Introduction
Lung cancer is the leading cause of cancer-related
death in the world, with non-small cell lung cancer (NSCLC)
accounting for 85% (1,2). Management of advanced NSCLC has changed
drastically over the past 15 years. Specific targeted therapies
have been available for the treatment of advanced NSCLC. Tyrosine
kinase inhibitors (TKIs) for driver mutations such as epidermal
growth factor receptor (EGFR) mutations, anaplastic lymphoma kinase
(ALK) translocations, and c-ros oncogene 1 (ROS-1) are superior to
conventional platinum based cytotoxic agents in clinical trials
(3–7). Moreover, immune checkpoint inhibitors
(ICIs) which target programmed death-1 (PD-1) and programmed death
ligand-1 (PD-L1) have been shown to contribute to overall survival
(OS) as first or second line therapy in advanced NSCLC (8–11).
However, subset analysis of phase III clinical trials indicated
that ICIs might be less effective in advanced NSCLC harboring
driver mutations (12). A
retrospective study showed that ICIs had progression-free survival
(PFS) as short as 1.2–2.1 months in those with EGFR mutations
(13). It has been reported that the
overall response rate (ORR) was 3.6% when ICIs were used for the
patients with EGFR mutation (14).
In addition, a basic research suggested that the expression of
PD-L1 in patients with EGFR/ALK wild type is lower than that of
patients with EGFR/ALK mutated patients (15). In this study, we analyzed a real
world cohort of patients with NSCLC harboring driver mutations who
were treated with ICIs.
Patients and methods
Study design
In this retrospective observational study, we aimed
to evaluate the efficacy of ICIs in advanced NSCLC harboring driver
mutations. The medical records were collected from two
institutions, Hirosaki University (Hirosaki, Japan) and Aomori
Prefectural Central Hospital (Aomori, Japan). This study was
approved by the Hirosaki University institutional review board and
Aomori Prefectural Central Hospital institutional review board.
Target lesion assessment
The efficacy of ICIs was assessed according to
Response Evaluation Criteria in Solid Tumors (RECIST), version 1.1.
The size of the target lesions were measured by imaging studies
(i.e, chest radiography, computed tomography, magnetic resonance
imaging).
Evaluation and statistical
analysis
The PFS was estimated using the Kaplan-Meier method.
The PFS has been defined as the time from the date of treatment
initiation to the date of disease progression, death or the last
contact. If neither event was observed, it was considered to be
censored with the latest observation date. In case, post-treatment
was started, it was considered to be censored with the date of
initiation of next line chemotherapy. If the event was unknown in
the case of transfer or non-arrival, it was censored with the final
date when the patient survival was confirmed. Statistical analyses
were performed using JMP 10 (SAS Institute, Cary, NC, USA).
Intergroup comparisons of response rate and other parameters were
made using the log-rank and chi-square tests. The significance
level was set at P<0.05.
Results
Patient characteristics
Among 139 patients who were treated previously with
molecular targeted therapy at Hirosaki University and Aomori
Prefectural Central Hospital from September 2014 to January 2017,
24 received ICI. The characteristics of the 24 eligible patients
were listed in Table I. Five male
(20.8%) patients and 19 female (79.2%) patients, with a median age
of 68 years (range, 39–82 years), were included. Twenty (83.3%)
patients had Eastern Cooperative Oncology Group (ECOG) performance
status (PS) of 0–1, and 23 (95.8%) had adenocarcinoma histology.
Only one patient had squamous cell carcinoma histology. Two
patients had stage IIIB, 12 had stage IV, and 10 had recurrent
disease. The driver mutation status was as follows: Exon 19
del/exon 21 L858R/ALK/ROS-1/Rearranged during transfection (RET) in
14/7/1/1/1, respectively. The reason for discontinuation of prior
TKI treatment for all patients was caused by progressive disease
(PD). All patients except one patient who had ROS-1 positive
patients received prior molecular target therapy. There were 7
patients (29.2%) who had resistant mutation. Twenty two (91.7%)
patients were treated with nivolumab, 2 (8.3%) patients were
treated with pembrolizumab.
 | Table I.Patient characteristics (n=24). |
Table I.
Patient characteristics (n=24).
| Variable | Number |
|---|
| Sex, n (%) |
|
| Male | 5 (20.8) |
|
Female | 19 (79.2) |
| Age, years
(range) | 68 (39–82) |
| ECOG PS, n (%) |
|
| 0–1 | 20 (83.3) |
| 2 | 4 (16.7) |
| Clinical stage, n
(%) |
|
| IIIB | 2 (8.3) |
| IV | 12 (50.0) |
|
Recurrence | 10 (41.7) |
| Histological type, n
(%) |
|
|
Adenocarcinoma | 23 (95.8) |
| Squamous
cell carcinoma | 1 (4.2) |
| Smoking history, n
(%) |
|
|
Smoker | 6 (25.0) |
| Non
smoker | 18 (75.0) |
| Driver mutation, n
(%) |
|
| Exon 19
del | 14 (58.3) |
| Exon 21
L858R | 7 (29.1) |
| ALK | 1 (4.2) |
|
ROS-1 | 1 (4.2) |
| RET | 1 (4.2) |
| Resistance gene, n
(%) |
|
| T790M
positive | 7 (29.2) |
| Negative
or unknown | 17 (70.8) |
| Treatment, n (%) |
|
|
Nivolumab | 22 (91.7) |
|
Pembrolizumab | 2 (8.8) |
Efficacy
The response to ICIs was shown in Table II. Four patients attained a partial
response (PR) but no patients attained a complete response (CR).
The ORR was 16.7%, and 4 patients (16.7%) had stable disease (SD).
Fifteen patients (62.5%) had PD. Subset analysis for the ORR by
baseline characteristics of patient was shown in Table III. There were no significant
relationships between patient characteristics and response to ICIs.
No patients (0%) with T90M achieved PR. On the other hand, 4
patients (23.5%) with negative or unknown of resistance gene
achieved PR, although the difference did not reach statistical
significance (P=0.10).
 | Table II.Response to immune checkpoint
inhibitors. |
Table II.
Response to immune checkpoint
inhibitors.
| Response | Number of
patients | Percentage, % |
|---|
| Complete
response | 0 | 0 |
| Partial response | 4 | 16.7 |
| Stable disease | 4 | 16.7 |
| Progressive
disease | 15 | 62.5 |
| Not evaluable | 1 | 4.2 |
| Response rate |
| 16.7 |
 | Table III.Univariate analysis of clinical
factors for ORR of immune checkpoint inhibitors. |
Table III.
Univariate analysis of clinical
factors for ORR of immune checkpoint inhibitors.
| Clinical factor | ORR (%) | P-value |
|---|
| Age, years |
| 0.95 |
|
<75 | 16.7 |
|
| ≥75 | 17.7 |
|
| Smoking status |
| 0.10 |
|
Smoker | 0 |
|
|
Never | 23.5 |
|
| PS |
| 0.67 |
|
0–1 | 15.8 |
|
| 2 | 25.0 |
|
| Resistance
gene |
| 0.10 |
| T790M
positive | 0 |
|
|
Negative or unknown | 23.5 |
|
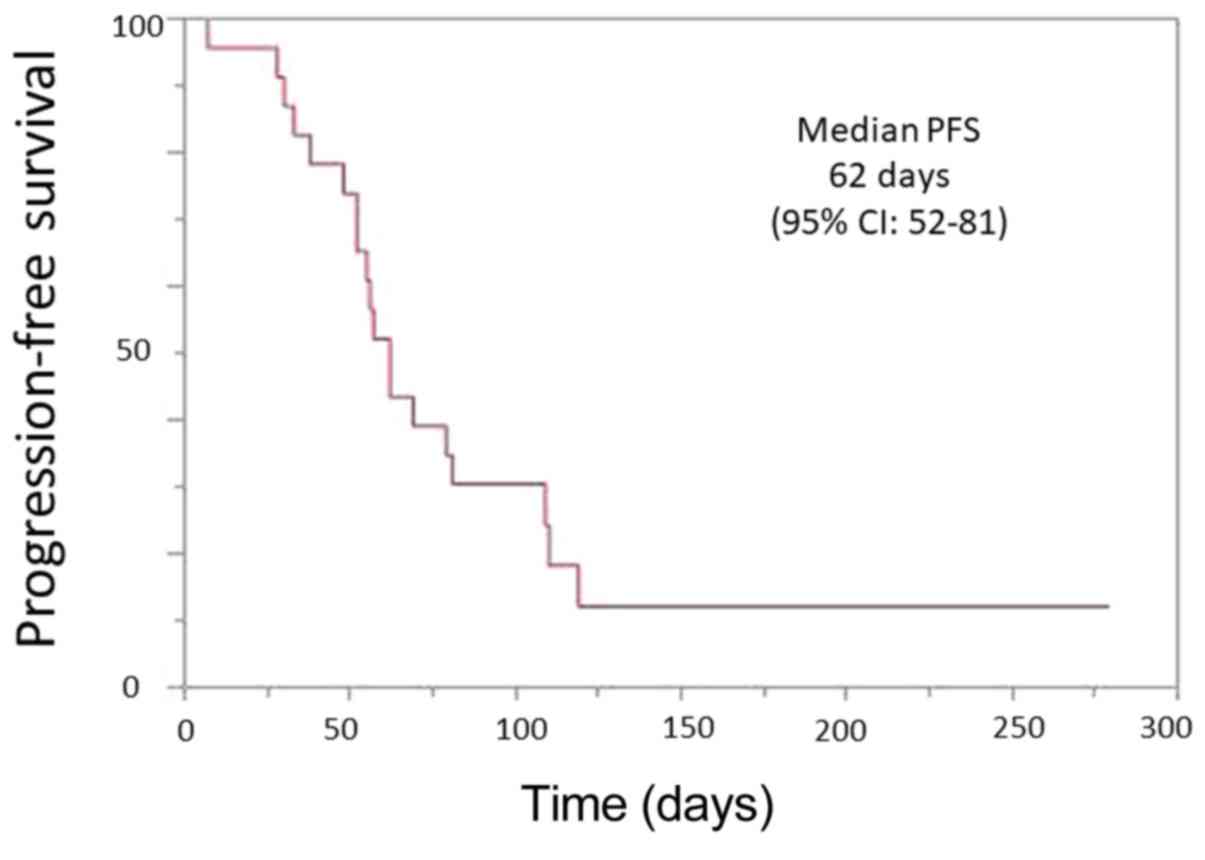
The median PFS was 62 days (95% CI, 52- 81)
(Fig. 1). A significant difference
was observed in the PFS between the patients with longer treatment
with TKI and those with shorter treatment (110 vs. 56 days;
P=0.012; Fig. 2). Of the 23 patients
who received prior TKI treatment, 9 patients had TKI treatment
period of 1 year or more, and the remaining 14 patients had TKI
administration period of less than 1 year. One patient who had
ROS-1 mutation was not treated with any TKI. There were no
significant correlations between other clinical characteristics and
PFS. In our study, there were 2 patients with rare mutation. One
patient had ROS-1 and another patients had RET.
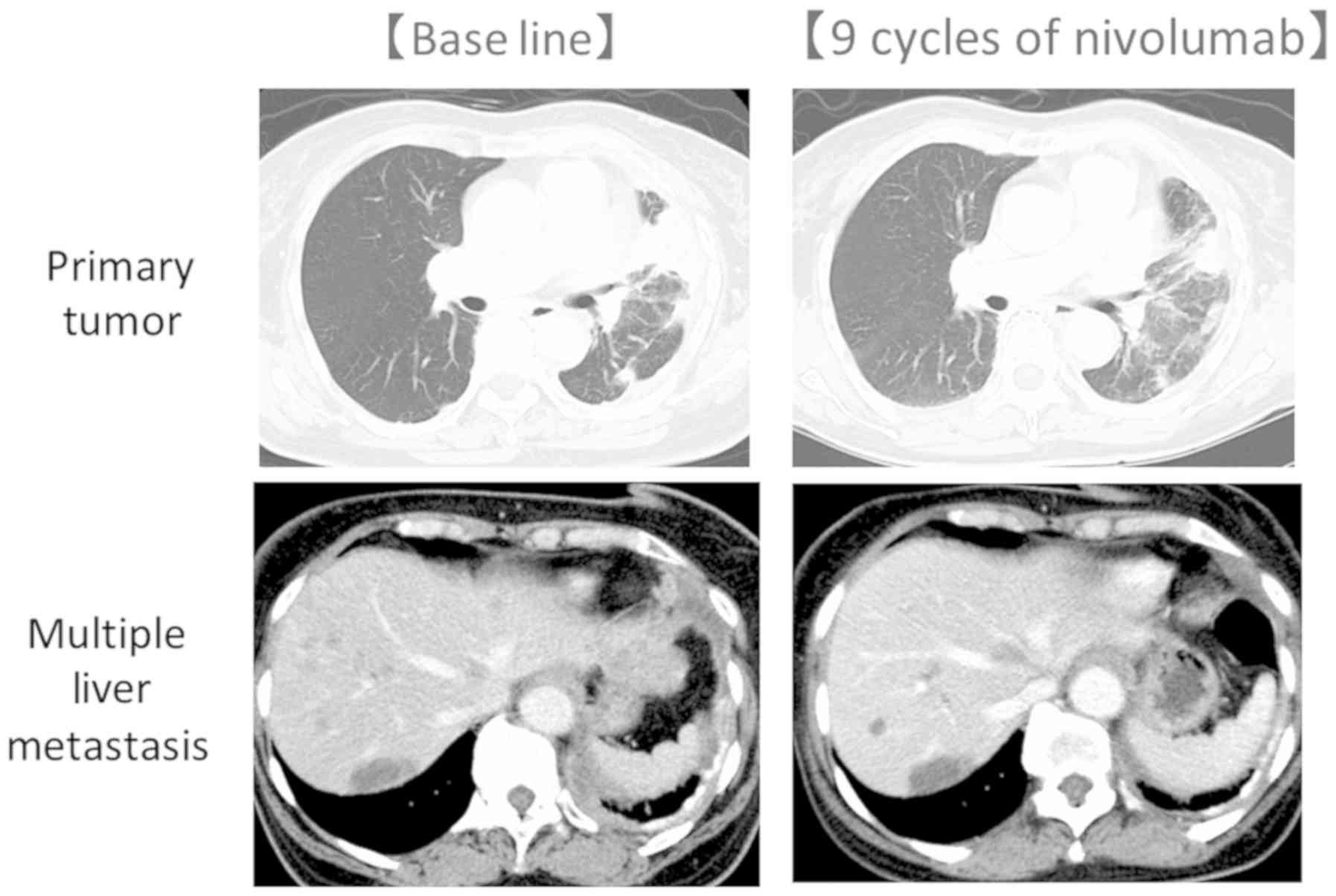
A 71-year old woman was diagnosed with stage IV lung
adenocarcinoma with ROS-1 in November 2015. After her four cycles
of carboplatin plus pemetrexed followed 4 cycles of maintenance
therapy, she had PD. Increased primary tumor and multiple liver
metastases appeared. She was treated with nivolumab and on 9 cycles
of treatment, a PR was confirmed (Fig.
3).
Discussion
In this retrospective study, we evaluated that the
efficacy of ICIs in patients with advanced NSCLC harboring driver
mutations and relationship between the efficacy and the patient
characteristics. We found that ORR was 16.7%, which was higher than
the numbers reported previously (14,16).
Moreover, a subset analysis revealed that patients who had received
TKIs for longer term had better PFS than those treated with shorter
terms of TKIs (110 vs. 56 days; P=0.012). Today, it is a serious
clinical problem that there are few treatment choices after
standard molecular target therapy. A previous subset analysis of
phase III trials of ICIs did not show the OS benefit compared with
docetaxel in patients with EGFR mutation (8,9,11). However, because the number of
patients included in these studies was small, it has been unclear
whether ICIs are effective or not.
Tumor mutation burden (TMB) and PD-L1 are key
biomarkers predictive of the effectiveness of ICIs treatment
(17). TMB does not correlate with
PD-L1 expression and both markers have similar predictive capacity
(18). In NSCLC with either EGFR
mutation, or ROS-1 or ALK oncogene, TMB is lower than wild type
(19–21). This is one of the main reasons why
ICIs are less effective for mutant NSCLC. Recently, 80 patients
with NSCLC harboring EGFR/ALK mutation were analyzed. The
population with PD-L1 tumor proportion score of 50% or higher was
reported to be 11.3% (22). Several
studies showed that PD-L1 expression is also regulated by oncogenic
drivers in NSCLC. EGFR activated by EGF stimulation, exon 19
deletion and L858R mutation induced PD-L1 expression, suggesting
that constitutive oncogene pathway activation can up-regulate
PD-L1. Chen and colleagues reported that inhibiting EGFR activation
could reduce PD-L1 expression (23).
Omori and colleagues showed that treatments with EGFR-TKIs may
increase PD-L1 expression in NSCLC harboring EGFR mutations. In
that report, 38% of the patients who were treated with EGFR-TKI
increased PD-L1 expression (24).
Other research group also suggested PD-L1 expression in tumor cells
markedly increased in a subset of patients after gefitinib
treatment (25). On the other hand,
Lin and colleagues reported that PD-L1 expression did not correlate
with treatment response and PFS in EGFR mutant patients, suggesting
that PD-L1 status may not be associated with the efficacy of ICIs
in patients with driver mutation (26). In our study, PD-L1 expression was not
determined in most patients, which is one of the major limitations
of our study. In our study, there were no patients who achieved
tumor PR in the patients with T790M. Haratani et al reported
that T790M-negative patients had longer PFS than T790M-positive
patients (2.1 vs. 1.3 months) after EGFR-TKI treatment (27). They suggested that prospective
clinical trials are required to confirm the efficacy of PD-1
inhibitors in T790M-negative patients with EGFR mutation-positive
NSCLC. Clinical trial is in progress (UMIN000021133). In our study,
one patient with ROS-1 was included. The patient had achieved PR.
Treatments of ICIs for the patients with rare mutations such as
ROS-1, RET has been unclear yet.
Our study had some limitations. First, this study
was retrospective study. Second, PD-L1 expression and T790M were
not evaluated in every patient. Finally, sample size was small.
Further analyses are warranted to determine the efficacy of ICIs in
patients with driver mutations after TKI treatment. In conclusion,
Even in NSCLC harboring driver mutation, there were some patient
who could receive benefit of ICI and the treatment duration of TKI
might be related to the efficacy.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used in this current study are
available from the corresponding author on reasonable request.
Authors' contributions
HS prepared the manuscript and made contributions to
acquisition of data. HT was invovled in the conception of this
study. HT and KT conducted statistical analysis. TS, KB, YI and MI
treated and observed patients in Hirosaki University. YH treated
and observed patients in Aomori Prefectural Central Hospital. ShT
advised and revised the statistical analysis. SaT contributed in
evaluating the tumor efficacy on the CT scan and was a major
contributor in writing the manuscript. All authors read and
approved the final manuscript.
Ethics approval and consent to
participate
All procedures performed in studies involving human
participants were in accordance with the ethical standards of the
institutional and/or national research committee and with the 1964
Helsinki declaration and its later amendments or comparable ethical
standards. The study protocol was approved by the institutional
review boards of all participating institutions.
Patient consent for publication
The present study was performed on a retrospective
observational cohort. Therefore, informed consent was not obtained.
The opt out approach was used.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Ettinger DS, Wood DE, Aisner DL, Akerley
W, Bauman J, Chirieac LR, D'Amico TA, DeCamp MM, Dilling TJ,
Dobelbower M, et al: Non-small cell lung cancer, version 5.2017,
NCCN clinical practice guidelines in oncology. J Natl Compr Canc
Netw. 15:504–535. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Siegel RL, Miller KD and Jemal A: Cancer
statistics, 2017. CA Cancer J Clin. 67:7–30. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Maemondo M, Inoue A, Kobayashi K, Sugawara
S, Oizumi S, Isobe H, Gemma A, Harada M, Yoshizawa H, Kinoshita, et
al: Gefitinib or chemotherapy for non-small-cell lung cancer with
mutated EGFR. N Engl J Med. 362:2380–2388. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Wu YL, Zhou C, Hu CP, Feng J, Lu S, Huang
Y, Li W, Hou M, Shi JH, Lee KY, et al: Afatinib versus cisplatin
plus gemcitabine for first-line treatment of Asian patients with
advanced non-small-cell lung cancer harbouring EGFR mutations
(LUX-Lung 6): An open-label, randomised phase 3 trial. Lancet
Oncol. 15:213–222. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Mok TS, Wu Y-L, Ahn MJ, Garassino MC, Kim
HR, Ramalingam SS, Shepherd FA, He Y, Akamatsu H, Theelen WS, et
al: Osimertinib or platinum-pemetrexed in EGFR T790M-positive lung
cancer. N Engl J Med. 376:629–640. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Solomon BJ, Mok T, Kim DW, Wu YL, Nakagawa
K, Mekhail T, Felip E, Cappuzzo F, Paolini J, Usari T, et al:
First-line crizotinib versus chemotherapy in ALK-positive lung
cancer. N Engl J Med. 371:2167–2177. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Shaw AT, Ou SH, Bang YJ, Camidge DR,
Solomon BJ, Salgia R, Riely GJ, Varella-Garcia M, Shapiro GI, Costa
DB, et al: Crizotinib in ROS1-rearranged non-small-cell lung
cancer. N Engl J Med. 371:1963–1971. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Borghaei H, Paz-Ares L, Horn L, Spigel DR,
Steins M, Ready NE, Chow LQ, Vokes EE, Felip E, Holgado E, et al:
Nivolumab versus docetaxel in advanced nonsquamous non-small-cell
lung cancer. N Engl J Med. 373:1627–1639. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Herbst RS, Baas P, Kim DW, Felip E,
Pérez-Gracia JL, Han JY, Molina J, Kim JH, Arvis CD, Ahn MJ, et al:
Pembrolizumab versus docetaxel for previously treated,
PD-L1-positive, advanced non-small-cell lung cancer (KEYNOTE-010):
A randomised controlled trial. Lancet. 387:1540–1550. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Reck M, Rodríguez-Abreu D, Robinson AG,
Hui R, Csőszi T, Fülöp A, Gottfried M, Peled N, Tafreshi A, Cuffe
S, et al: Pembrolizumab versus chemotherapy for PD-L1-positive
non-small-cell lung cancer. N Engl J Med. 375:1823–1833. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Rittmeyer A, Barlesi F, Waterkamp D, Park
K, Ciardiello F, von Pawel J, Gadgeel SM, Hida T, Kowalski DM, Dols
MC, et al: Atezolizumab versus docetaxel in patients with
previously treated non-small-cell lung cancer (OAK): A phase 3,
open-label, multicentre randomised controlled trial. Lancet.
389:255–265. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Lee CK, Man J, Lord S, Links M, Gebski V,
Mok T and Yang JC: Checkpoint inhibitors in metastatic EGFR-mutated
non-small cell lung cancer-a meta-analysis. J Thorac Oncol.
12:403–407. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Bylicki O, Paleiron N, Margery J, Guisier
F, Vergnenegre A, Robinet G, Auliac JB, Gervais R and Chouaid C:
Targeting the PD-1/PD-L1 immune checkpoint in EGFR-Mutated or
ALK-Translocated non-small-cell lung cancer. Target Oncol.
12:563–569. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Gainor JF, Shaw AT, Sequist LV, Fu X,
Azzoli CG, Piotrowska Z, Huynh TG, Zhao L, Fulton L, Schultz KR, et
al: EGFR mutations and ALK rearrangements are associated with low
response rates to PD-1 pathway blockade in non-small cell lung
cancer: A retrospective analysis. Clin Cancer Res. 22:4585–4593.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Azuma K, Ota K, Kawahara A, Hattori S,
Iwama E, Harada T, Matsumoto K, Takayama K, Takamori S, Kage M, et
al: Association of PD-L1 overexpression with activating EGFR
mutations in surgically resected nonsmall-cell lung cancer. Ann
Oncol. 25:1935–1940. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Kobayashi K, Nakachi I, Naoki K, Satomi R,
Nakamura M, Inoue T, Tateno H, Sakamaki F, Sayama K, Terashima T,
et al: Real-world efficacy and safety of nivolumab for advanced
non-small-cell lung cancer: A retrospective multicenter analysis.
Clin Lung Cancer. 19:e349–e358. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Carbone DP, Reck M, Paz-Ares L, Creelan B,
Horn L, Steins M, Felip E, van den Heuvel MM, Ciuleanu TE, Badin F,
et al: First-line nivolumab in stage IV or recurrent non-small-cell
lung cancer. N Engl J Med. 376:2415–2426. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Gandara DR, Paul SM, Kowanetz M,
Schleifman E, Zou W, Li Y, Rittmeyer A, Fehrenbacher L, Otto G,
Malboeuf C, et al: Blood-based tumor mutational burden as a
predictor of clinical benefit in non-small-cell lung cancer
patients treated with atezolizumab. Nat Med. 24:1441–1448. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Rozenblum AB, Ilouze M, Dudnik E, Dvir A,
Soussan-Gutman L, Geva S and Peled N: Clinical impact of hybrid
capture-based next-generation sequencing on changes in treatment
decisions in lung cancer. J Thorac Oncol. 12:258–268. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Hatakeyama K, Nagashima T, Urakami K,
Ohshima K, Serizawa M, Ohnami S, Shimoda Y, Ohnami S, Maruyama K,
Naruoka A, et al: Tumor mutational burden analysis of 2,000
Japanese cancer genomes using whole exome and targeted gene panel
sequencing. Biomed Res. 39:159–167. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Spiegel DR, Schrock AB, Fabrizio D,
Frampton GM, Sun J and He J: Total mutation burden (TMB) in lung
cancer (LC) and relationship with response to PD-1/PD-L1 targeted
therapies. J Clin Oncol. 34 (Suppl 15):S90172016. View Article : Google Scholar
|
|
22
|
Yoneshima Y, Ijichi K, Anai S, Ota K,
Otsubo K, Iwama E, Tanaka K, Oda Y, Nakanishi Y and Okamoto I:
PD-L1 expression in lung adenocarcinoma harboring EGFR mutations or
ALK rearrangements. Lung Cancer. 118:36–40. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Chen N, Fang W, Zhan J, Hong S, Tang Y,
Kang S, Zhang Y, He X, Zhou T, Qin T, et al: Upregulation of PD-L1
by EGFR activation mediates the immune escape in EGFR-Driven NSCLC:
Implication for optional immune targeted therapy for NSCLC Patients
with EGFR mutation. J Thorac Oncol. 10:910–923. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Omori S, Kenmotsu H, Abe M, Watanabe R,
Sugino T, Kobayashi H, Nakashima K, Wakuda K, Ono A, Taira T, et
al: Changes in programmed death ligand 1 expression in non-small
cell lung cancer patients who received anticancer treatments. Int J
Clin Oncol. 23:1052–1059. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Han JJ, Kim DW, Koh J, Keam B, Kim TM,
Jeon YK, Lee SH, Chung DH and Heo DS: Change in PD-L1 expression
after acquiring resistance to gefitinib in EGFR-Mutant
non-small-cell lung cancer. Clin Lung Cancer. 17:263–270. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Lin SY, Yang CY, Liao BC, Ho CC, Liao WY,
Chen KY, Tsai TH, Hsu CL, Hsu WH, Su KY, et al: Tumor PD-L1
expression and clinical outcomes in advanced-stage non-small cell
lung cancer patients treated with nivolumab or pembrolizumab:
Real-world data in Taiwan. J Cancer. 9:1813–1820. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Haratani K, Hayashi H, Tanaka T, Kaneda H,
Togashi Y, Sakai K, Hayashi K, Tomida S, Chiba Y, Yonesaka K, et
al: Tumor immune microenvironment and nivolumab efficacy in EGFR
mutation-positive non-small-cell lung cancer based on T790M status
after disease progression during EGFR-TKI treatment. Ann Oncol.
28:1532–1539. 2017. View Article : Google Scholar : PubMed/NCBI
|