery, conformal brachytherapy, and
intensity-modulated radiation therapy for prostate cancer.
Radiother Oncol. 118:85–91. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Hayashi S, Tanaka H, Kajiura Y, Ohno Y and
Hoshi H: Stereotactic body radiotherapy for very elderly patients
(age, greater than or equal to 85 years) with stage I non-small
cell lung cancer. Radiat Oncol. 9:1382014. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Shao YH, Demissie K, Shih W, Mehta AR,
Stein MN, Roberts CB, Dipaola RS and Lu-Yao GL: Contemporary risk
profile of prostate cancer in the United States. J Natl Cancer
Inst. 101:1280–1283. 2009. View Article : Google Scholar : PubMed/NCBI
|