Introduction
Synovial sarcoma (SS) is a soft tissue sarcoma (STS)
that can occur at many different locations. In Japan, SS has been
ranked as the fifth most common STS, with the exception of
well-differentiated liposarcoma, and its occurrence has been shown
to peak in the third and fourth decades of life (1). The standard treatment for SS is wide
surgical resection with or without radiotherapy, which is similar
to the approach for other STSs (2).
The role of chemotherapy is highly debated. A review of 15 clinical
trials demonstrated that advanced SS had a better response to
systemic chemotherapy than other subtypes of STS (3). Hence, neoadjuvant and/or adjuvant
chemotherapy (N/AC) is frequently used for localized SS, especially
in young patients (4). However, the
role of N/AC is still controversial. Several large studies have
demonstrated a survival benefit with N/AC (5–7), whereas
others have not demonstrated such a benefit (4,8–10). Further, the dose efficacy of N/AC
remains unclear. The present study aimed to review the clinical
outcomes of surgically treated localized SS and investigate the
effects of N/AC with long-term observation.
Patients and methods
We assessed our institutional database and
identified 54 patients with histological diagnosis of localized SS
treated between 2000 and 2016. All patients were staged according
to computed tomography (CT) and/or magnetic resonance imaging (MRI)
findings. The 8th American Joint Committee on Cancer (AJCC) staging
system was used for disease staging (11). The greatest dimension of the tumor
was defined as tumor size. Tumor grading was evaluated using the
French Federation of Cancer Centres Sarcoma Group (FNCLCC) grading
system (12). According to the
FNCLCC grading system, the tumor could be either grade 2 or grade
3, depending on the mitotic rate, the extent of necrosis, or both;
however, no grading information was available. The resection margin
status was classified as R0 (macroscopically and microscopically
clear), R1 (macroscopically clear and microscopically involved),
and R2 (macroscopically involved). Inclusion criteria of N/AC were
deep located and ≥5 cm tumors. If tumor was attached to critical
structures, or seemed to contaminate surrounding tissue after
unplanned surgery, the use of N/AC depended on the physician's
choice. Exclusion criteria of N/AC was superficial located and
<5 cm tumors and patients >70 years old. The administered
regimen was doxorubicin (60 mg/m2) in combination with
ifosfamide (6–10 g/m2), high-dose ifosfamide (12–15
g/m2), or both. When histology showed the existence of
poorly differentiated component, regimens administered were
doxorubicin (60 mg/m2) in combination with vincristine
(1.5 mg/m2) and cyclophosphamide (1,000
mg/m2), ifosfamide (5 g/m2) in combination
with etoposide (300 mg/m2). If surgical margin was
contaminated, or close to the tumor, radiotherapy was used based on
physician's choice. Patients were followed up at our outpatient
clinic every 3 months during first 2 years, every 6 months during
next 3 years, then annually until 10 years had passed or death. At
each visit, chest radiographs were obtained. Chest CT and/or local
MRI were performed at intervals of 3–6 months until 5 years. This
study was approved by Osaka University Clinical Research Review
Committee, and waived off the requirement for written informed
consent from the subjects (certificate no. 14240-2).
Statistical analysis
We assessed local recurrence free survival (LRFS),
overall survival (OS), and metastasis-free survival (MFS) using the
Kaplan-Meier method with 95% confidence intervals (CIs).
Differences between LRFS, OS, and MFS were compared using the
log-rank test. Descriptive statistics were used to show the
distribution of variables in the population. All statistical
analyses were performed using SPSS 23.0 software (IBM Corp.,
Armonk, NY, USA). P<0.05 was considered to indicate a
statistically significant difference.
Results
Patient and treatment
demographics
The study included 54 patients (23 male and 31
female patients). The median patient age was 42 years (range, 8–81
years), and the median follow-up period was 94 months for survivors
(range, 7–220 months). Fifteen patients were referred after
unplanned surgery, 36 had initial definitive treatment at our
institutions, and 3 had no records about initial treatment before
reference. All patients underwent surgical resection of the primary
tumor. Six patients (11%) had positive surgical margins. Of the 6
patients, 3 had R2 resection and 3 had R1 resection. Fourteen
patients (26%) received radiotherapy after surgery. Thirty-eight
patients (70%) were treated with chemotherapy. Neoadjuvant
chemotherapy and adjuvant chemotherapy were used in 32 patients
(59%) and 33 patients (61%), respectively. Twenty-seven (50%)
patients received both neoadjuvant and adjuvant chemotherapy. All
patients with stage III tumors, except 3 patients, received N/AC.
One patient did not receive chemotherapy because of high age (81
years) and the other patients because of superficial location.
Their tumor size were 8 and 10 cm. The factors associated with
receipt of N/AC were grade 3 histology, deep-seated location, tumor
size ≥5 cm, and stage III tumor (Table
I). The factors associated with receipt of radiotherapy were
positive surgical margins, age ≥40, and grade 3 tumor (P=0.001,
P=0.062, and P=0.08, respectively; data not shown).
 | Table I.Clinical and treatment
characteristics. |
Table I.
Clinical and treatment
characteristics.
|
| All patients |
|
|---|
|
|
|
|
|---|
| Variables | N/AC (−) | N/AC (+) | P-value |
|---|
| Sex |
|
| 0.911 |
| Male | 7 | 16 |
|
|
Female | 9 | 22 |
|
| Age |
|
| 0.233 |
|
<40 | 6 | 21 |
|
| 40≤ | 10 | 17 |
|
| Unplanned
surgery |
|
| 0.559 |
| Yes | 6 | 9 |
|
| No | 9 | 27 |
|
| ND | 1 | 2 |
|
| Grade |
|
| 0.005 |
| 2 | 11 | 15 |
|
| 3 | 1 | 20 |
|
| ND | 4 | 3 |
|
| Depth |
|
| 0.008 |
|
Superficial | 6 | 3 |
|
| Deep | 10 | 35 |
|
| Size |
|
| <0.001 |
| <5
cm | 12 | 9 |
|
| 5 cm
≤ | 3 | 29 |
|
| Site |
|
| 0.981 |
|
Limbs | 11 | 26 |
|
|
Axial | 5 | 12 |
|
| Stage |
|
| <0.001 |
| II | 13 | 9 |
|
| III | 3 | 29 |
|
| Surgical margin |
|
| 0.246 |
|
Negative | 13 | 35 |
|
|
Positive | 3 | 3 |
|
| RT |
|
| 0.562 |
| Yes | 5 | 9 |
|
| No | 11 | 29 |
|
Outcomes
Three patients (6%) had local recurrence (LR) and 13
patients (24%) developed distant metastasis. Of the 13 patients who
developed distant metastasis, 8 died of SS, 4 showed no evidence of
disease after metastasectomy, and one lost follow-up due to
shifting to palliative care. One patient died of another cause. The
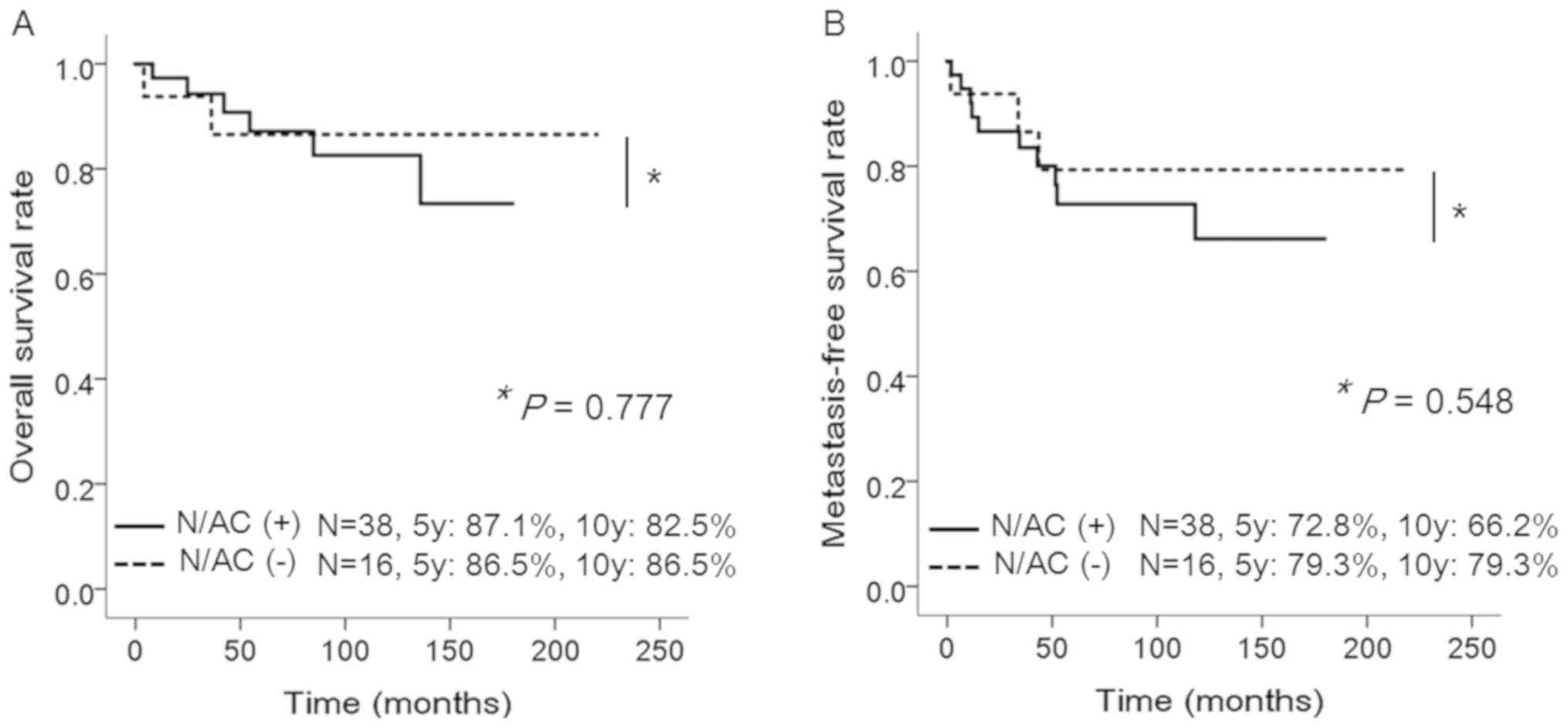
5- and 10-year LR rates were 93.7% (95% CI, 86.8–100) and 93.7%
(95% CI, 86.8–100), respectively. Tumor size <5 cm was
associated with worse LRFS (P=0.033). The 5- and 10-year MFS rates
were 74.7% (95% CI, 62.2–87.2) and 70.0% (95% CI, 55.3–84.7),
respectively. Tumor size ≥5 cm, and receipt of radiotherapy were
significantly associated with worse MFS (P=0.045, and P=0.02,
respectively). The 5- and 10-year OS rates were 87.1% (95% CI,
77.6–96.9) and 83.7% (95% CI, 72.3–95.1), respectively. Receipt of
radiotherapy was significantly associated with worse OS (P=0.02)
(Table II), probably due to
selection bias. Because receipt of radiotherapy was associated with
positive surgical margins, age ≥40, and grade 3 tumor (P=0.001,
P=0.062, and P=0.08, respectively; data not shown) N/AC did not
have significant effects on LR, MFS, and OS (P=0.131, P=0.548, and
P=0.777, respectively; Fig. 1).
 | Table II.Univariate analysis of factors
influencing LRFS, MFS, and OS. |
Table II.
Univariate analysis of factors
influencing LRFS, MFS, and OS.
| Variables | LRFS P-value | MFS P-value | OS P-value |
|---|
| Sex |
|
Male | 0.703 | 0.511 | 0.239 |
|
Female |
|
|
|
| Age |
|
<40 | 0.608 | 0.598 | 0.357 |
|
40≤ |
|
|
|
| Unplanned
surgery |
|
Yes | 0.331 | 0.061 | 0.234 |
| No |
|
|
|
| ND |
|
|
|
| Site |
|
Limbs | 0.198 | 0.071 | 0.343 |
|
Axial |
|
|
|
| Size |
| <5
cm | 0.033 | 0.045 | 0.093 |
| 5 cm
≤ |
|
|
|
| Depth |
|
Superficial | 0.42 | 0.084 | 0.193 |
|
Deep |
|
|
|
| Histology |
|
Monophasic | 0.635 | 0.272 | 0.915 |
|
Biphasic |
|
|
|
|
NOS |
|
|
|
| Grade |
| 2 | 0.513 | 0.191 | 0.340 |
| 3 |
|
|
|
| ND |
|
|
|
| Surgical
margin |
|
Negative | 0.12 | 0.068 | 0.118 |
|
Positive |
|
|
|
| RT |
|
Yes | 0.601 | 0.02 | 0.020 |
| No |
|
|
|
| N/AC |
|
Yes | 0.131 | 0.548 | 0.777 |
| No |
Discussion
In the present study, the 5- and 10-year OS rates
were found to be 87.1 and 83.7%, respectively. To our knowledge,
this is the best long-term result among published large series
involving adult SS patients (Table
III), although Krieg et al (13) reported that SS tends to develop
metastasis late with high mortality. There are 3 possible factors
to explain the favorable outcomes. The first is patient and tumor
demographics. A number of prognostic factors for localized SS have
been reported, and among them, the relatively common favorable
factors were small tumor size and young age at diagnosis (4,5,7–9,14). It remains unclear whether the
prognostic significance of age is related to biological variables
or to historically different treatment approaches adapted in
pediatric vs. adult patients (15).
The proportion of tumors measuring ≥5 cm and the median age at
diagnosis in this cohort were comparable to the values in previous
studies (Table III). Thus, patient
and tumor demographics appear to have less effect on the outcomes.
The second is primary treatment. In this study, the status of the
surgical margin was comparable to that in previous studies and the
rate of adjuvant radiotherapy use was lower than that in previous
studies (Table III). On the
contrary, the proportion of patients who received N/AC was higher
than that in previous studies (Table
III). The third is treatment after tumor relapse. For the last
3–4 decades, there have been few options other than doxorubicin and
ifosfamide for the treatment of advanced STS. Recently, 3 new drugs
(pazopanib, trabectedin, and eribulin) were approved in Japan for
the treatment of advanced STS. The effects of these new drugs on SS
have not been clarified yet, but it is possible that these new
drugs will prolong the survival of patients with relapsed SS.
Furthermore, 4 patients achieved disease free survival after
metastasectomy. Hence aggressive treatment after tumor relapse
including local and systematic therapy likely contributed to
prolong the survival. However, considering that the rates of local
recurrence and distant metastasis were lower in this study than
those in previous studies (Table
III), primary treatment appears to have a more vital role than
treatment after tumor relapse. Taken together, the effect of N/AC
on survival is considered important in this cohort.
 | Table III.Published large series on localized
synovial sarcoma including adult patients. |
Table III.
Published large series on localized
synovial sarcoma including adult patients.
|
| Number of
patients | Proportion of
tumors measuring ≥5 cm | Age (median) | Follow-up
(months) | Surgical margin (%,
positive) | N/AC (%) | RT (%) | LR (%) | DM (%) | 5-y OS (%) | 10-y OS (%) |
|---|
| Bergh et al
(14) | 121 | ND | 34 | 118 | ND | 32 | 27 | 31 | 54 | 60 | 50 |
| Lewis et al
(8) | 112 | 45 | 35 | 72 | 14 | 37 | 46 | 15 | 39 | 75 | ND |
| Trassard et
al (9) | 128 | ND | 33 | 37 | 24 | 57 | 80 | 24 | 48 | 63 | ND |
| Ferrari et
al (5) | 215 | 52 | ND | 65 | ND | 28 | 50 | 37 | 49 | 72 | ND |
| Canter et al
(7) | 255 | 56 | 34 | 72 | 14 | 39 | 63 | ND | 45 | 72 | 60 |
| Palmaerini et
al (4) | 204 | 51 | 36 | 66 | 12 | 52 | 52 | 18 | 27 | 76 | ND |
| Italiano et
al (10) | 237 | ND | 35 | 58 | 14< | 60 | 76 | 24 | 45 | 64 | <46 |
| Current study | 54 | 59 | 42 | 78 | 11 | 70 | 26 | 6 | 24 | 87 | 84 |
The effect of N/AC on SS has not been proven
previously, and study results have been conflicting. Italiano et
al (10) demonstrated that N/AC
did not have a significant impact on OS, using the French Sarcoma
Group Database. On the contrary, Chen et al (16) reported that adjuvant chemotherapy
improved disease-specific survival and prolonged the time to
metastasis in stage IIB/III SS patients. Recently, Vining et
al (17) reported improved
outcomes with adjuvant chemotherapy in stage III SS patients from
an analysis of the National Cancer Database and recommended less
restricted use of adjuvant chemotherapy for stage III SS. These
differences might come from varying use of chemotherapy regimens
among patients and different proportions of N/AC administration.
Besides which population of stage III SS could improve survival by
N/AC is still unclear. In this study, all patients with stage III
SS, except 3 patients, received N/AC. Of the 3 patients, one died
of disease 4 months after surgery, 2 were continuous disease free
for 69 and 50 months after surgery. Thus, we could not compare the
effects of N/AC in high-risk patients properly and recommend that
all stage III patients should have N/AC. Nonetheless, the treatment
resulted in better long-term outcomes than those of previous
studies. Collectively, our results suggest that patients with stage
III SS include the population of patients who benefit from N/AC and
high utilization of N/AC in SS might improve long-term
outcomes.
Some studies have shown that adjuvant radiotherapy
improved survival in SS patients (18,19).
However, we showed that radiotherapy had adverse effects on OS and
MFS. Receipt of radiotherapy was associated with positive surgical
margins, age ≥40, and grade 3 tumor (P=0.001, P=0.062, and P=0.08,
respectively; data not shown); thus, the adverse effects of
radiotherapy observed in this study resulted from selection bias
and we were not able to evaluate the impact of radiotherapy
appropriately. In this study, radiotherapy and surgical margin did
not correlate with LRFS probably due to low rate of LR.
Surprisingly, tumor size <5 cm was associated with LRFS. Because
2 of the 3 patients who developed LR had unplanned surgery, we
speculated the correlation might be affected by unplanned
surgery.
The present study has several limitations. The
number of patients was too small to draw a definitive conclusion.
There were no information of chromosomal translocation. We did not
use fluorescent in situ hybridization for diagnosis, and
that might affect the patient population. We were not able to
obtain histological grading information in some cases. It was
sometimes difficult to determine FNCLCC grading after neoadjuvant
chemotherapy. Administration regimens were not uniform. The rate of
positive surgical margins was comparable to that reported in
previous series, but the rate was the lowest among previously
reported rates. This might be associated with lower local
recurrence rates and might influence favorable outcomes. The rate
of radiotherapy use was low. Usually, radiotherapy is considered
useful for local control, and it can improve survival in SS
patients (19). We considered that
the low rate of radiotherapy use did not lead to overestimation of
the effects of N/AC. Finally, there was possible selection bias
with regard to receipt of N/AC. We delivered N/AC to almost all
high-risk patients, and we failed to assess the effects of N/AC
appropriately.
In conclusion, we demonstrated satisfactory
long-term outcomes in localized SS patients with a high utilization
rate of N/AC. We failed to show the impact of N/AC on survival
probably due to high use of N/AC on stage III patients. Further
study should be necessary to evaluate which population of SS would
benefit from N/AC.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used in the present study are available
from the corresponding author on reasonable request.
Authors' contributions
HO conceived and designed the study, collected,
analyzed, and interpreted the data, and wrote the manuscript. SK
analyzed data and revised the manuscript. KH and ST collected data
and reviewed the manuscript. SN reviewed the histological grading
of the tumor specimens. YI, TT, HT, and KO collected and
interpreted data. NN, IK, NA, TU, and HY analyzed and interpreted
the data and reviewed and revised the manuscript. All authors read
and approved the final manuscript.
Ethics approval and consent to
participate
The present study was approved by Osaka University
Clinical Research Review Committee, and waived off the requirement
for written informed consent from the subjects (certificate no.
14240-2).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no conflict of
interest.
References
|
1
|
Ogura K, Higashi T and Kawai A: Statistics
of soft-tissue sarcoma in Japan: Report from the bone and soft
tissue tumor registry in Japan. J Orthop Sci. 22:755–764. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Pisters PW, O'Sullivan B and Maki RG:
Evidence-based recommendations for local therapy for soft tissue
sarcomas. J Clin Oncol. 25:1003–1008. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Vlenterie M, Litière S, Rizzo E, Marréaud
S, Judson I, Gelderblom H, Le Cesne A, Wardelmann E, Messiou C,
Gronchi A and van der Graaf WT: Outcome of chemotherapy in advanced
synovial sarcoma patients: Review of 15 clinical trials from the
European organisation for research and treatment of cancer soft
tissue and bone sarcoma group; setting a new landmark for studies
in this entity. Eur J Cancer. 58:62–72. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Palmerini E, Staals EL, Alberghini M,
Zanella L, Ferrari C, Benassi MS, Picci P, Mercuri M, Bacci G and
Ferrari S: Synovial sarcoma: Retrospective analysis of 250 patients
treated at a single institution. Cancer. 115:2988–2998. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Ferrari A, Gronchi A, Casanova M, Meazza
C, Gandola L, Collini P, Lozza L, Bertulli R, Olmi P and Casali PG:
Synovial sarcoma: A retrospective analysis of 271 patients of all
ages treated at a single institution. Cancer. 101:627–634. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Eilber FC, Brennan MF, Eilber FR, Eckardt
JJ, Grobmyer SR, Riedel E, Forscher C, Maki RG and Singer S:
Chemotherapy is associated with improved survival in adult patients
with primary extremity synovial sarcoma. Ann Surg. 246:105–113.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Canter RJ, Qin LX, Maki RG, Brennan MF,
Ladanyi M and Singer S: A synovial sarcoma-specific preoperative
nomogram supports a survival benefit to ifosfamide-based
chemotherapy and improves risk stratification for patients. Clin
Cancer Res. 14:8191–8197. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Lewis JJ, Antonescu CR, Leung DH, Blumberg
D, Healey JH, Woodruff JM and Brennan MF: Synovial sarcoma: A
multivariate analysis of prognostic factors in 112 patients with
primary localized tumors of the extremity. J Clin Oncol.
18:2087–2094. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Trassard M, Le Doussal V, Hacène K,
Terrier P, Ranchère D, Guillou L, Fiche M, Collin F, Vilain MO,
Bertrand G, et al: Prognostic factors in localized primary synovial
sarcoma: A multicenter study of 128 adult patients. J Clin Oncol.
19:525–534. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Italiano A, Penel N, Robin YM, Bui B, Le
Cesne A, Piperno-Neumann S, Tubiana-Hulin M, Bompas E, Chevreau C,
Isambert N, et al: Neo/adjuvant chemotherapy does not improve
outcome in resected primary synovial sarcoma: A study of the French
sarcoma group. Ann Oncol. 20:425–430. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Amin M, Edge S, Greene F, Byrd DR,
Brookland RK, Washington MK, Gershenwald JE, Compton CC, Hess KR,
Sullivan DC, et al: AJCC Cancer Staging Manual (8th edition). (New
York). Springer. 2017.
|
|
12
|
Trojani M, Contesso G, Coindre JM, Rouesse
J, Bui NB, de Mascarel A, Goussot JF, David M, Bonichon F and
Lagarde C: Soft-tissue sarcomas of adults; study of pathological
prognostic variables and definition of a histopathological grading
system. Int J Cancer. 33:37–42. 1984. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Krieg AH, Hefti F, Speth BM, Jundt G,
Guillou L, Exner UG, von Hochstetter AR, Cserhati MD, Fuchs B,
Mouhsine E, et al: Synovial sarcomas usually metastasize after
>5 years: A multicenter retrospective analysis with minimum
follow-up of 10 years for survivors. Ann Oncol. 22:458–467. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Bergh P, Meis-Kindblom JM, Gherlinzoni F,
Berlin O, Bacchini P, Bertoni F, Gunterberg B and Kindblom LG:
Synovial sarcoma: Identification of low and high risk groups.
Cancer. 85:2596–2607. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Vlenterie M, Ho VK, Kaal SE, Vlenterie R,
Haas R and van der Graaf WT: Age as an independent prognostic
factor for survival of localised synovial sarcoma patients. Br J
Cancer. 113:1602–1606. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Chen Y, Yang Y, Wang C and Shi Y: Adjuvant
chemotherapy decreases and postpones distant metastasis in
extremity stage IIB/III synovial sarcoma patients. J Surg Oncol.
106:162–168. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Vining CC, Sinnamon AJ, Ecker BL, Kelz RR,
Fraker DL, Roses RE and Karakousis GC: Adjuvant chemotherapy in
resectable synovial sarcoma. J Surg Oncol. 116:550–558. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Song S, Park J, Kim HJ, Kim IH, Han I, Kim
HS and Kim S: Effects of adjuvant radiotherapy in patients with
synovial sarcoma. Am J Clin Oncol. 40:306–311. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Naing KW, Monjazeb AM, Li CS, Lee LY, Yang
A, Borys D and Canter RJ: Perioperative radiotherapy is associated
with improved survival among patients with synovial sarcoma: A SEER
analysis. J Surg Oncol. 111:158–164. 2015. View Article : Google Scholar : PubMed/NCBI
|