Introduction
Lung cancer is frequently accompanied by bone
metastasis. A prospective observation study conducted in Japan
revealed that bone metastasis was present at the time of diagnosis
in 48% of patients with stage IV non-small-cell lung cancer (NSCLC)
and in 40% of those with extensive-stage small-cell lung cancer
(1). It has also been demonstrated
that skeletal-related events (SREs) such as pain, bone fracture,
and spinal cord compression in cases of bone metastases lower the
patients' quality of life and worsen their prognosis. In cases of
lung cancer with bone metastases at an advanced stage, SREs are
reportedly found in approximately 20–30% of cases at the time of
diagnosis (1–3). The time between the occurrence of bone
metastases and the onset of SREs is reportedly shorter in patients
with lung cancer than in those with breast or prostate cancer.
Therefore, it is important to initiate treatment as soon as
possible after the diagnosis of bone metastases to prevent
SREs.
Under normal conditions, osteoclasts resorb bone and
release growth factors accumulated in the bone matrix, such as
insulin-like growth factor (IGF) and transforming growth factor-β
(TGF-β), into the marrow, thereby facilitating osteogenesis by
osteoblasts. On the other hand, osteoblasts produce the receptor
activator of nuclear factor κB ligand (RANKL), promote the
differentiation of osteoclast precursors into osteoclasts, and
activate mature osteoclasts. The process of this series of events
is called bone remodeling. If cancer cells invade this environment,
osteolysis by osteoclasts causes IGF and TGF-β to be released from
the bone matrix in order to serve as growth factors for cancer
cells. Cancer cells release cytokines such as parathyroid
hormone-related peptide and prostaglandin E2, which subsequently
induce the expression of RANKL. In turn, RANKL activates
osteoclasts, and the activated osteoclasts accelerate bone
resorption. This secures a space for cancer cells to proliferate
and survive and allows IGF and TGF-β accumulated in the bone matrix
to be released as a means to promote the proliferation of cancer
cells. The proliferation of cancer cells increases cytokines that
stimulate osteoblasts and further facilitates the activity of
osteoclasts, creating a vicious cycle (4).
Bone-modifying agents (BMAs) aim to inhibit the
progression of bone metastases and to reduce SREs by severing this
vicious cycle of bone metastases. At present, BMAs used for lung
cancer include the bisphosphonate zoledronic acid and denosumab,
which is a monoclonal antibody for RANKL.
Zoledronic acid accumulates in the surface of the
bone matrix and exerts its SRE-inhibiting action by causing
apoptosis of osteoclasts that have incorporated zoledronic acid,
thereby inhibiting bone resorption (5,6). In a
phase III randomized placebo-controlled trial in patients with
solid cancers, including non-small cell lung cancer, the incidence
rate of SREs (irradiation to bone, pathological fracture, surgical
treatment of bone lesions, spinal cord compression, and
hypercalcemia) by the 87th week was significantly decreased by
administration of zoledronic acid (7,8).
Based on the results of this study, administration
of zoledronic acid in patients with lung cancer accompanied by bone
metastases has become the standard treatment in daily clinical
practice. The Japanese Society of Medical Oncology (JSMO)
guidelines for the diagnosis and treatment of bone metastases also
recommend this therapy. However, although osteonecrosis of the jaw
(ONJ) is known as an adverse event of zoledronic acid therapy,
there have been no comprehensive reports in the field of lung
cancer. With this in mind, we conducted the present retrospective
multi-center survey study to better understand the clinical course
of NSCLC accompanied by bone metastases, paying particular
attention to the frequency of ONJ, during a period when zoledronic
acid was becoming widely used.
Materials and methods
Patients
This study covered the observation period between
January 2008 and December 2009, and included 12 medical facilities
(Table I) that were routinely
prescribing zoledronic acid for patients with NSCLC accompanied by
bone metastases and that consented to cooperate in the study. All
patients with NSCLC with bone metastases who received at least 2
doses of zoledronic acid were enrolled. The protocol was approved
by the Clinical Trial Review Committee of the Thoracic Oncology
Research Group (TORG) and the Institutional Review Board of
TokyoMetropolitan Cancer and Infectious disease Center Komagome
Hospital (September 13, 2010; approval no. 863) and by each
participating institution. Due to the retrospective nature of the
study, the requirement for written informed consent was waived.
 | Table I.Institutions involved in the
study. |
Table I.
Institutions involved in the
study.
| Institution name | No. of patients
recruited |
|---|
| Tokyo Metropolitan
Cancer and Infectious disease Center Komagome Hospital | 34 |
| Keio University
School of Medicine | 26 |
| Shikoku Cancer
center | 25 |
| Yokohama Municipal
Citizen's Hospital | 24 |
| Tokyo Medical
University | 18 |
| Kitasato University
School of Medicine | 15 |
| Fujisawa city
hospital | 14 |
| National Kyushu
Cancer Center | 14 |
| Gunma Prefectural
Cancer Center | 10 |
| Graduate School of
Medicine, Chiba University | 7 |
| Teikyo University
School of Medicine | 5 |
| Kanagawa Cancer
Center | 4 |
The purpose of the study was to evaluate the
incidence of ONJ. The study items included age, sex, performance
status (PS), histological type, disease stage, treatment regimen,
number of zoledronic acid doses, duration of zoledronic acid
therapy, types of SREs, time until SRE occurrence, and
presence/absence of ONJ.
SREs were defined as bone fracture, spinal cord
compression (spinal paralysis), surgery for bone lesions, radiation
therapy for bone lesions, and hypercalcemia.
Statistical analysis
Differences in characteristics were evaluated using
the χ2 test. Survival was estimated using the
Kaplan-Meier method. All the tests were two-sided, and P<0.05
was considered to indicate a statistically significant difference.
All the data were analyzed using JMP software, version 10 (SAS
Institute, Inc.).
Results
A total of 198 patients were enrolled from the 12
facilities. Table II shows the
patient characteristics. The subjects comprised 126 men and 72
women, with a median age of 64 (44–89) years. The histological type
was adenocarcinoma in 131 patients, squamous cell carcinoma in 30,
and not otherwise specified in 37. Seventy-eight patients had
experienced SREs prior to the beginning of zoledronic acid therapy,
whereas 120 patients had not. Table
III shows the regimens of zoledronic acid therapy. The median
duration of zoledronic acid therapy was 106 days [95% confidence
interval (CI): 92–133 days] in 196 patients, excluding two in whom
data collection was not possible. The median number of zoledronic
acid doses was 4 (2–41).
 | Table II.Patients characteristics. |
Table II.
Patients characteristics.
| Characteristics | No. of patients |
|---|
| Total no. of
patients | 198 |
| Sex |
|
| Male | 126 |
|
Female | 72 |
| Age, years |
|
|
Median | 64 |
|
Range | 44–89 |
| Histology |
|
|
Adenocarcinoma | 131 |
| Squamous
cell carcinoma | 30 |
|
NSCLC-NOS | 37 |
| Prior SREs |
|
| No | 120 |
| Yes | 78 |
 | Table III.Duration and number of ZA
administrations. |
Table III.
Duration and number of ZA
administrations.
| Variable | Duration of ZA
administration | No. of ZA
administrations |
|---|
| Total no. of
patients | 196 | 197 |
| Median, days or
n | 106 | 4 |
| Range, days | – | 2–41 |
| 95% CI | 92–133 | – |
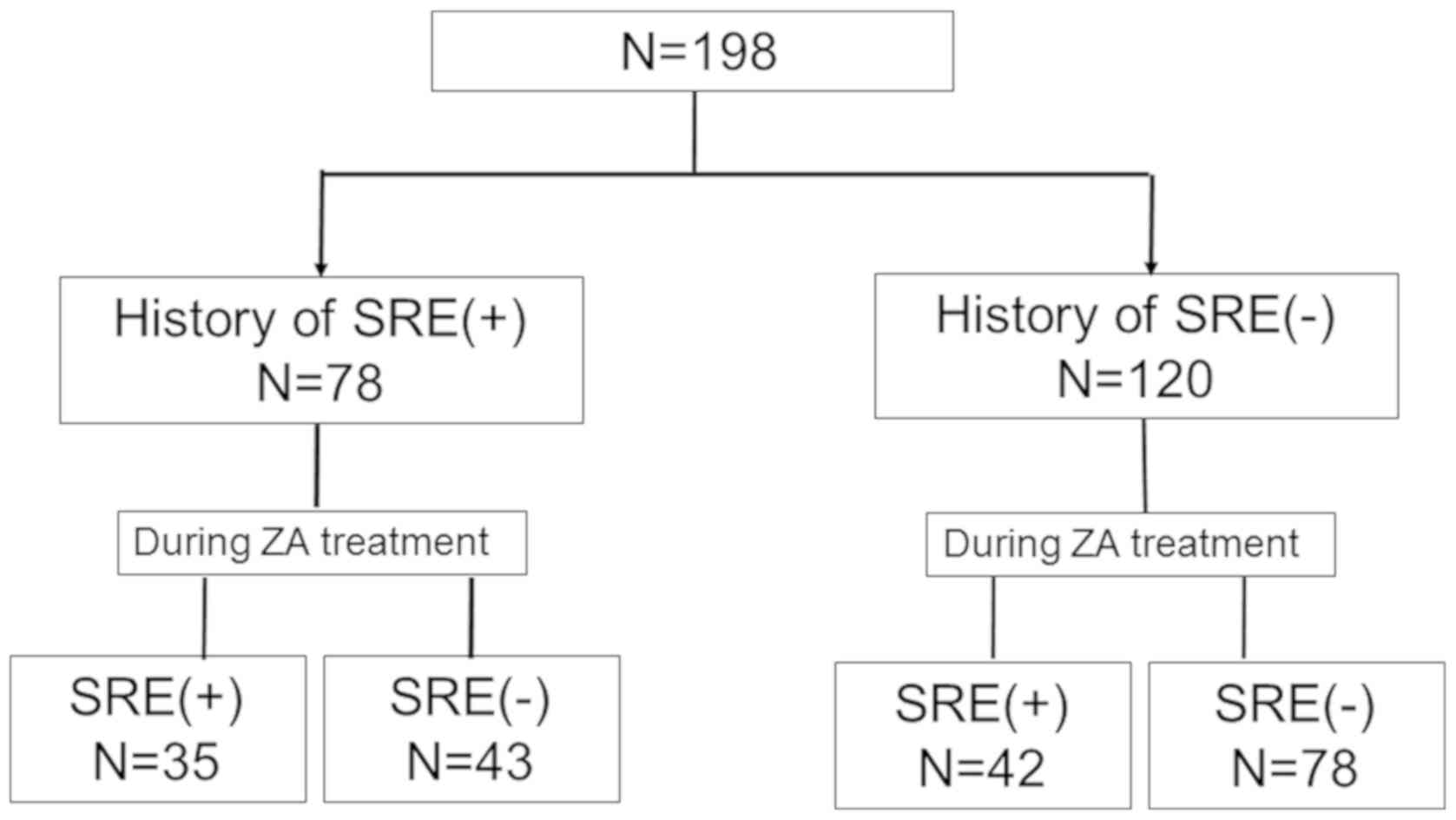
A breakdown of the SREs observed is shown in
Fig. 1. Overall, SREs occurred in 77
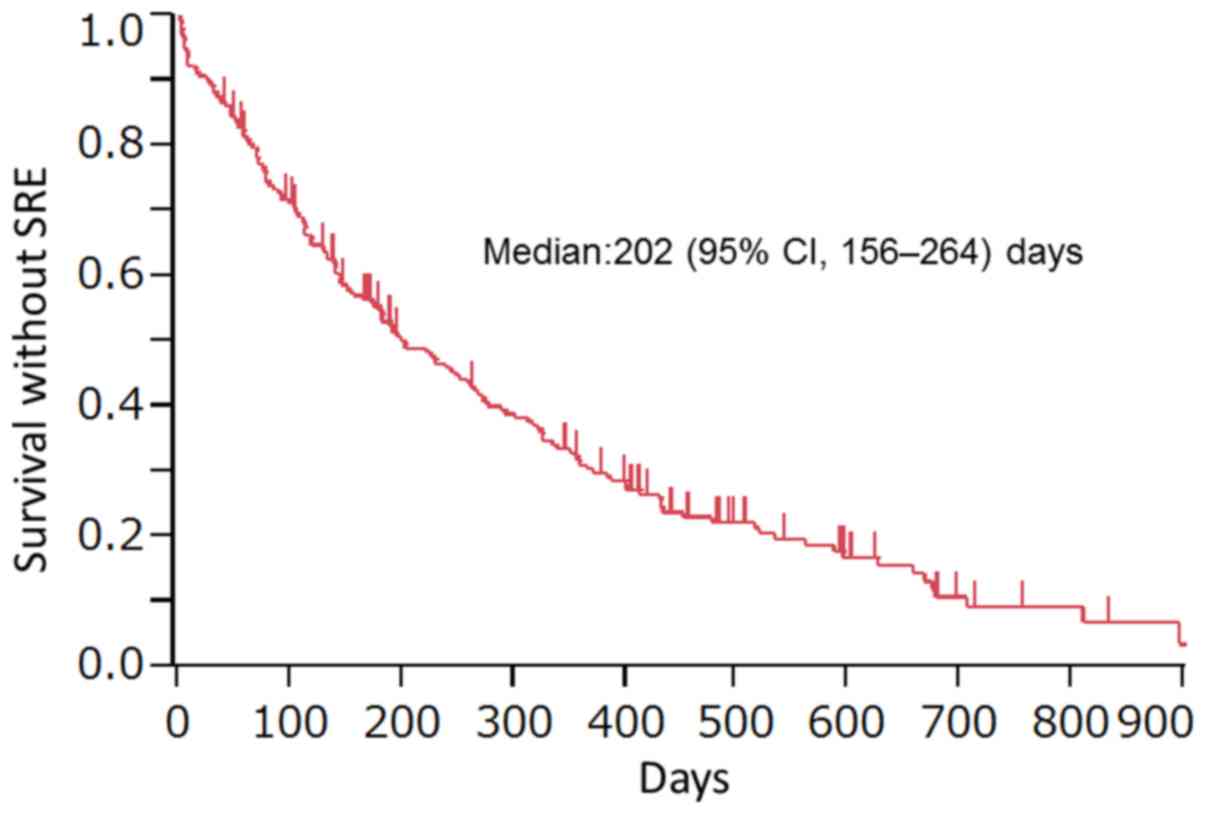
of 198 patients. The period of survival until the occurrence of the
first SRE or death after zoledronic acid therapy (survival without
SREs) was 202 (95% CI: 156–246) days (Fig. 2). In relation to the presence or
absence of a history of SREs prior to zoledronic acid therapy, SREs
reoccurred during zoledronic acid therapy in 35 of 78 patients
(45%) with a history of SREs, whereas SREs occurred in 42 of 120
patients (35%) without a history of SREs (Fig. 1). There was no significant difference
in the rate of SRE occurrence between the two groups (P=0.16). No
ONJ occurred in any of the 198 patients (95% CI: 0–1.9%).
Discussion
This study was a retrospective study in
consecutively enrolled patients with advanced NSCLC with bone
metastases who received zoledronic acid therapy. The median period
of survival until the first SRE or death was 202 days. In a
randomized study conducted by Rosen et al (8), the time to the first SRE was 236 days.
In our study, the number of events was judged to be insufficient
for analysis of the time to the first SRE, and the time to the
first SRE, including death as an event, was therefore examined. A
subgroup analysis of patients with NSCLC from the aforementioned
randomized study showed that the median survival period was 177
days. Our present study showed a longer survival period, but this
may have reflected the characteristic features of lung cancer in
Japanese patients, who are often positive for driver gene mutations
such as EGFR gene mutation. It has also been reported that the risk
of SRE occurrence is decreased by 38% and that the time to the
first SRE is significantly longer in patients on zoledronic acid
therapy (9).
The-above mentioned subgroup analysis of the
randomized controlled study also revealed that the risk of
developing SREs was 1.41-fold higher in patients with a history of
SREs than in those without such a history (10). In the study, the incidence rates of
SREs after zoledronic acid therapy were 42 and 34% in patients with
a history of SREs and in those without such a history. In our
study, there was no significant difference in the incidence rate of
SREs after the initiation of zoledronic acid therapy between
patients who had prior SREs and those who did not. The incidence
rates of SREs after zoledronic acid therapy in our study were
similar to the-above mentioned analysis (10). In other words, our study showed that
zoledronic acid has a certain level of efficacy regardless of the
presence or absence of prior SREs.
In addition, Hirsh et al (10) reported that zoledronic acid is
slightly more effective in patients with a history of SREs. More
specifically, the risk of SREs was decreased by 31%, and the time
to the first SRE was increased by 4 months in patients with a
history of SREs, whereas the risk of SREs was decreased by 23% and
the time to the first SRE was increased by 2.5 months in those
without a history of SREs. We did not examine this issue because no
randomization was used in this study (10).
In this study, ONJ was not found in any patient. ONJ
associated with the administration of BMAs is defined as follows:
The presence of bone fistula occurring from inside or outside the
oral cavity or exposed bone persisting for at least 8 weeks in the
mouth, jaw, or face area in patients who were on current or prior
bone-modifying agent (BMA) therapy and who have no history of
radiation therapy or evident metastatic lesions in the jawbone.
Although rare, ONJ is a serious adverse event. It has been reported
that the incidence rate of ONJ associated with the use of
injectable bisphosphonate for bone metastases depends on the type
of bisphosphonate agent, total dose, dosing period, and history of
dental disease (11), and the
incidence rate is particularly high, at 1.3%, when zoledronic acid,
which contains nitrogen, is used (12). Risk factors of ONJ include invasive
dental treatment applied to the bone such as tooth extraction, poor
status of oral hygiene, periodontal disease, periodontal abscess,
and a history of inflammatory disease such as apical periodontitis
(13). It has been reported that
proper management of oral hygiene reduces the risk of ONJ
occurrence during BMA therapy, and it is thus recommended to
routinely perform dental checkups and prophylactic dental
procedures before the initiation of BMA therapy (14). All facilities that participated in
this study were general hospitals that had full-time dentists who
provided appropriate oral care at the time of zoledronic acid
therapy. The degree of intervention by dentists are various between
facilities. However, for example, dentists performed dental checkup
for all patients before the administration of zoledronic acid in
the Department of Thoracic Oncology and Respiratory Medicine,
TokyoMetropolitan Cancer and Infectious disease Center Komagome
Hospital. These interventions by expert dentists might have led to
the lack of occurrence of ONJ among the subjects in this study. In
addition, the longer the duration of the use of injectable
bisphosphonate, the higher the risk of ONJ occurrence; the
incidence rate of ONJ is reportedly 1.5% after 4–12 months of
bisphosphonate therapy, whereas the corresponding rate is 7.7%
after 27–48 months (11). In our
study, the median duration of zoledronic acid therapy was only 106
days, and this may be another reason for why there was no
occurrence of ONJ.
This study has some limitations. First, this was a
retrospective study including a small number of patients. Second,
this study did not use a randomized design in relation to the
presence or absence of zoledronic acid therapy. Third, the duration
of zoledronic acid therapy was short for the clinical situation of
Japanese patients, in whom the involvement of driver mutations is
frequent. Fourth, this study did not include the data about the
treatment for lung cancer and the lesion and radiologic appearance
of bone metastases.
Despite these limitations, we consider that this
study is valuable, because the clinical course of NSCLC accompanied
by bone metastases and the related occurrence of ONJ have rarely
been reported in a comprehensive manner since BMAs have become
widely used. In the treatment of NSCLC, many novel drugs, including
new molecular-targeted drugs, immune checkpoint inhibitors, and
angiogenesis inhibitors, are currently available, achieving marked
prolongation of survival. However, the development of new drugs for
bone metastases has been limited, and it is expected that
zoledronic acid will continue to play an important role in the
treatment of bone metastases. In this regard, further accumulation
of data on the long-term prognosis and incidence rates of ONJ and
other late complications of zoledronic acid therapy seems to be
particularly important.
Our study showed that zoledronic acid has a certain
level of efficacy regardless of the presence or absence of prior
SREs. However, the duration of zoledronic acid therapy was short in
this study, further accumulation of data on the long-term prognosis
and incidence rates of ONJ and other late complications of
zoledronic acid therapy seems to be particularly important.
Acknowledgements
The authors would like to thank Ms Yumiko Tanabe and
Mr. Hiroyuki Kashiro (Thoracic Oncology Research Group, Yokohama,
Japan) for assisting with data management during this study.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
YH and MS conceived and designed the study. YN, YH,
HM, MK, KN, KS, NN, SN, MN, KM, YT, NS, KY, TS and HO acquired the
data. YN and YH analyzed the data and wrote the manuscript. All
authors gave approval of the final version of the manuscript.
Ethics approval and consent to
participate
The protocol was approved by the Clinical Trial
Review Committee of the Thoracic Oncology Research Group (TORG) and
the Institutional Review Board of TokyoMetropolitan Cancer and
Infectious disease Center Komagome Hospital (September 13, 2010;
approval no. 863) and by each participating institution. Due to the
retrospective nature of the study, the requirement for written
informed consent was waived.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
BMA
|
bone-modifying agent
|
|
BMAs
|
bone-modifying agents
|
|
IGF
|
insulin-like growth factor
|
|
JSMO
|
Japanese Society of Medical
Oncology
|
|
NSCLC
|
non-small-cell lung cancer
|
|
ONJ
|
osteonecrosis of the jaw
|
|
PS
|
performance status
|
|
RANKL
|
receptor activator of nuclear factor
κB ligand
|
|
SRE
|
skeletal-related event
|
|
SREs
|
skeletal-related events
|
|
TGF-β
|
transforming growth factor-β
|
|
ZA
|
zoledronic acid
|
References
|
1
|
Katakami N, Kunikane H, Takeda K, Takayama
K, Sawa T, Saito H, Harada M, Yokota S, Ando K, Saito Y, et al:
Prospective study on the incidence of bone metastasis (BM) and
skeletal-related events (SREs) in patients (pts) with stage IIIB
and IV lung cancer-CSP-HOR 13. J Thorac Oncol. 9:231–238. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Tsuya A, Kurata T, Tamura K and Fukuoka M:
Skeletal metastases in non-small cell lung cancer: A retrospective
study. Lung Cancer. 57:229–232. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Oster G, Lamerato L, Glass AG, Richert-Boe
KE, Lopez A, Chung K, Richhariya A, Dodge T, Wolff GG, Balakumaran
A and Edelsberg J: Natural history of skeletal-related events in
patients with breast, lung, or prostate cancer and metastases to
bone: A 15-year study in two large US health systems. Support Care
Cancer. 21:3279–3286. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Yoneda T and Hiraga T: Crosstalk between
cancer cells and bone microenvironment in bone metastasis. Biochem
Biophys Res Commun. 328:679–687. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Saylor PJ and Smith MR: Bisphosphonates
and other bone-targeted therapies. Cancer Chemotherapy and
Biotherapy: Principles and Practice. 5th. Chabner BA and Longo DL:
Wolters Kluwer Lippincott Williams & Wilkins; Philadelphia: pp.
732–745. 2011
|
|
6
|
Roelofs AJ, Thompson K, Gordon S and
Rogers MJ: Molecular mechanisms of action of bisphosphonates:
Current status. Clin Cancer Res. 12:6222s–6230s. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Rosen LS, Gordon D, Tchekmedyian S,
Yanagihara R, Hirsh V, Krzakowski M, Pawlicki M, de Souza P, Zheng
M, Urbanowitz G, et al: Zoledronic acid versus placebo in the
treatment of skeletal metastases in patients with lung cancer and
other solid tumors: A phase III, double-blind, randomized trial-the
Zoledronic acid lung cancer and other solid tumors study group. J
Clin Oncol. 21:3150–3157. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Rosen LS, Gordon D, Tchekmedyian NS,
Yanagihara R, Hirsh V, Krzakowski M, Pawlicki M, De Souza P, Zheng
M, Urbanowitz G, et al: Long-term efficacy and safety of zoledronic
acid in the treatment of skeletal metastases in patients with
nonsmall cell lung carcinoma and other solid tumors: A randomized,
Phase III, double-blind, placebo-controlled trial. Cancer.
100:2613–2621. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Hirsh V, Major PP, Lipton A, Cook RJ,
Langer CJ, Smith MR, Brown JE and Coleman RE: Zoledronic acid and
survival in patients with metastatic bone disease from lung cancer
and elevated markers of osteoclast activity. J Thorac Oncol.
3:228–236. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Hirsh V, Tchekmedyian NS, Rosen LS, Zheng
M and Hei YJ: Clinical benefit of zoledronic acid in patients with
lung cancer and other solid tumors: Analysis based on history of
skeletal complications. Clin Lung Cancer. 6:170–174. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Bamias A, Kastritis E, Bamia C,
Moulopoulos LA, Melakopoulos I, Bozas G, Koutsoukou V, Gika D,
Anagnostopoulos A, Papadimitriou C, et al: Osteonecrosis of the jaw
in cancer after treatment with bisphosphonates: Incidence and risk
factors. J Clin Oncol. 23:8580–8587. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Khosla S, Burr D, Cauley J, Dempster DW,
Ebeling PR, Felsenberg D, Gagel RF, Gilsanz V, Guise T, Koka S, et
al Bisphosphonate-associated osteonecrosis of the jaw, : Report of
a task force of the American Society for Bone and Mineral Research.
J Bone Miner Res. 22:1479–1491. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Vahtsevanos K, Kyrgidis A, Verrou E,
Katodritou E, Triaridis S, Andreadis CG, Boukovinas I, Koloutsos
GE, Teleioudis Z, Kitikidou K, et al: Longitudinal cohort study of
risk factors in cancer patients of bisphosphonate-related
osteonecrosis of the jaw. J Clin Oncol. 27:5356–5362. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Van Poznak CH, Temin S, Yee GC, Janjan NA,
Barlow WE, Biermann JS, Bosserman LD, Geoghegan C, Hillner BE,
Theriault RL, et al: American Society of Clinical Oncology
executive summary of the clinical practice guideline update on the
role of bone-modifying agents in metastatic breast cancer. J Clin
Oncol. 29:1221–1227. 2011. View Article : Google Scholar : PubMed/NCBI
|