Introduction
Hepatocellular carcinoma (HCC), one of the most
commonly occurring malignant tumors worldwide (1,2), often
develops in patients with hepatitis C virus (HCV) or hepatitis B
virus (HBV) infection (3,4), who are defined as high-risk for HCC and
are recommended to undergo protocol screening for tumor development
with a surveillance program. Detection of HCC at an early stage is
important to improve patient prognosis, as tumor burden is an
important prognostic factor along with hepatic reserve function
(5–7). The progression of surgical resection
(8) and/or radiofrequency ablation
(RFA) (9,10) as curative treatments have improved
patient prognosis when diagnosed at an earlier stage. In addition,
the development of tyrosine kinase inhibitors (11–14) has
contributed to a better HCC prognosis in patients who are allocated
an unresectable status. Ultrasonography (US) is a popular and
economical examination method that does not involve X-ray exposure,
which is deemed to be suitable for HCC surveillance. Early stage
HCC diagnosis is important for treatment (15), meaning that a suitable surveillance
program should be evaluated.
Although the surveillance program for HCC, which
primarily utilizes US, has been performed in patients with a high
risk of HCC in Japan, few reports of surveillance efficacy for the
improvement of prognosis have been performed. Only a limited number
of studies have examined the effectiveness of HCC surveillance
performed at regional hub hospitals in Japan (16). Herein, the outcomes of HCC patients
with and without surveillance using with US were analyzed to
elucidate its usefulness and impact for improving prognosis.
Materials and methods
Patients and definition of
surveillance for HCC
The records of 872 patients with naïve HCC who were
examined from October 2006 to December 2014 were analyzed,
following the exclusion of 170 cases without information regarding
HCC surveillance. The enrolled patients were divided into those who
did (S-group, n=398) and did not (non-S group, n=474) undergo
surveillance. In addition, the S-group was divided into two
sub-groups: i) Those who underwent surveillance at Ehime
Prefectural Central Hospital (EPCH), an expert medical institution
(SE-group, n=189); and ii) those who received surveillance at
general clinics (SG-group, n=209; Fig.
1). Patients whose surveillance of HCC was performed only with
AFP examination or whose HCC was pointed out incidentally were
classified as the non-S group. The outcomes and clinical
characteristics of the S- and non-S groups were compared in a
retrospective manner, while those of the SE- and SG-groups were
also compared as a sub-analysis.
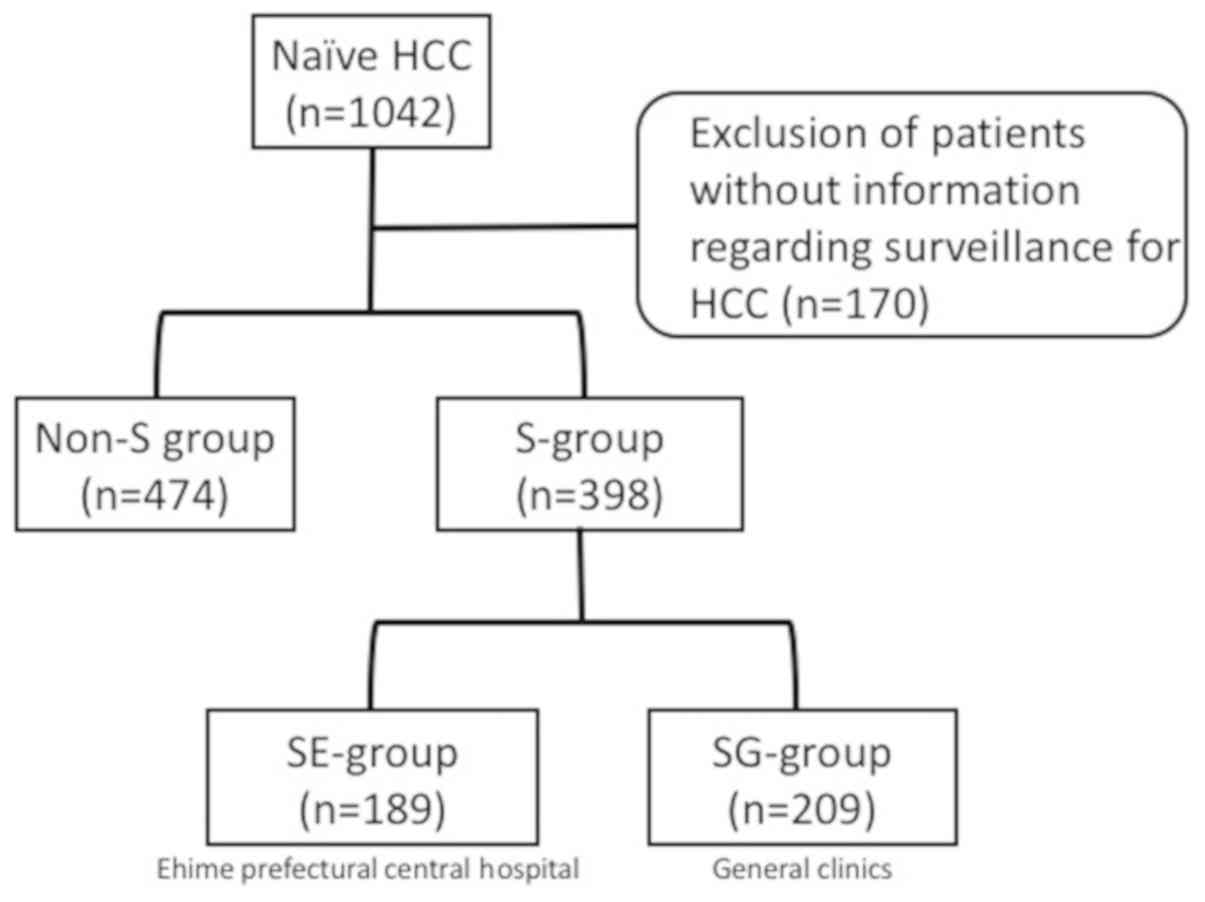 | Figure 1.Patients with naïve HCC enrolled in
the present study. In total, 872 patients with naïve HCC were
enrolled, who were examined from October 2006 to December 2014,
following the exclusion of cases without information regarding
surveillance findings. They were divided into those who did
(S-group, n=398) and did not (non-S group, n=474) undergo
surveillance. The S-group was further subdivided into patients who
underwent surveillance at Ehime Prefectural Central Hospital
(SE-group, n=189) and general clinics (SG-group, n-209). HCC,
hepatocellular carcinoma; S-group, surveillance group; non-S,
non-surveillance group; SE-group, follow-up surveillance at Ehime
Prefectural Central Hospital; SG-group, follow-up surveillance at
general clinics. |
Surveillance program
EPCH is a qualified regional hub hospital, where
surveillance for HCC is performed for a fixed interval (3 or 6
months) with US and α-fetoprotein (AFP) in accordance with liver
disease grade [chronic hepatitis (CH; n=200) or liver cirrhosis
(LC; n=198)], following practical guidelines for HCC used in Japan
since 2005 (17,18).
Diagnosis of HCC
When HCC is suspected based on US findings, contrast
enhanced US (CEUS), dynamic CT, or
gadolinium-ethoxybenzyl-diethylenetriamine pentaacetic acid
(Gd-EOB-DTPA)-enhanced magnetic resonance imaging (EOB-MRI)
(19) was typically performed as an
additional examination. HCC was diagnosed in patients based on
increasing AFP expression, as well as dynamic CT (20), magnetic resonance imaging (MRI),
and/or CEUS with perflubutane (Sonazoid®; Daiichi Sankyo
Co., Ltd., Tokyo, Japan) (21,22)
findings. Tumor, Node, Metastasis (TNM) stage was determined
according to the Liver Cancer Study Group of Japan, 6th edition
(23).
The study protocol was conducted in compliance with
the Helsinki Declaration and was approved by the Institutional
Ethics Committee of EPCH (approval no. 26-11). Values are expressed
as the mean ± standard deviation. Statistical analyses were
performed using Student's t test, Welch's test, Fischer's exact
test, Mann-Whitney's U test, and the Kaplan-Meier method with a
log-rank test using EZR version 1.29 (24), a graphical user interface for R (The
R Foundation for Statistical Computing, Vienna, Austria). P<0.05
was considered to indicate a statistically significant
difference.
Results
Although age was not significantly different between
the S- (n=398) and non-S (n=474) groups (S, 70.2±9.2 vs. non-S,
70.4±10.7 years; P=0.689), the frequency of male gender (S, 66.1%
vs. non-S, 76.8%; P<0.001), surgical resection (S, 21.1% vs.
non-S, 31.4%; P<0.001), NBNC-HCC (S, 14.5% vs. non-S, 36.7%;
P<0.001), lower grade of Child-Pugh classification (B and C; S,
23.1% vs. non-S, 36.4%; P<0.001), and TNM stage IV (S, 1.3% vs.
non-S, 29.1%; P<0.001) was greater, while tumor size (S, 2.3±1.2
vs. non-S, 5.4±3.7 cm; P<0.001) and tumor number (S, 1.6±1.1 vs.
non-S, 2.5±2.0; P<0.001) were significantly larger in the non-S
group. Furthermore, those non-S patients had higher levels of tumor
markers, including AFP (S, 201.8±1228.6 ng/ml vs. non-S,
15,123.4±77,037.9 ng/ml; P<0.001), fucosylated-AFP (AFP-L3; S,
9.2±17.0% vs. non-S, 19.9±25.8%; P<0.001), des-γ-carboxy
prothrombin (DCP; S, 757.2±4918.0 mAU/ml vs. non-S,
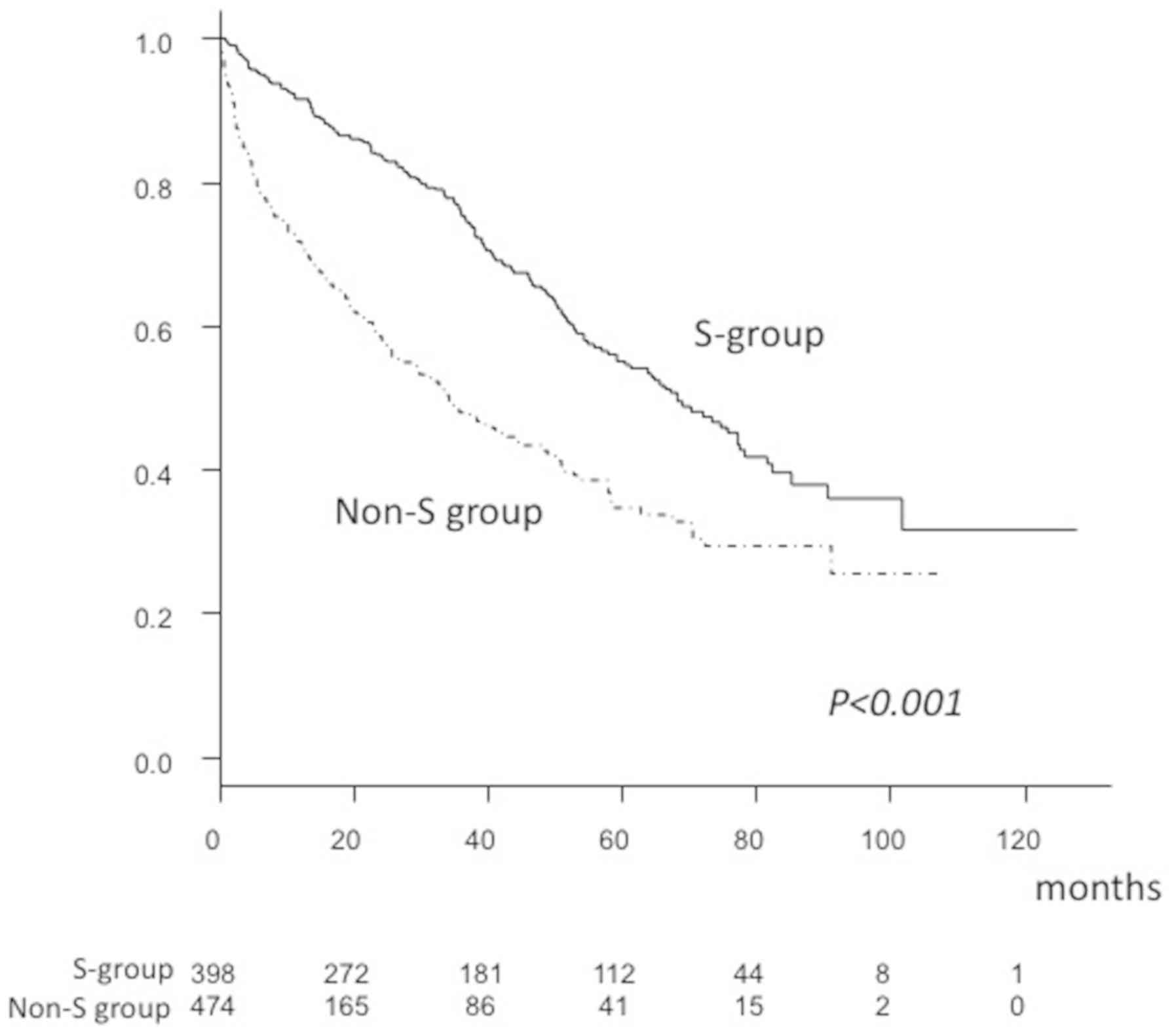
17,472.5±46,451.4 mAU/ml; P<0.001; Table I). In addition, the overall survival
rate (OSR) after 1 (S, 91.6% vs. non-S, 71.8%), 3 (S, 75.3% vs.
non-S, 47.8%) and 5 years (S, 55.2% vs. non-S, 34.7%) was
significantly higher in the S group, compared with the non-S group,
as was median survival time (MST; S, 68.2 vs. non-S, 34.1 months;
P<0.001; Fig. 2).
 | Table I.Comparison for the characteristics of
S- and non-S group. |
Table I.
Comparison for the characteristics of
S- and non-S group.
| Patient
characteristics | S-group
(n=398) | Non-S group
(n=474) | P-value |
|---|
| Age (years) | 70.2±9.2 | 70.4±10.7 | 0.689 |
| Gender
(male:female) | 263:135 | 364:110 | <0.001 |
| Etiology
(HCV:HBV:HBV+HCV:NBNC) | 310:25:5:58 | 250:46:4:174 | <0.001 |
| Number of HCV
patients obtained SVR | 8 | 7 | 1.000 |
| Number of HBV
patients treated with NA | 18 | 0 | <0.001 |
| Maximum tumor
diameter (cm) | 2.3±1.2 | 5.4±3.7 | <0.001 |
| Tumor number | 1.6±1.1 | 2.5±2.0 | <0.001 |
| AFP (ng/ml) | 201.8±1228.6 |
15,123.4±77,037.9 | <0.001 |
| AFP-L3 (%) | 9.2±17.0 | 19.9±25.8 | <0.001 |
| DCP (mAU/ml) | 757.2±4918.0 |
17,472.5±46,451.4 | <0.001 |
| Child-Pugh
classification (A:B:C) | 306:78:14 | 301:123:50 | <0.001 |
| TNM classification
of LCSGJ 6th (I:II:III:IV) | 143:183:67:5 | 42:172:122:138 | <0.001 |
| JIS score
(0:1:2:3:4:5) |
115:161:91:27:3:1 |
26:139:119:113:54:23 | <0.001 |
| Treatment
(resection:RFA:PEIT:others) | 84:246:2:66 | 149:64:1:260 | <0.001 |
| MST (months) | 68.2 | 34.1 | <0.001 |
Sub-analyses were also performed for comparison of
patient characteristics and prognosis. The S-group was divided into
those who underwent surveillance at EPCH, an expert medical
institution (SE-group, n=189), and at a general clinic (SG-group,
n=209). It was determined that average age was older (SG-group,
71.4±8.8 vs. SE-group, 68.8±9.5 years), tumor size was larger
(SG-group, 2.5±1.3 vs. SE-group, 2.0±1.0 cm), and frequency of TNM
stage II and III (SG-group, 71.8 vs. SE-group, 52.9%) was greater
in the SG-group (all P<0.001; Table
II). Although there were significant differences in tumor size
and frequency of TNM stage II and III, tumor number was not
significantly different (SE-group, 1.5±1.1 vs. SG-group, 1.7±1.2;
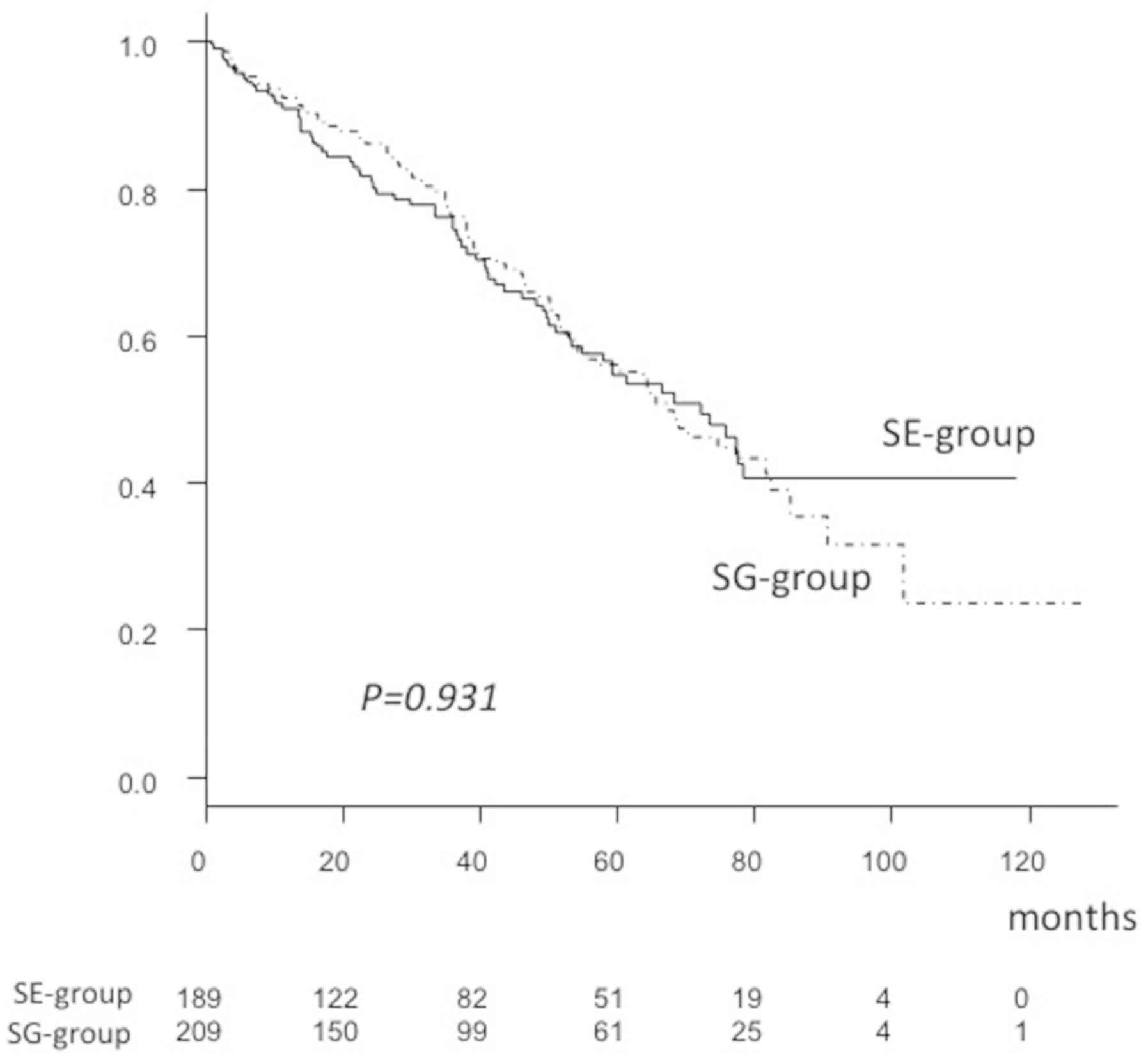
P=0.164). As a result, the 1-, 3- and 5-year OSR (SG-group, 92.4,
76.0 and 55.8% vs. SE-group, 90.8, 74.5 and 54.6%, respectively)
and MST (SG-group, 67.1 vs. SE-group, 72.1 months) were not
significantly different between the SG- and SE-groups (P=0.931;
Fig. 3).
 | Table II.Characteristics of SE- and
SG-groups. |
Table II.
Characteristics of SE- and
SG-groups.
| Patient
characteristics | SE-group
(n=189) | SG-group
(n=209) | P-value |
|---|
| Age (years) | 68.8±9.5 | 71.4±8.8 | <0.001 |
| Gender
(male:female) | 120:69 | 143:66 | 0.301 |
| Etiology
(HCV:HBV:HBV&HCV:NBNC) | 141:12:2:34 | 168:13:3:25 | 0.204 |
| Number of HCV
patients obtained SVR | 6 | 2 | 0.145 |
| Number of HBV
patients treated with NA | 11 | 7 | 0.073 |
| Maximum tumor
diameter (cm) | 2.0±1.0 | 2.5±1.3 | <0.001 |
| Tumor number | 1.5±1.1 | 1.7±1.2 | 0.164 |
| AFP (ng/ml) | 169.0±929.5 | 231.3±1447.5 | 0.614 |
| AFP-L3 (%) | 7.4±13.4 | 10.7±19.6 | 0.055 |
| DCP (mAU/ml) | 369.5±1812.9 | 1107.0±6549.6 | 0.130 |
| Child-Pugh
classification (A:B:C) | 140:42:7 | 166:36:7 | 0.218 |
| TNM classification
of LCSGJ 6th (I:II:III:IV) | 88:77:23:1 | 55:106:44:4 | <0.001 |
| JIS score
(0:1:2:3:4:5) | 68:72:39:8:1:1 |
47:89:52:19:2:0 | 0.003 |
| Treatment
(resection:RFA:PEIT:others) | 34:125:1:29 | 50:121:1:37 | 0.540 |
| MST (months) | 72.1 | 67.1 | 0.931 |
Discussion
Surveillance for HCC in high-risk patients, such as
those with viral hepatitis or LC has been expected to improved
patient prognosis due to earlier tumor detection. There are a
number of reasons why a surveillance program improves prognosis,
including advancements in imaging modalities such as CEUS (21,22,25) and
EOB-MRI (19), allowing the rapid
detection and diagnosis of smaller HCC. In addition, the
development of therapeutic modalities and assistance methods with
low invasiveness, such as RFA (10,26),
virtual US (VUS) (27), and
artificial effusion in RFA (28)
have bettered small HCC treatment. Surveillance for smaller HCC
detection in high-risk patients is important to increase the
likelihood of successful curative treatment, thus obtaining a
better prognosis.
Singal et al (29), reported that the combination of US
and AFP maximizes the sensitivity for HCC detection at an early
stage, compared to surveillance with US or AFP alone (combination,
US alone, AFP alone sensitivity: 90, 44 and 66%, respectively;
specificity: 83, 92 and 91%, respectively) (29). In a randomized control trial (RCT),
it was demonstrated that biannual screening for HCC with US and AFP
in HBV patients reduced mortality by 37%, as compared to the
control group that did not undergo screening (30). In addition, surveillance with US and
AFP in patients with LC significantly improved survival as compared
to patients with incidentally detected HCC (15). On the other hand, in another report,
biannual AFP screening in HBV patients did not result in an overall
reduction in mortality (31). Thus,
it is thought that surveillance with both US and AFP is most
effective. Costentin et al (32) reported that compliance with HCC
surveillance guidelines (fewer than 7 months between image
evaluations) lead to early diagnosis, allocation of curative
treatment, and a longer adjusted OS of patients with compensated
HCV- or HBV-associated cirrhosis and a diagnosis of HCC (32). In the present study, average tumor
size of the SG-group was larger than that of the SE-group
(P<0.001). This result may have depended on an easier access
system to CEUS, CT or MRI in the SE-group. However, the frequency
of curative treatments (resection, RFA and PEIT) did not show a
significant difference between the groups, and prognosis was
similar. Surveillance with US and AFP improves the prognosis of
patients with chronic liver disease. The clinical usefulness of
AFP-L3 and DCP other than AFP as tumor markers for HCC surveillance
should be examined in additional future studies. Lead-time bias
should be taken into consideration for this type of investigation.
Toyoda et al (33) reported
that the surveillance group exhibited a significantly better
survival than the non-surveillance group after adjusting for
lead-time bias (MST: Surveillance vs. non-surveillance group, 7.18
vs. 5.65 years; P<0.0001). Further study is required to confirm
the influence of lead-time bias to survival.
In the present cohort, the frequency of NBNC-HCC was
greater in the non-S group, compared with the S-group. Along with
an increasing aging population in Japan, the frequency of patients
without HCV or HBV (NBNC-HCC) has increased (34–36),
thus establishment of a method for identifying affected patients
without viral hepatitis or alcohol abuse is becoming increasingly
important. Recently, non-alcoholic steatohepatitis (NASH) has been
recognized to increase the risk for development of HCC; therefore,
patients with NASH must be enrolled in a HCC screening program
(37). Nevertheless, many patients
with diabetes mellitus (DM), who often also have NASH, are
receiving regular medical check-ups at local clinics. To control
DM, it may be possible to focus high-risk patients, who require HCC
surveillance with US, from those with DM. It has been proposed that
age and fibrosis-4 index (38,39) are
factors that indicate individuals at high risk for HCC among DM
patients without alcohol abuse (alcohol intake: >60 g/day)
(37). Additional investigations to
form a surveillance strategy for detection of HCC at an earlier
stage in patients without viral hepatitis or alcohol abuse are
required.
US is easily introduced due to its lower cost
relative to other radiological modalities, such as enhanced CT and
EOB-MRI (40). Furthermore,
examinations may be repeatedly performed, although some issues
remain. The accuracy of US findings for HCC surveillance is
strongly dependent on the quality of the equipment and expertise of
the performing operator (41), thus
special training is warranted for ultrasonographers. Although tumor
diameter and TNM stage in the SG-group of the present study were
greater compared with the SE-group, tumor number and OSR were not
significantly different between those groups. It was speculated
that the reason why repeated surveillance screening for HCC using
the combination of US and AFP was effective, was because of the
increased chance for detection of smaller sized and fewer tumors,
resulting in the possibility to be treated with a curative
method.
The present study has certain limitations. First,
the present study was retrospective. Second, the quality of US
examination of each institution could not be exactly compared.
Additional analyses with a prospective study or RCT, if possible,
are required. However, physicians and their patients should be
notified of the importance of surveillance for HCC with a
combination of US and AFP for high-risk patients with viral
hepatitis and/or alcohol abuse. In addition, management of
high-risk patients without viral hepatitis or alcohol abuse is
necessary. It was concluded that repeated surveillance with US and
AFP leads to diagnosis of HCC at an earlier stage, and improved
prognosis of affected patients compared with patients who did not
undergo surveillance.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets of this study are not publicly
available, but are available from the corresponding author on
reasonable request.
Authors' contributions
AH and HY conceived the present study, analyzed the
data and wrote the manuscript. YH, BM, AM and MH conceived,
reviewed and edited the manuscript of the current study. TM, HI,
HU, MO, TA, TO, RI, YS, KMo, HM, ET, MK, TN and KMi acquired the
data.
Ethics approval and consent to
participate
The study protocol was conducted in compliance with
the Helsinki Declaration and was approved by the Institutional
Ethics Committee of EPCH (approval no. 26-11).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Song TJ, Ip EW and Fong Y: Hepatocellular
carcinoma: Current surgical management. Gastroenterology 127 (5
Suppl 1). S248–S260. 2004.
|
|
2
|
Parkin DM, Bray F, Ferlay J and Pisani P:
Global cancer statistics, 2002. CA Cancer J Clin. 55:74–108. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Hiraoka A, Hidaka S, Shimizu Y, Utsunomiya
H, Imai Y, Tatsukawa H, Tazuya N, Yamago H, Yorimitsu N, Tanihira
T, et al: Recent trends of Japanese hepatocellular carcinoma due to
HCV in aging society. Hepatogastroenterology. 59:1893–1895.
2012.PubMed/NCBI
|
|
4
|
Tada T, Kumada T, Toyoda H, Tsuji K,
Hiraoka A and Tanaka J: Impact of FIB-4 index on hepatocellular
carcinoma incidence during nucleos(t)ide analogue therapy in
patients with chronic hepatitis B: An analysis using time-dependent
receiver operating characteristic. J Gastroenterol Hepatol.
32:451–458. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Kudo M, Chung H, Haji S, Osaki Y, Oka H,
Seki T, Kasugai H, Sasaki Y and Matsunaga T: Validation of a new
prognostic staging system for hepatocellular carcinoma: The JIS
score compared with the CLIP score. Hepatology. 40:1396–1405. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Hiraoka A, Kumada T, Michitaka K, Toyoda
H, Tada T, Ueki H, Kaneto M, Aibiki T, Okudaira T, Kawakami T, et
al: Usefulness of albumin-bilirubin grade for evaluation of
prognosis of 2584 Japanese patients with hepatocellular carcinoma.
J Gastroenterol Hepatol. 31:1031–1036. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Hiraoka A, Kumada T, Kudo M, Hirooka M,
Tsuji K, Itobayashi E, Kariyama K, Ishikawa T, Tajiri K, Ochi H, et
al: Albumin-Bilirubin (ALBI) grade as part of the evidence-based
clinical practice guideline for HCC of the Japan society of
hepatology: A comparison with the liver damage and child-pugh
classifications. Liver Cancer. 6:204–215. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Miyagawa S, Makuuchi M, Kawasaki S and
Kakazu T: Criteria for safe hepatic resection. Am J Surg.
169:589–594. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Shiina S, Tateishi R, Arano T, Uchino K,
Enooku K, Nakagawa H, Asaoka Y, Sato T, Masuzaki R, Kondo Y, et al:
Radiofrequency ablation for hepatocellular carcinoma: 10-year
outcome and prognostic factors. Am J Gastroenterol. 107:569–577;
quiz 578. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Hiraoka A, Michitaka K, Horiike N, Hidaka
S, Uehara T, Ichikawa S, Hasebe A, Miyamoto Y, Ninomiya T, Sogabe
I, et al: Radiofrequency ablation therapy for hepatocellular
carcinoma in elderly patients. J Gastroenterol Hepatol. 25:403–407.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Llovet JM, Ricci S, Mazzaferro V, Hilgard
P, Gane E, Blanc JF, de Oliveira AC, Santoro A, Raoul JL, Forner A,
et al: Sorafenib in advanced hepatocellular carcinoma. N Engl J
Med. 359:378–390. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Bruix J, Qin S, Merle P, Granito A, Huang
YH, Bodoky G, Pracht M, Yokosuka O, Rosmorduc O, Breder V, et al:
Regorafenib for patients with hepatocellular carcinoma who
progressed on sorafenib treatment (RESORCE): A randomised,
double-blind, placebo-controlled, phase 3 trial. Lancet. 389:56–66.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Kudo M, Finn RS, Qin S, Han KH, Ikeda K,
Piscaglia F, Baron A, Park JW, Han G, Jassem J, et al: Lenvatinib
versus sorafenib in first-line treatment of patients with
unresectable hepatocellular carcinoma: A randomised phase 3
non-inferiority trial. Lancet. 391:1163–1173. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Hiraoka A, Kumada T, Kariyama K, Takaguchi
K, Itobayashi E, Shimada N, Tajiri K, Tsuji K, Ishikawa T, Ochi H,
et al: Therapeutic potential of lenvatinib for unresectable
hepatocellular carcinoma in clinical practice: Multicenter
analysis. Hepatol Res. 49:111–117. 2019.PubMed/NCBI
|
|
15
|
Bolondi L, Sofia S, Siringo S, Gaiani S,
Casali A, Zironi G, Piscaglia F, Gramantieri L, Zanetti M and
Sherman M: Surveillance programme of cirrhotic patients for early
diagnosis and treatment of hepatocellular carcinoma: A cost
effectiveness analysis. Gut. 48:251–259. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Tanaka H, Nouso K, Kobashi H, Kobayashi Y,
Nakamura S, Miyake Y, Ohnishi H, Miyoshi K, Iwado S, Iwasaki Y, et
al: Surveillance of hepatocellular carcinoma in patients with
hepatitis C virus infection may improve patient survival. Liver
Int. 26:543–551. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Makuuchi M, Kokudo N, Arii S, Futagawa S,
Kaneko S, Kawasaki S, Matsuyama Y, Okazaki M, Okita K, Omata M, et
al: Development of evidence-based clinical guidelines for the
diagnosis and treatment of hepatocellular carcinoma in Japan.
Hepatol Res. 38:37–51. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Kokudo N, Hasegawa K, Akahane M, Igaki H,
Izumi N, Ichida T, Uemoto S, Kaneko S, Kawasaki S, Ku Y, et al:
Evidence-based clinical practice guidelines for hepatocellular
carcinoma: The Japan society of hepatology 2013 update (3rd JSH-HCC
Guidelines). Hepatol Res. 45:2015. View Article : Google Scholar
|
|
19
|
Sano K, Ichikawa T, Motosugi U, Sou H,
Muhi AM, Matsuda M, Nakano M, Sakamoto M, Nakazawa T, Asakawa M, et
al: Imaging study of early hepatocellular carcinoma: Usefulness of
gadoxetic acid-enhanced MR imaging. Radiology. 261:834–844. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Bruix J and Sherman M; Practice Guidelines
Committee, American Association for the Study of Liver Diseases, :
Management of hepatocellular carcinoma. Hepatology. 42:1208–1236.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Hiraoka A, Hiasa Y, Onji M and Michitaka
K: New contrast enhanced ultrasonography agent: Impact of Sonazoid
on radiofrequency ablation. J Gastroenterol Hepatol. 26:616–618.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Hiraoka A, Ichiryu M, Tazuya N, Ochi H,
Tanabe A, Nakahara H, Hidaka S, Uehara T, Ichikawa S, Hasebe A, et
al: Clinical translation in the treatment of hepatocellular
carcinoma following the introduction of contrast-enhanced
ultrasonography with Sonazoid. Oncol Lett. 1:57–61. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
The Liver Cancer Study Group of Japan. The
general rules for the clinical and pathological study of primary
liver cancer. 6th. Kanehara; Tokyo: 26. 2015
|
|
24
|
Kanda Y: Investigation of the freely
available easy-to-use software ‘EZR’ for medical statistics. Bone
Marrow Transplant. 48:452–458. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Kan M, Hiraoka A, Uehara T, Hidaka S,
Ichiryu M, Nakahara H, Ochi H, Tanabe A, Kodama A, Hasebe A, et al:
Evaluation of contrast-enhanced ultrasonography using
perfluorobutane [Sonazoid(®)] in patients with small
hepatocellular carcinoma: Comparison with dynamic computed
tomography. Oncol Lett. 1:485–488. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Hiraoka A, Horiike N, Yamashita Y, Koizumi
Y, Doi K, Yamamoto Y, Hasebe A, Ichikawa S, Yano M, Miyamoto Y, et
al: Efficacy of radiofrequency ablation therapy compared to
surgical resection in 164 patients in Japan with single
hepatocellular carcinoma smaller than 3 cm, along with report of
complications. Hepatogastroenterology. 55:2171–2174.
2008.PubMed/NCBI
|
|
27
|
Hirooka M, Iuchi H, Kumagi T, Shigematsu
S, Hiraoka A, Uehara T, Kurose K, Horiike N and Onji M: Virtual
sonographic radiofrequency ablation of hepatocellular carcinoma
visualized on CT but not on conventional sonography. AJR Am J
Roentgenol. 186 (5 Suppl):S255–S260. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Uehara T, Hirooka M, Ishida K, Hiraoka A,
Kumagi T, Kisaka Y, Hiasa Y and Onji M: Percutaneous
ultrasound-guided radiofrequency ablation of hepatocellular
carcinoma with artificially induced pleural effusion and ascites. J
Gastroenterol. 42:306–311. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Singal AG, Conjeevaram HS, Volk ML, Fu S,
Fontana RJ, Askari F, Su GL, Lok AS and Marrero JA: Effectiveness
of hepatocellular carcinoma surveillance in patients with
cirrhosis. Cancer Epidemiol Biomarkers Prev. 21:793–799. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Zhang BH, Yang BH and Tang ZY: Randomized
controlled trial of screening for hepatocellular carcinoma. J
Cancer Res Clin Oncol. 130:417–422. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Chen JG, Parkin DM, Chen QG, Lu JH, Shen
QJ, Zhang BC and Zhu YR: Screening for liver cancer: Results of a
randomised controlled trial in Qidong, China. J Med Screen.
10:204–209. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Costentin CE, Layese R, Bourcier V, Cagnot
C, Marcellin P, Guyader D, Pol S, Larrey D, De Lédinghen V, Ouzan
D, et al: Compliance with hepatocellular carcinoma surveillance
guidelines associated with increased lead-time adjusted survival of
patients with compensated viral cirrhosis: A multi-center cohort
study. Gastroenterology. 155:431–442.e10. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Toyoda H, Kumada T, Tada T, Mizuno K,
Hiraoka A, Tsuji K, Ishikawa T, Akita T and Tanaka J: Impact of
hepatocellular carcinoma aetiology and liver function on the
benefit of surveillance: A novel approach for the adjustment of
lead-time bias. Liver Int. 38:2260–2268. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Tateishi R, Okanoue T, Fujiwara N, Okita
K, Kiyosawa K, Omata M, Kumada H, Hayashi N and Koike K: Clinical
characteristics, treatment, and prognosis of non-B, non-C
hepatocellular carcinoma: A large retrospective multicenter cohort
study. J Gastroenterol. 50:350–360. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Urata Y, Yamasaki T, Saeki I, Iwai S,
Kitahara M, Sawai Y, Tanaka K, Aoki T, Iwadou S, Fujita N, et al:
Clinical characteristics and prognosis of non-B non-C
hepatocellular carcinoma patients with modest alcohol consumption.
Hepatol Res. 46:434–442. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Hiraoka A, Ochi M, Matsuda R, Aibiki T,
Okudaira T, Kawamura T, Yamago H, Nakahara H, Suga Y, Azemoto N, et
al: Ultrasonography screening for hepatocellular carcinoma in
Japanese patients with diabetes mellitus. J Diabetes. 8:640–646.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Kolly P and Dufour JF: Surveillance for
hepatocellular carcinoma in patients with NASH. Diagnostics
(Basel). 6(pii): E222016. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Sterling RK, Lissen E, Clumeck N, Sola R,
Correa MC, Montaner J, S Sulkowski M, Torriani FJ, Dieterich DT,
Thomas DL, et al: Development of a simple noninvasive index to
predict significant fibrosis in patients with HIV/HCV coinfection.
Hepatology. 43:1317–1325. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Vallet-Pichard A, Mallet V, Nalpas B,
Verkarre V, Nalpas A, Dhalluin-Venier V, Fontaine H and Pol S:
FIB-4: An inexpensive and accurate marker of fibrosis in HCV
infection. Comparison with liver biopsy and fibrotest. Hepatology.
46:32–36. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Smajerova M, Petrasova H, Little J, Ovesna
P, Andrasina T, Valek V, Nemcova E and Miklosova B:
Contrast-enhanced ultrasonography in the evaluation of incidental
focal liver lesions: A cost-effectiveness analysis. World J
Gastroenterol. 22:8605–8614. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Ogawa C, Minami Y, Morioka Y, Noda A,
Arasawa S, Izuta M, Kubo A, Matsunaka T, Tamaki N, Shibatouge M and
Kudo M: Virtual sonography for novice sonographers: Usefulness of
SYNAPSE VINCENT® with pre-check imaging of tumor
location. Oncology. 87 (Suppl 1):S50–S54. 2014. View Article : Google Scholar
|