Introduction
Colorectal cancer (CRC) is the third most common
malignancy and the fourth leading cause of cancer-related mortality
worldwide (1,2). The improvement in treatments for
unresectable advanced or metastatic CRC (mCRC) has markedly changed
its prognosis over the past few decades (3–13), and
the median overall survival (OS) and progression-free survival
(PFS) are currently approaching 30 and 10 months, respectively
(14,15). Although PFS has been recognized as a
surrogate parameter for OS, the possible effect of post-progression
treatments on OS is often considered. A randomized phase 3 trial
(First-Line Treatment For Patients With Metastatic Colorectal
Cancer-3) examining first-line chemotherapy for mCRC demonstrated
that the depth of response (DpR) was correlated with survival time
(16). Thus, DpR may also be
considered as a surrogate endpoint for OS (4,16,17).
Controlling liver metastases is an important factor
for improving OS in CRC patients with limited liver metastases, as
several studies have demonstrated that the resection of liver
metastases led to a better prognosis compared with hepatectomy
after chemotherapy (18–22). In addition, adequate control of
extrahepatic lesions is not necessarily associated with favorable
survival when liver metastases persist (23). Furthermore, regardless of recurrence
in the liver or extrahepatic organs following hepatectomy,
hepatectomized patients had a significantly better prognosis
compared with patients not undergoing resection (22,24–27). The
Japanese Society for Cancer of the Colon and Rectum 2014 guidelines
for the treatment of CRC recommend surgical resection of liver
metastases when the liver lesions become resectable following
systemic chemotherapy (28–32). However, it remains unknown whether it
is also meaningful to control liver metastases by chemotherapy in
CRC patients with metastases to multiple organs.
The Response Evaluation Criteria In Solid Tumors
(RECIST) has been adopted as a widely accepted method for assessing
the objective response of solid tumors to treatment (18). In RECIST version 1.0, the definition
of response is a 30% decrease in the sum of diameters of measurable
lesions in up to five organs (18).
The number of lesions required to assess tumor burden for response
determination was reduced from a maximum of 10 to 5 in the 2009
revision of RECIST guidelines (18),
based on evidence that 5 measurable lesions was the minimum number
of target lesions that did not cause meaningful changes in the
reduction ratio (RR) (33). This
revision may provide convenient and reproducible lesion
measurements in clinical trials. Assessing the echange in tumor
burden is crucial for evaluating tumor response, as both objective
tumor response and PFS time are used as endpoints in clinical
trials; these parameters are also key to assessing the
effectiveness and appropriate selection of treatment in clinical
practice. There are no data that analyze the RR for each metastatic
organ. Particularly in mCRC, liver metastases are subdivided as
H1-H3 according to tumor volume; however, it remains unknown how
the degree of shrinkage differs for each case with various tumor
volumes of liver metastases.
The aim of the present study was to investigate CRC
cases with multiple organ metastases, including the liver, in order
to analyze how the TRR is correlated with the LRR and to evaluate
whether tumor reduction and the control of liver metastases with
systemic chemotherapy can improve patient prognosis.
Patients and methods
Patients and definition of
response
This was a retrospective study on the primary
systemic chemotherapy of CRC patients with multiple metastases to
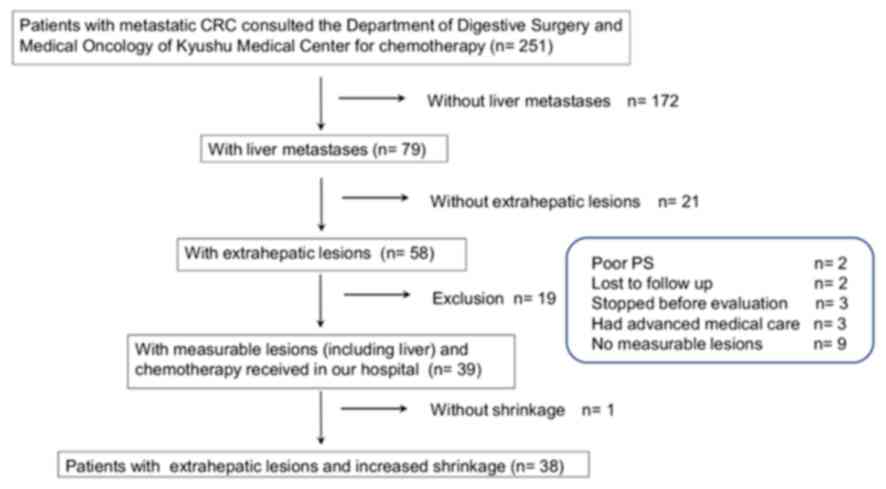
the liver and other organs. Between April 2013 and April 2016, we
screened 251 patients with CRC who received consult for the purpose
of chemotherapy at the Department of Gastrointestinal Surgery and
the Department of Medical Oncology of Kyushu Medical Center
Hospital. Among those, 172 patients without liver metastases, 21
patients without extrahepatic lesions and 9 patients without
measurable lesions in the liver and extrahepatic organs were
excluded. Other patients who were excluded from the analyses were
as follows: 2 patients who were unsuitable for chemotherapy due to
poor performance status (PS), 2 patients who were lost to
follow-up, 3 patients who did not receive chemotherapy prior to the
evaluation, 3 patients whose insurance did not cover combined
advanced healthcare services, and 1 patient who did not achieve
tumor reduction by chemotherapy, as the present study evaluated RR.
Finally, 38 patients who had measurable lesions in both the liver
and extrahepatic organs, and who were able to continue chemotherapy
at our hospital, were analyzed (Fig.
1). Clinical information, including age, sex, Eastern
Cooperative Oncology Group PS, RAS mutation status,
chemotherapy regimen, primary tumor site, H stage of liver
metastases, tumor RR, treatment duration of primary chemotherapy
and survival time, was obtained from medical records. H stage was
determined by the number of liver metastases and size of the
largest liver metastatic lesion: H1; ≤4 lesions, and lesions ≤5 cm
in diameter, H2; ≥5 lesions, or lesions ≥5 cm in diameter, and H3;
≥5 lesions, and lesions ≥5 cm in diameter (17,34). The
study protocol was approved by the ethics committee of Kyushu
Medical Center Hospital.
The treatment duration of primary chemotherapy was
defined as the time from the first day of chemotherapy to the day
on which the regimen was changed, or to the date of death from any
cause. OS was defined as the time from the first day of
chemotherapy to the last day on which the patient was confirmed to
be alive, or to the date of death from any cause. For the total
lesion reduction ratio (TRR), the selection of measurable lesions
and calculation of the RR were conducted according to the RECIST.
To evaluate the liver lesions, measurable lesions were selected (up
to two lesions in each organ) according to the RECIST, the sum of
the major diameters of the hepatic and extrahepatic lesions (short
diameter for lymph nodes) was measured, and the reduction ratio at
the time of maximum reduction against pretreatment was calculated.
The reduction ratio of all lesions assessed by RECIST was expressed
as the TRR and the reduction ratio of only liver lesions was
expressed as the liver lesion reduction ratio (LRR). Patients who
developed a recurrence following hepatectomy are included in this
study. In our hospital, the indications of hepatectomy for each
case in which the surgeons consider it technically feasible to
resect both the primary tumor and liver metastases, and which can
withstand surgery and the disease state is stable, are discussed in
a multidisciplinary joint conference.
Statistical analysis
Pearson's correlations were applied to determine the
association between TRR and LRR. Partial correlation analysis was
used to assess this association controlling for H stage. Univariate
and multivariate analyses of factors associated with OS were
calculated with Cox regression survival analysis. OS and duration
of primary chemotherapy were calculated with the Kaplan-Meier
method, and differences between survival curves were analyzed by
the log-rank test. A difference was considered statistically
significant when the two-sided P-value was <0.05. Patient
categorical variables and characteristics between H1/2 and H3 liver
metastases were compared using the Fisher's exact test and
Student's t-test, respectively.
Results
Patient characteristics
The clinical characteristics of the 38 patients who
were finally enrolled in this study are listed in Table I. The study population included 18
(47%) men. The median age at diagnosis was 65 years (range, 44–84
years). The majority of the patients had a PS of 0 or 1, but the PS
of 3 patients was 2 (8%). The primary sites were as follows: 10
cases in the ascending colon (26%), 5 in the transverse colon
(13%), 1 in the descending colon (3%), 13 in the sigmoid colon
(34%), and 9 in the rectum (24%). The RAS status was
wild-type in 22 patients (29%), and 4 patients were not
investigated. Regarding the H stage of liver metastases, 9 patients
(24%) were H1, 11 (27%) were H2, and 18 (47%) were H3. Measurable
lesions other than those in the liver included lesions in the lung,
lymph nodes, peritoneum, ovary, and soft tissue. The median of the
maximum diameter of liver lesions was 81.2 mm (range, 8.39–228.7
mm), and the median liver occupancy rate in all measurable lesions
of each of the 38 patients was 76% (range, 16–94%). Among these
patients, 18 underwent resection of the primary site before primary
chemotherapy, 11 underwent resection to manage their symptoms, and
the remaining 7 underwent radical surgery; 22 of the 38 patients
developed recurrence after hepatectomy. A two-drug combination
chemotherapy was administered to 33 (86%) patients, and 29 (76%)
were administered bevacizumab, a molecular-targeted agent. Five
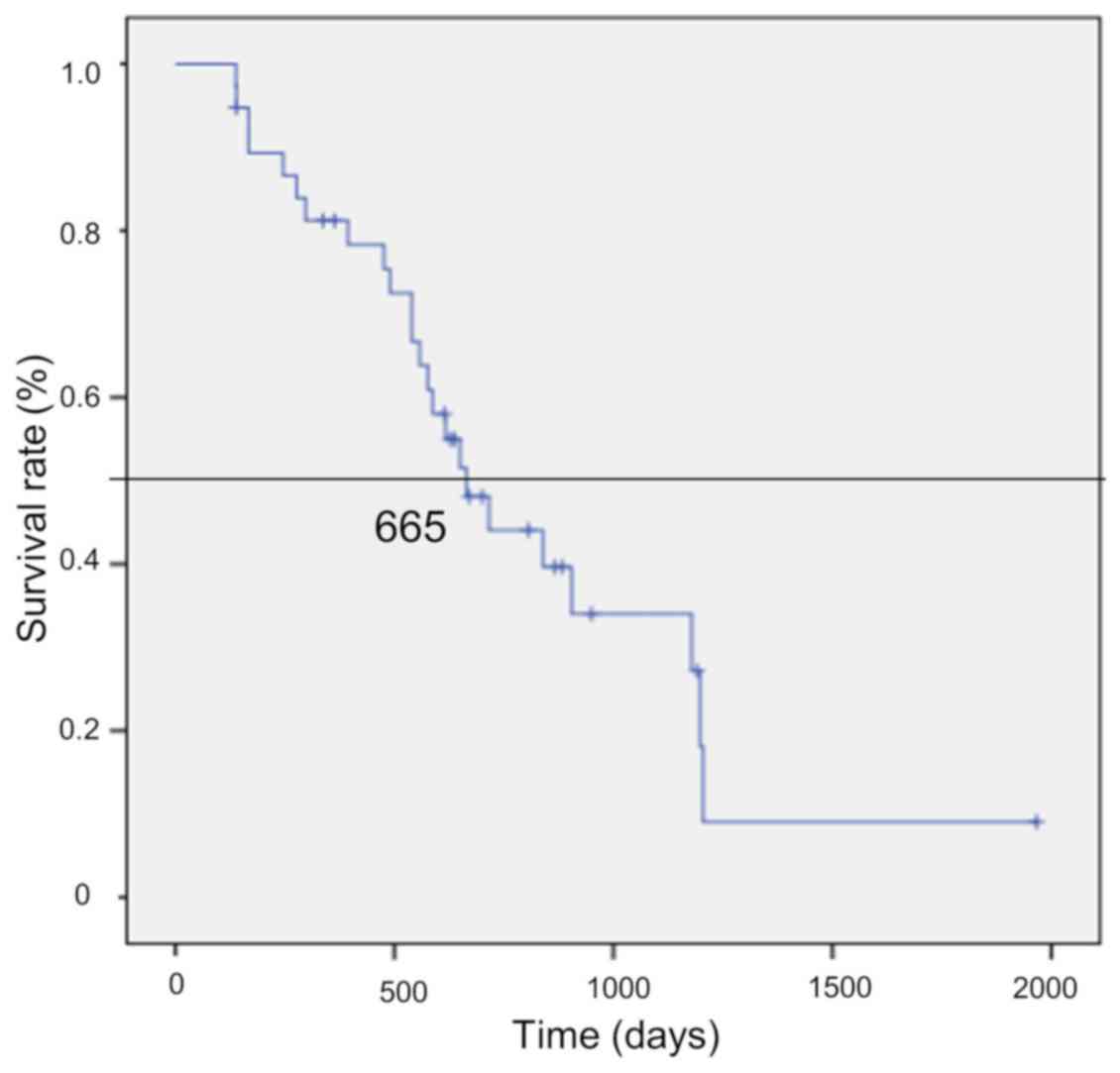
patients (14%) received monotherapy. The median survival time of
the 38 patients was 665 days [95% confidence interval (CI):
548–767] (Fig. 2).
 | Table I.Clinical characteristics of enrolled
patients (n=38). |
Table I.
Clinical characteristics of enrolled
patients (n=38).
|
Characteristics | Number (%) |
|---|
| Age, years |
|
|
<65 | 18 (47) |
|
≥65 | 20 (53) |
| Mean
66.2, median 65 |
|
| Sex |
|
|
Male | 18 (47) |
|
Female | 20 (53) |
| ECOG-PS |
|
| 0 | 18 (47) |
| 1 | 17 (45) |
| 2 | 3 (8) |
| Primary tumor
site |
|
|
Ascending colon | 10 (26) |
|
Transverse colon | 5 (13) |
|
Descending colon | 1 (3) |
| Sigmoid
colon | 13 (34) |
|
Rectum | 9 (24) |
| H stage |
|
| H1 | 9 (24) |
| H2 | 11 (29) |
| H3 | 18 (47) |
| RAS |
|
|
Wild-type | 22 (58) |
|
Mutation | 12 (32) |
|
Unknown | 4 |
| Resection of
primary site |
|
|
Resection | 18 (47) |
| No
resection | 20 (53) |
| Liver
resection |
|
|
Resection | 22 (58) |
| No
resection | 16 (42) |
| Targeted
agents |
|
|
Bevacizumab | 29 (76) |
|
Anti-EGFR antibody | 0 |
|
None | 9 (24) |
| Chemotherapy |
|
|
Two-drug combination | 33 (86) |
|
Single-agent | 5 (14) |
| Extrahepatic
measurable lesions |
|
|
Lung | 11 |
|
Peritoneum | 5 |
| Lymph
nodes | 23 |
|
Other | 4 |
| Median liver
occupancy | 76% (16–94%) |
| Median OS
(days) | 665 (95% CI:
507.9–822) |
Association between TRR and LRR
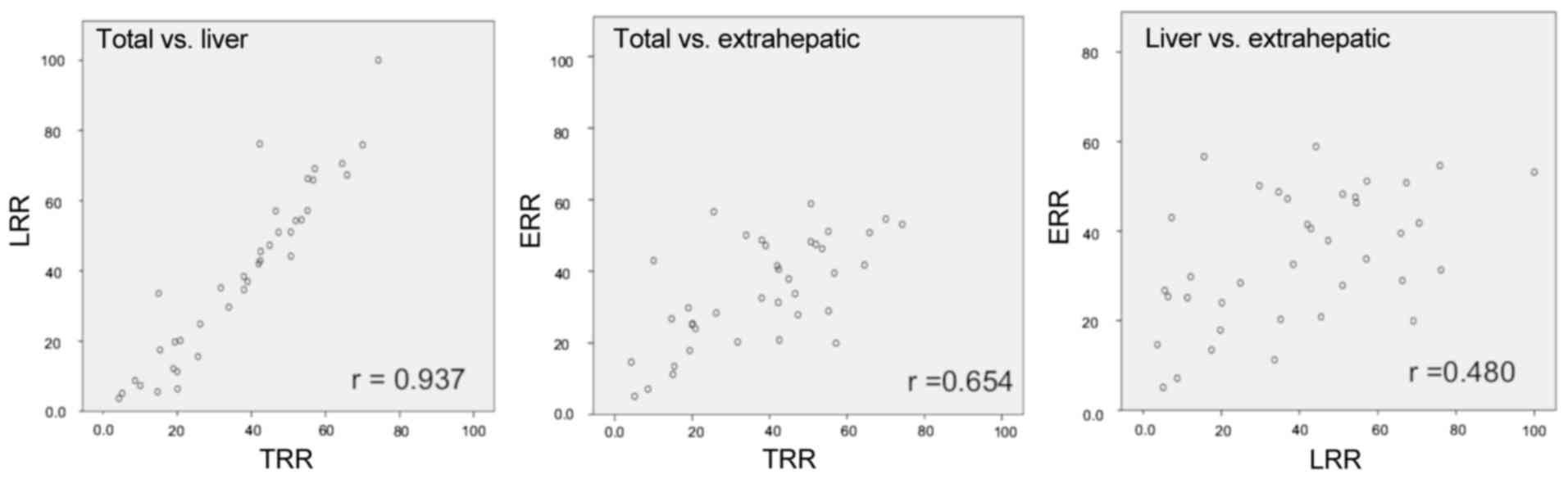
The mean of the TRR was 37% (95% CI: 31–43), and the
mean of the LRR was 39% (95% CI: 31–47). TRR and LRR were strongly
correlated (r=0.937, P<0.0001) in any H stage (H1/H2: r=0.911,
H3: r=0.915; Fig. 3). The results of
univariate and multivariate analyses for predictors of OS are
summarized in Table II. On
univariate analysis, RAS wild-type status, >30% of TRR
and >30% of LRR were associated with a better OS. On
multivariate analysis, RAS wild-type status [hazard ratio
(HR)=0.281, P=0.016] and >30% TRR (HR=0.23, P=0.006) were
independent predictors of better OS.
 | Table II.Univariate and multivariate analyses
for predictors of overall survival. |
Table II.
Univariate and multivariate analyses
for predictors of overall survival.
|
| Univariate | Multivariate |
|---|
|
|
|
|
|---|
| Variables | P-value | HR | 95% CI | P-value |
|---|
| Age, years |
|
|
|
|
| ≥65 vs.
<65 | 0.517 |
|
|
|
| Sex |
|
|
|
|
| Male
vs. female | 0.517 |
|
|
|
| ECOG PS score |
|
|
|
|
| 0–1 vs.
2–4 | 0.333 |
|
|
|
| Liver metastases
time |
|
|
|
|
|
Synchronous vs.
metachronous | 0.451 |
|
|
|
| Resection of
primary site |
|
|
|
|
|
Resection vs. no
resection | 0.265 |
|
|
|
| H stage of liver
metastases |
|
|
|
|
| H1 or 2
vs. H3 | 0.118 |
|
|
|
| RAS status |
|
|
|
|
|
Wild-type vs. mutation | 0.041 | 0.016 | 0.281 | 0.100–0.786 |
| CEA level |
|
|
|
|
| High
vs. normal | 0.539 |
|
|
|
| CA19-9 level |
|
|
|
|
| High
vs. normal | 0.246 |
|
|
|
| RECIST RR |
|
|
|
|
| ≥30 vs.
<30% | 0.002 |
0.006 | 0.238 | 0.086–0.657 |
| Liver RR |
|
|
|
|
| ≥30 vs.
<30% | 0.004 | (0.024) | (0.35) | (0.141–0.871) |
| RR other than
liver |
|
|
|
|
| ≥30 vs.
<30% | 0.012 |
|
|
|
| No. of liver
metastases |
|
|
|
|
| ≥5 vs.
<5 | 0.327 |
|
|
|
| Primary site |
|
|
|
|
| Right
vs. left colon | 0.848 |
|
|
|
| Albumin level |
|
|
|
|
| Low vs.
normal | 0.855 |
|
|
|
| Targeted
agents |
|
|
|
|
| Yes vs.
no | 0.158 |
|
|
|
Association between reduction ratio
and OS
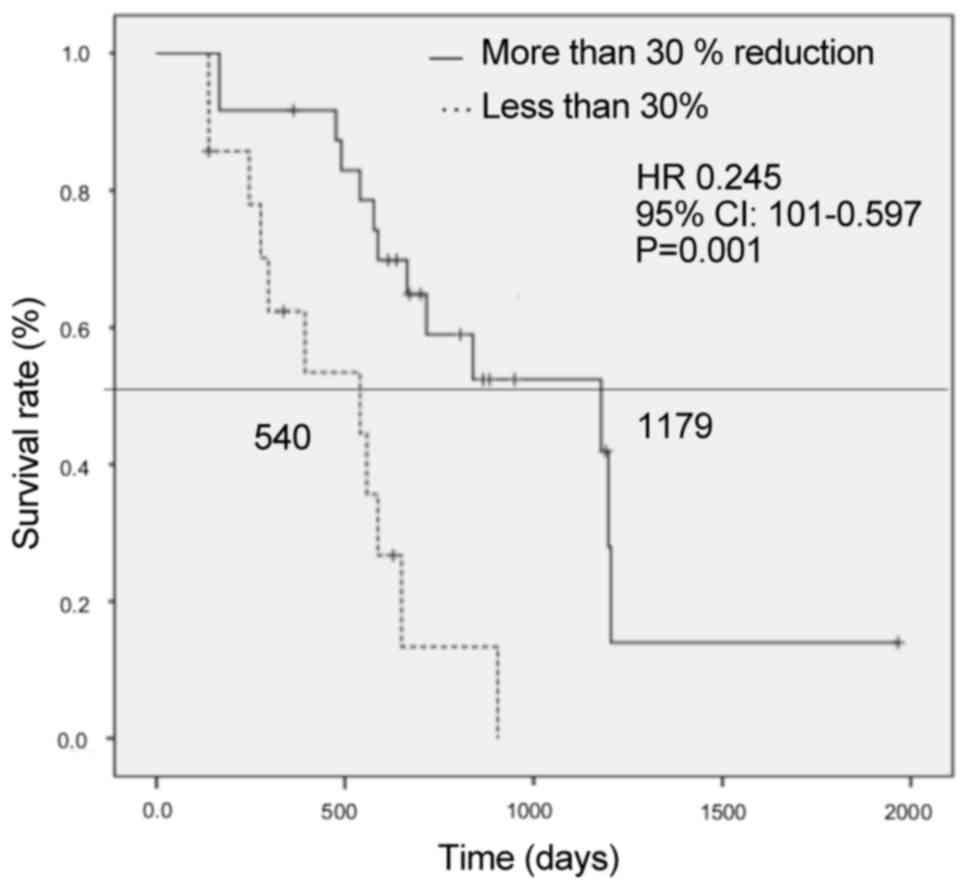
Patients with >30% TRR had a significantly better
OS compared with those with <30% (1,179 vs. 540 days,
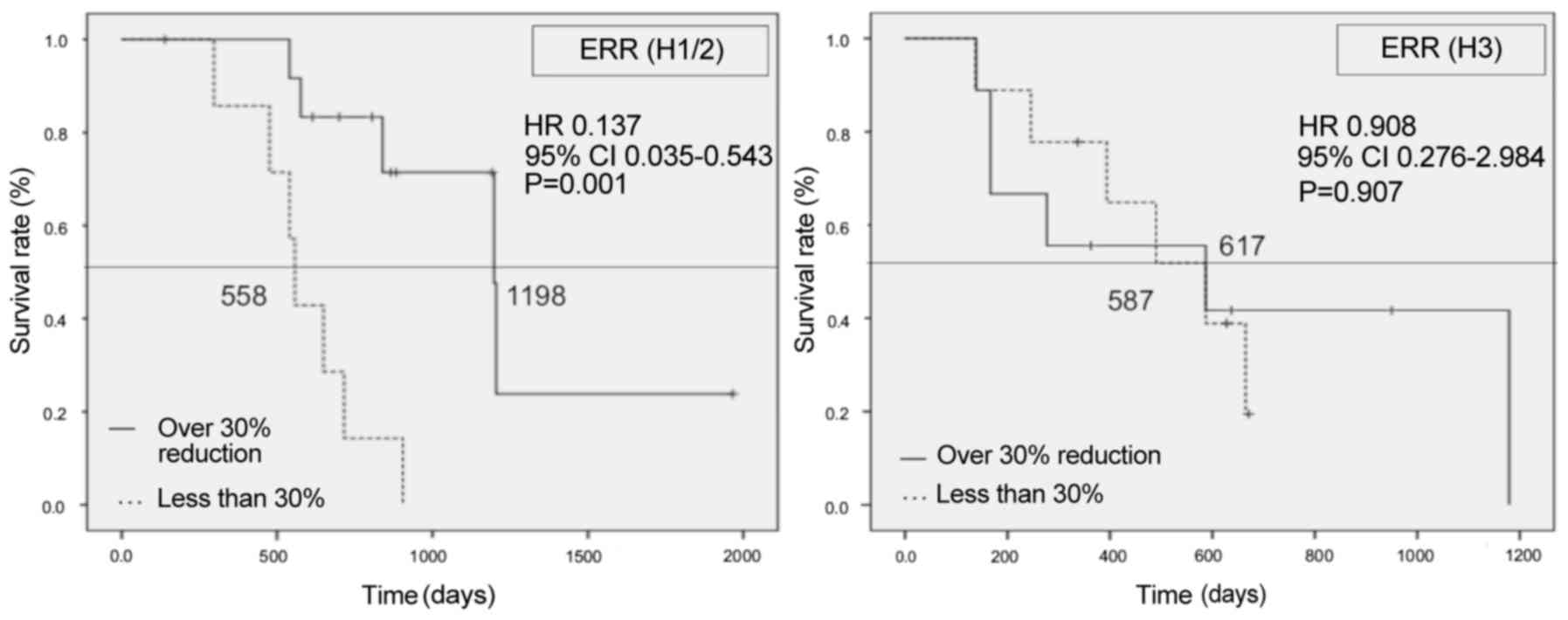
respectively; HR=0.245, 95% CI: 0.101–0.597, P=0.001) (Fig. 4). Patients with >30% LRR also had
a significantly better OS compared with those with <30% (1,179
vs. 540 days, respectively; HR=0.27, 95% CI: 0.111–0.656, P=0.002)
(Fig. 5). However, in patients with
H3 liver metastases, no significant statistical differences in OS
were observed between these two groups of patients upon analysis
for each H stage of liver metastasis (H1/2, HR=0.225, 95% CI:
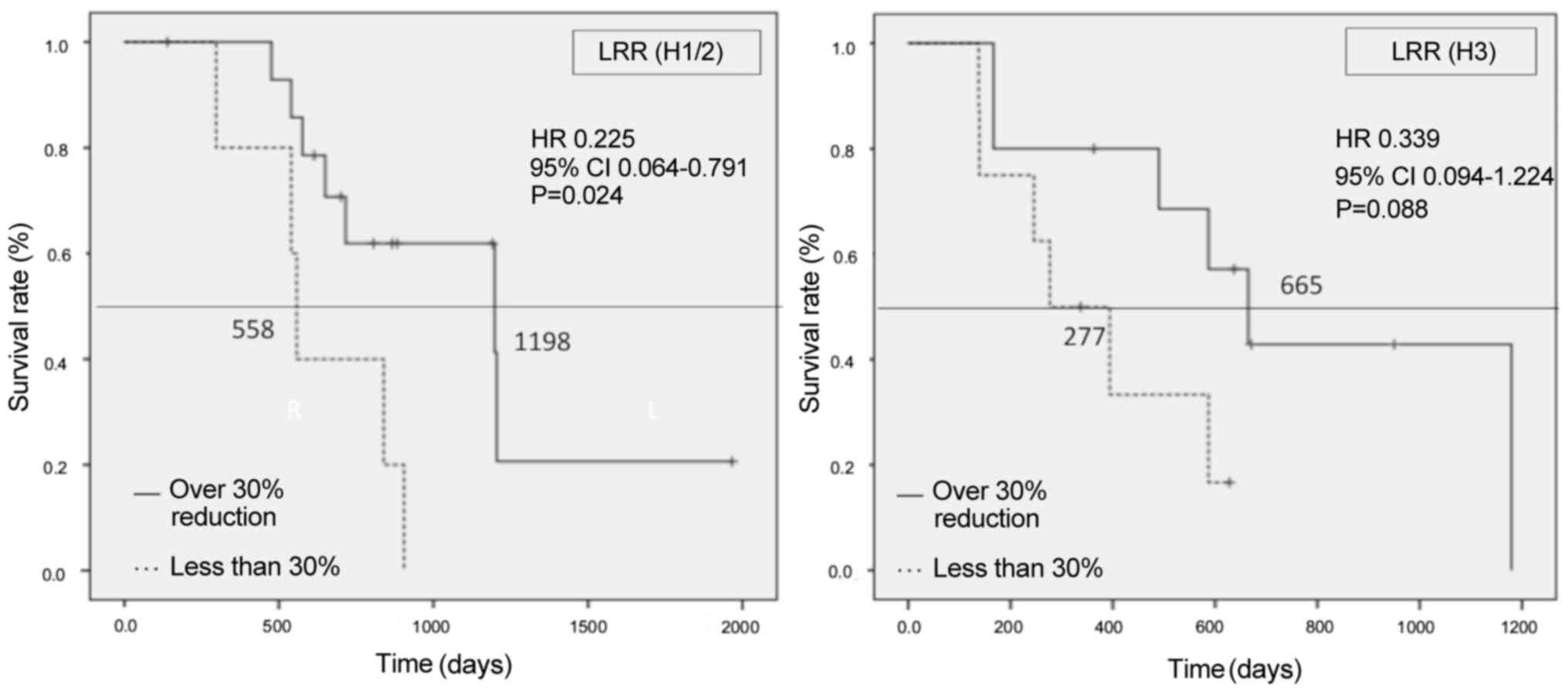
0.064–0.791; H3, HR=0.339, 95% CI: 0.094–1.224) (Fig. 6). The treatment duration of primary
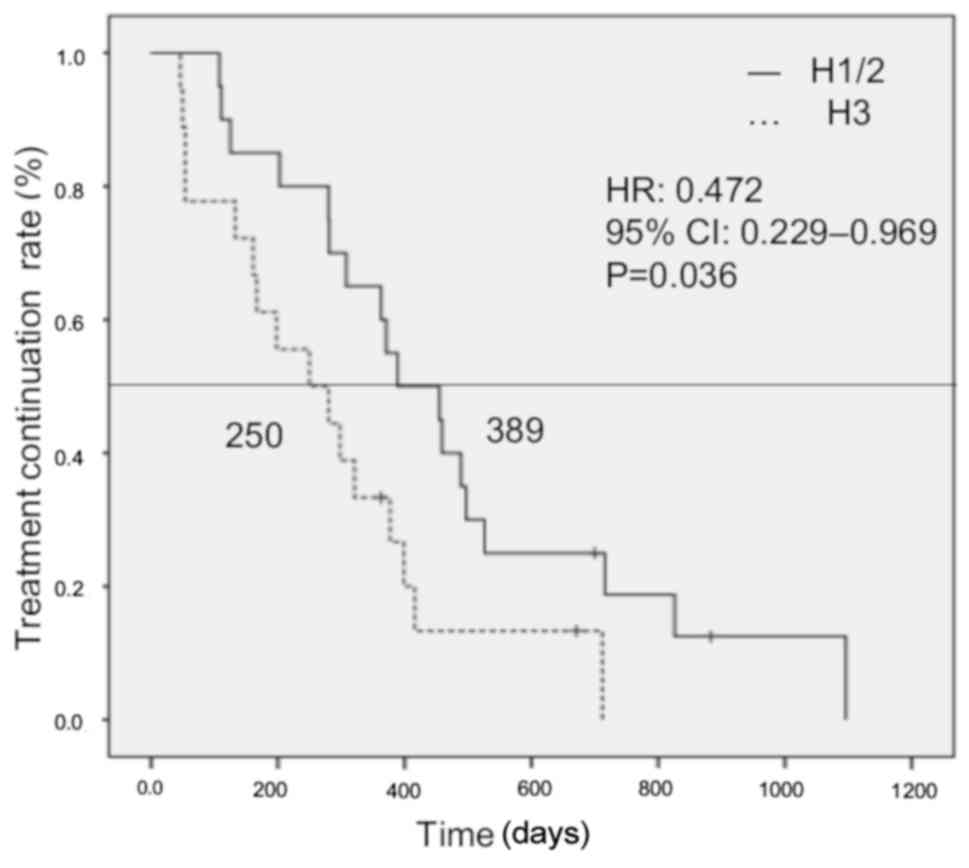
chemotherapy in patients with H1/2 liver metastases was
significantly longer compared with that in patients with H3 (389
vs. 250 days, respectively; HR=0.472, 95% CI: 0.339–0.969, P=0.036)
(Fig. 7). In comparison to the
characteristics of patients with H1/2 and H3 liver metastases
immediately prior to chemotherapy, the maximum diameter of liver
metastases, number of liver metastases, and levels of serum
carcinoembryonic antigen, serum cancer antigen 19-9, aspartate
transaminase, lactate dehydrogenase, alkaline phosphatase and
γ-glutamyl transpeptidase, were higher in H3 patients. On the other
hand, the serum albumin levels were higher in H1/2 patients.
Furthermore, more patients with RAS mutations were H3
(Table III).
 | Table III.Comparison of characteristics between
H1/2 and H3 liver metastases. |
Table III.
Comparison of characteristics between
H1/2 and H3 liver metastases.
|
Characteristics | H1/H2 (n=20) | H3 (n=18) | P-value |
|---|
| PS score |
|
| 0.594 |
|
0-1 | 19 (95) | 16 (89) |
|
| 2 | 1 (5) | 2 (11) |
|
| Age, years |
|
| 0.531 |
|
<65 | 10 (50) | 7 (39) |
|
|
≥65 | 10 (50) | 11 (61) |
|
| Sex |
|
| 0.351 |
|
Male | 11 (55) | 7 (39) |
|
|
Female | 9 (45) | 11 (61) |
|
| Liver
metastases |
|
| 0.72 |
|
Synchronous | 14 (70) | 18 (100) |
|
|
Metachronous | 6 (30) | 0 (0) |
|
| Targeted drug |
|
| 0.13 |
|
Yes | 13 (65) | 16 (89) |
|
| No | 7 (35) | 2 (11) |
|
| Shrinkage |
|
| 0.503 |
|
≥30% | 14 (70) | 10 (56) |
|
|
<30% | 6 (30) | 8 (44) |
|
| Pathology |
|
| 1.00 |
|
Tubular | 6 (30) | 6 (33) |
|
|
Others | 14 (70) | 12 (67) |
|
| Primary site |
|
| 0.468 |
|
Colon | 16 (80) | 12 (67) |
|
|
Rectum | 4 (20) | 6 (33) |
|
| Resection of
primary site |
|
| 0.058 |
|
Resection | 12 (60) | 5 (28) |
|
| No
resection | 8 (40) | 13 (72) |
|
| Number of liver
metastases |
|
| 0.025 |
| ≥6 | 8 (40) | 14 (78) |
|
|
<6 | 12 (60) | 4 (22) |
|
| RAS status |
|
| 0.089 |
|
Wild-type | 14 (70) | 7 (39) |
|
|
Mutation | 5 (25) | 10 (61) |
|
|
Unknown | 1 | 1 |
|
| Maximum diameter of
hepatic metastases (mm) | 29.45
(8.39–54.79) | 29.45
(8.39–54.79) | <0.001 |
| CEA (ng/ml) | 13.5
(6.2–1,314.5) | 221.1
(4.3–1,471.1) | 0.045 |
| CA19-9 (U/ml) | 12 (1–2,937) | 873
(4.1–10,590) | 0.0007 |
| AST (U/ml) | 20 (12–85) | 39 (14–210) | 0.011 |
| ALT (U/ml) | 19 (10–62) | 25.5 (8–85) | 0.41 |
| LDH (U/ml) | 225 (141–406) | 415
(175–2,400) | 0.0076 |
| ALP (U/ml) | 244 (141–815) | 462 (7–1,291) | <0.001 |
| γ-GTP (U/ml) | 31 (13–255) | 109.5 (24–495) | 0.023 |
| Serum albumin
(g/dl) | 3.7 (2.6–4.6) | 3.2 (2.0–4.0) | 0.012 |
| Lymph node
metastases |
|
| 1.00 |
|
Yes | 10 (50) | 9 (50) |
|
| No | 10 (50) | 9 (50) |
|
Discussion
To the best of our knowledge, this was the first
study to investigate the correlation between RECIST RR and the LRR
in mCRC patients, and to analyze the association between tumor
reduction of liver metastases and prognosis. A strong correlation
between TRR and LRR in any H stage was observed. In mCRC cases with
liver metastases, regardless of the tumor volume of the liver (H
stage), the TRR reflected the LRR (Fig.
3). As 70–87% of patients with unresectable or recurrent CRC
harbored liver metastases (32,33,35–38), the
RR reported in clinical trials for unresectable or recurrent CRC
mostly reflected the LRR.
In the present study, no patients received
anti-epidermal growth factor receptor (EGFR) antibody, and only
bevacizumab was used as a molecular-targeted agent. It was reported
that anti-EGFR antibody combination therapy exerted a higher tumor
shrinkage effect compared with anti-vascular endothelial growth
factor combination antibodies (35–37).
These cases, even in patients with wild-type RAS, did not
require an immediate reduction effect, but rather a sustained
treatment strategy of sequential therapies.
Multivariate analysis revealed that >30% tumor
reduction was correlated with a favorable prognosis. The OS was
significantly better in patients with >30% reduction ratio in
the log-rank test (Figs. 4 and
5), and DpR was suggested to improve
the prognosis. However, in the analysis of the association between
reduction of liver metastases and prognosis of each H stage, no
statistically significant difference was observed in patients with
H3 liver metastases, even with >30% reduction of the lesions
(Fig. 6). Although there may not be
a significant difference due to the small number of cases, it is
suggested there are some factors that affect the prognosis in
addition to tumor reduction in the H3 group.
In the H3 group, the duration of primary
chemotherapy was clearly shorter compared with the H1/2 group.
Liver function prior to treatment of H3 patients was significantly
worse compared with that of H1/2 patients (Table III), but there was no difference in
the dose intensity and treatment intensity of chemotherapy.
Considering the rapid regrowth of lesions and appearance of new
lesions in the H3 group, the poor prognosis may be attributed to
the biological characteristics of the tumor. An example is the
RAS mutation. In this study, RAS mutation was a
contributing factor to the poor prognosis on multivariate analysis
(HR=3.5, P=0.02; Table II), and
more patients in the H3 group tended to harbor RAS
mutations, although the difference was not statistically
significant. Several studies have reported that mutations in the
RAS gene itself is a poor prognostic factor (38–42), and
that prognosis following hepatectomy is poor in the RAS
mutation group. Thus, mutations in the RAS gene may lead to
an aggressive tumor phenotype (43–45).
Although BRAF mutations were not examined in this study,
further progress in gene research is expected to provide more
detailed prognostic predictions and treatment strategies.
These results suggest that >30% reduction did not
always improve the prognosis in CRC patients with H3 liver
metastasis. For such cases, systemic chemotherapy alone may not
improve the prognosis, and other treatment strategies may be
required to control liver metastases, such as combination with
local treatment, including debulking liver resection. There are
some reports that liver metastasis control contributes to the
improvement of survival. For example, Elias et al (24) reported that the prognosis was
improved by curative resection of liver lesions, even if the
patients had extrahepatic metastases, and Bokemeyer et al
(41) reported that a good prognosis
was obtained by combining resection with chemotherapy, even with R1
resection of liver metastases. However, Passot et al
(40) reported that node-positive
primary tumors, a tumor diameter of >3 cm, and >7 cycles of
preoperative chemotherapy, were factors associated with worse OS
for mCRC patients who had hepatectomy, and the more of these
prognostic factors the patients had, the worse their OS, even if R0
hepatectomy was performed. Maughan et al (42) reported that the response to
preoperative chemotherapy was likely to be a significant prognostic
factor affecting survival time following curative hepatectomy.
Therefore, it is hypothesized that the prognosis after hepatectomy
may be associated with various factors, and further studies on the
therapeutic indications for local control of liver metastases are
needed.
There were certain limitations to the present study.
First, this was a retrospective single-center study, and the number
of cases was limited. Second, recurrence cases after hepatectomy
are included, whereas time to relapse was not considered. Third,
the treatment regimen was not uniform. Fourth, tumor reduction was
evaluated only by the tumor diameter, and the possible reduction
effect of changes such as tumor necrosis and lumen formation could
not be evaluated in this manner.
It may be that larger tumors are less likely to
exhibit shrinkage due to tumor necrosis and lumen formation;
therapeutic effect evaluation may be difficult in these cases.
Taken together, the results of the present study
demonstrated that the LRR was strongly reflected by the TRR in mCRC
cases with liver metastases. The correlation of DpR with both TRR
and LRR and prognosis was suggested; however, H3 patients did not
achieve prolonged survival, even in DpR cases. Multidisciplinary
treatments, including local therapy, may improve the prognosis of
H3 patients. However, further studies with a larger number of cases
are needed to draw more definitive conclusions.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
All the datasets generated and analyzed in the
present study are included in this published manuscript.
Authors' contributions
SK and KU conceived the present study. SK designed
the current study and performed the necessary calculations. SK, KU
and MN verified the analytical methods used. EB and KU performed
the experiments. All authors discussed the results and contributed
to the final manuscript.
Ethics approval and consent to
participate
The study protocol was approved by the Ethics
Committee of Kyushu Medical Center Hospital.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Jemal A, Bray F, Center MM, Ferlay J, Ward
E and Forman D: Global cancer statistics. CA Cancer J Clin.
61:69–90. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Ferlay J, Shin HR, Bray F, Forman D,
Mathers C and Parkin DM: Estimates of Worldwide burden of cancer in
2008: GLOBOCAN 2008. Int J Cancer. 127:2893–2917. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Manfredi S, Lepage C, Hatem C, Coatmeur O,
Faivre J and Bouvier AM: Epidemiology and management of liver
metastases from colorectal cancer. Ann Surge. 244:254–259. 2006.
View Article : Google Scholar
|
|
4
|
Heinemann V, Stintzing S, Modest DP,
Giessen-Jung C, Michl M and Mansmann UR: Early tumor shrinkage
(ETS) and depth of response (DpR) in the treatment of patients with
metastatic colorectal cancer (mCRC). Eur J Cancer. 51:1927–1936.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Schmoll HJ, Van Cutsem E, Stein A,
Valentini V, Glimelius B, Haustermans K, Nordlinger B, van de Velde
CJ, Balmana J, Regula J, et al: ESMO consensus guidelines for
management of patients with colon and rectal cancer. A personalized
approach to clinical decision making. Ann Oncol. 23:2479–2516.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Van Cutsem E, Dicato M, Arber N, Berlin J,
Cervantes A, Ciardiello F, De Gramont A, Diaz-Rubio E, Ducreux M,
Geva R, et al: Molecular markers and biological targeted in
metastasis colorectal cancer: Expert opinion and recommendations
derived from the 11th ESNO/World Congress on Gastrointestinal
Cancer, Barcelona, 2009. Ann Oncol. 21 (Suppl 6):vi1–10. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Ye LC, Liu TS, Ren L, Wei Y, Zhu DX, Zai
SY, Ye QH, Yu Y, Xu B, Qin XY and Xu J: Randomized controlled trial
of cetuximab plus chemotherapy for patients with LRAS wild-type
unresectable colorectal liver-limited metastases. J Clin Oncol.
31:1931–1938. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Folprecht G, Gruenberger T, Bechstein WO,
Raab HR, Lordick F, Hartmann JT, Lang H, Frilling A, Stoehlmacher
J, Weitz J, et al: Tumor response and secondary resectability of
colorectal liver metastases following neoadjuvant chemotherapy with
cetuximab: The CELIM randomized phase II trial. Lancet Oncol.
11:38–47. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Van Cutsem E, Köhne CH, Hitre E, Zaluski
J, Chang Chien CR, Makhson A, D'Haens G, Pintér T, Lim R, Bodoky G,
et al: Cetuximab and chemotherapy as initial treatment for
metastatic colorectal cancer. N Engl J Med. 360:1408–1417. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Bokemeyer C, Bondarenko I, Makhson A,
Hartmann JT, Aparicio J, de Braud F, Donea S, Ludwig H, Schuch G,
Stroh C, et al: Fluororacil, leucovorin, and oxaliplatin with and
without cetuximab in the first line treatment of metastatic
colorectal cancer. J Clin Oncol. 27:663–671. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Saltz LB, Clarke S, Díaz-Rubio E,
Scheithauer W, Figer A, Wong R, Koski S, Lichinitser M, Yang TS,
Rivera F, et al: Bevacizumab in combination with oxaliplatin-based
chemotherapy as first-line therapy in metastatic colorectal cancer:
A randomized phase III study. J Clin Oncol. 26:2013–2019. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Douillard JY, Oliner KS, Siena S,
Tabernero J, Burkes R, Barugel M, Humblet Y, Bodoky G, Cunningham
D, Jassem J, et al: Panitumumab-FOLFOX4 treatment and RAS mutations
in colorectal cancer. N Engl J Med. 369:1023–1034. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Hurwitz H, Fehrenbacher L, Novotny W,
Cartwright T, Hainsworth J, Heim W, Berlin J, Baron A, Griffing S,
Holmgren E, et al: Bevacizumab plus irinotecan, fluoriuracil, and
leucovolin for metastatic colorectal cancer. N Engl J Med.
350:2335–2342. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Fakih MG: Metastatic colorectal cancer:
Current state and future directions. J Clin Oncol. 33:1809–1824.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Heinemann V, von Weikersthal LF, Decker T,
Kiani A, Vehling-Kaiser U, Al-Batran SE, Heintges T, Lerchenmüller
C, Kahl C, Seipelt G, et al: FOLFIRI plus cetuximab versus FOLFIRI
plus bevacizumab as first-line treatment for patients with
metastatic colorectal cancer (FIRE-3): A randomized, open-label,
phase III trial. Lancet Oncol. 15:1065–1075. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Osumi H, Matsusaka S, Suenaga M, Wakatsuki
T, Ogura M, Ozaka M, Shinozaki E, Chin K and Mizunuma N:
Quantitative analysis of the impact of deepness of response on
survival time following patients with metastatic colorectal cancer
treated by chemotherapy and anti-EGFR monoclonal antibodies. J Clin
Oncol 32 (3 Suppl). S4932014. View Article : Google Scholar
|
|
17
|
Peeters M, Price TJ, Cervantes A, Sobrero
A, Ducreux MP, André T, Lordick F, Punt CJA, Koukakis R, Terwey J
and van Custem E: Tumour shrinkage and response outcomes during
second-line panitumumab (pmab) + FOLFIRI vs FOLFIRI treatment. Ann
Oncol. 25 (Suppl 4):iv186–iv187. 2014. View Article : Google Scholar
|
|
18
|
Eisenhauer EA, Therasse P, Bogaerts J,
Schwartz LH, Sargent D, Ford R, Dancey J, Arbuck S, Gwyther S,
Mooney M, et al: New response evaluation criteria in solid tumours:
Revised RECIST guideline (version 1.1). Eur J Cancer. 45:228–247.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Nordlinger B, Van Cutsem E, Gruenberger T,
Glimelius B, Poston G, Rougier P, Sobrero A and Ychou M; European
Colorectal Metastases Treatment Group; Sixth International
Colorectal Liver Metastases Workshop, : Combination of surgery and
chemotherapy and the role of targeted agents in the treatment of
patients with colorectal liver metastases: Recommendations from an
expert panel. Ann Oncol. 20:985–992. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Adam R, Lucidi V and Bismuth H: Hepatic
colorectal metastases: Methods of improving resectability. Surge
Clin North Am. 84:659–671. 2004. View Article : Google Scholar
|
|
21
|
Pawlik TM and Choti MA: Surgical therapy
for colorectal metastases to the liver. J Gastrointest Surg.
11:1057–1077. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Sharma S, Camci C and Jobbour N:
Management of hepatic metastasis from colorectal cancers: An
update. J Hepatobiliary Pancreat Surg. 15:570–580. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Himuro N, Minakata T, Oshima Y, Kataoka D,
Yamamoto S and Kadokura M: Prognostic indicators after resection of
pulmonary metastases from colon and rectal cancer. J Jpn Assoc
Chest Surg. 30:136–142. 2016. View Article : Google Scholar
|
|
24
|
Elias D, Sideris L, Pocard M, Ouellet JF,
Boige V, Lasser P, Pignon JP and Ducreux M: Results of R0 resection
for colorectal liver metastases associated with extrahepatic
disease. Ann Surg Oncol. 11:274–280. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Adam R, de Gramont A, Figueras J, Kokudo
N, Kunstlinger F, Loyer E, Poston G, Rougier P, Rubbia-Brandt L,
Sobrero A, et al: Managing synchronous liver metastases from
colorectal cancer: A multidisciplinary international consensus.
Cancer Treat Rev. 41:729–741. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Yoshidome H, kimura F, Shimizu H, Ohysuka
M and Miyazaki M: Advances in surgical treatment for colorectal
liver metastases. Nippon Shokakibyo Gakkai Zasshi. 106:1438–1446.
2009.(In Japanese). PubMed/NCBI
|
|
27
|
Yang YY, Fleshman JW and Strasberg SM:
Detection and management of extrahepatic colorectal cancer in
patients with resectable liver metastases. J Gastrointest Surg.
11:929–944. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Adam R: Developing strategies for liver
metastases from colorectal cancer. Semin Oncol Apr 34 (2 Suppl 1).
S7–S11. 2007.
|
|
29
|
Lam VW, Spiro C, Laurence JM, Johnston E,
Hollands MJ, Pleass HC and Richardson AJ: A systematic review of
clinical response and survival outcomes of downsizing systemic
chemotherapy and rescue liver surgery in patients with initially
unresectable colorectal liver metastases. Ann Surg Oncol.
19:1292–1301. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Watanabe T, Itabashi M, Shimada Y, Tanaka
S, Ito Y, Ajioka Y, Hamaguchi T, Hyodo I, Igarashi M, Ishida H, et
al: Japanese society for cancer of the colon and rectum (JSCCR)
guidelines 2014 for treatment of colorectal cancer. Int J Clin
Oncol. 20:207–239. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Borner MM: Neoadjuvant chemotherapy for
unresectable liver metastases of colorectal cancer-too good to be
true? Ann Oncol. 10:623–626. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Cummings LC, Payes JD and Cooper GS:
Survival after hepatic resection in metastatic colorectal cancer: A
population-based study. Cancer. 109:718–726. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Bogaerts J, Ford R, Sargent D, Schwartz
LH, Rubinstein L, Lacombe D, Eisenhauer E, Verweij J and Therasse
P; RECIST Working Party, : Individual patient data analysis to
assess modifications to the RECIST criteria. Eur J Cancer.
45:248–260. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Yamaguchi T, Mori T, Takahashi K,
Matsumoto H, Miyamoto H and Kato T: A new classification system for
liver metastases from colorectal cancer in Japanese multicenter
analysis. Hepatogastroenterology. 55:173–178. 2008.PubMed/NCBI
|
|
35
|
Cassidy J, Clarke S, Díaz-Rubio E,
Scheithauer W, Figer A, Wong R, Koski S, Lichinitser M, Yang TS,
Rivera F, et al: Randomized phase III study of capecitabine plus
oxaliplatin compared with fluorouracil/folinic acid plus
oxaliplatin as first-line therapy for metastatic colorectal cancer.
J Clin Oncol. 26:2006–2012. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Klinger M, Tamandl D, Eipeldauer S, Hacker
S, Herberger B, Kaczirek K, Dorfmeister M, Gruenberger B and
Gruenberger T: Bevacizumab improves pathological response of
colorectal cancer liver metastases treated with XELOX/FOLFOX. Ann
Surge Oncol. 17:2059–2065. 2010. View Article : Google Scholar
|
|
37
|
Zorzi D, Chun YS, Madoff DC, Abdalla EK
and Vauthey JN: Chemotherapy with bevacizumab does not affect liver
regeneration after portal vein embolization in the treatment of
colorectal liver metastases. Ann Surg Oncol. 15:2765–2772. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Veen T and Søreide K: Can molecular
biomarkers replace a clinical risk score for resectable colorectal
liver metastasis? World J Gastrointest Oncol. 9:98–104. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Brudvik KW, Kopetz SE, Li L, Conrad C,
Aloia TA and Vauthey JN: Meta-analysis of KRAS mutations and
survival after resection of colorectal liver metastases. Br J Surg.
102:1175–1183. 2015. View
Article : Google Scholar : PubMed/NCBI
|
|
40
|
Passot G, Denbo JW, Yamashita S, Kopetz
SE, Chun YS, Maru D, Overman MJ, Brudvik KW, Conrad C, Aloia TA and
Vauthey JN: Is Hepatectomy justified for Patients with RAS mutant
colorectal liver metastases? An analysis of 524 patients undergoing
curative liver resection. Surgery. 161:332–340. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Bokemeyer C, Kohne C, Rougier P, Stroh C,
Schlichting M and Van Cutsem E: Cetuximab with chemotherapy (CT) as
first-line treatment for metastatic colorectal cancer (mCRC):
Analysis of the CRYSTAL and OPUS studies according to KRAS and BRAF
mutation status. (ASCO Annual Meeting, abstract no. 3506). J Clin
Oncol. 28:15s2010. View Article : Google Scholar
|
|
42
|
Maughan TS, Adams RA, Smith CG, Meade AM,
Seymour MT, Wilson RH, Idziaszczyk S, Harris R, Fisher D, Kenny SL,
et al: Addition of cetuximab to oxaliplatin-based first-line
combination chemotherapy for treatment of advanced colorectal
cancer: Results of the randamised phase 3 MRC COIN trial. Lancet.
377:2103–2114. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Odisio BC, Yamashita S, Huang SY, Harmoush
S, Kopetz SE, Ahrar K, Shin Chun Y, Conrad C, Aloia TA, Gupta S, et
al: Local tumour progression after percutaneous ablation of
colorectal liver metastases according to RAS mutation status. Br J
Surge. 104:760–768. 2017. View Article : Google Scholar
|
|
44
|
Vauthey JN, Zimmitti G, Kopetz SE, Shindoh
J, Chen SS, Andreou A, Curley SA, Aloia TA and Maru DM: RAS
mutation status predicts survival and patterns of recurrence in
patients undergoing hepatectomy for colorectal liver metastases.
Ann Surg. 258:619–626; discussion 626–627. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Margonis GA, Kim Y, Spolverato G, Ejaz A,
Gupta R, Cosgrove D, Anders R, Karagkounis G, Choti MA, Pawlik TM,
et al: Association between specific mutations in KRAS Codon
12 and colorectal liver metastasis. JAMA Surg. 150:722–729. 2015.
View Article : Google Scholar : PubMed/NCBI
|