Introduction
Androgen deprivation therapy (ADT) is the standard
treatment for patients with newly diagnosed metastatic prostate
cancer and for those with recurrent prostate cancer after failure
of localized therapy (1). Most of
these patients have initially a good response to ADT, in which a
luteinizing hormone-releasing hormone (LH-RH) analogue is combined
with a nonsteroidal anti-androgen, such as bicalutamide or
flutamide. However, the disease usually progresses to a
castration-resistant state within 12–30 months (2).
No study so far, has demonstrated a definitive
survival benefit (3), but secondary
hormonal therapy, such as anti-androgen withdrawal, alternative
anti-androgens, estrogenic compounds, and adrenolytic agents, has
been used for patients with castration-resistant prostate cancer
(CRPC) prior to docetaxel (4).
However, the recent management of CRPC is sequential therapy using
new hormonal or cytotoxic agents that have a demonstrable survival
advantages in randomized clinical studies (5–11).
Therefore, with these new agents available, secondary hormonal
therapy is no longer recommended (12).
Nevertheless, in clinical practice, secondary
hormonal therapy has proven beneficial for some patients with CRPC.
In particular, alternative anti-androgen therapy (switching to
secondary anti-androgens) (1), is
still widely performed in Asian countries, and some CRPC patients
show a good response to it (13–18).
Furthermore, a previous retrospective study reported that a history
of switching to a second-line anti-androgen therapy did not
influence the overall survival (OS) with the new hormonal agent
abiraterone acetate (4). This may
suggest that the OS of patients with CRPC may be prolonged based on
the response duration of alternative anti-androgen therapy; which
may mean that patients with a long response to this therapy may
have a particular benefit. These findings suggest that alternative
anti-androgens may be beneficial for selected patients with CRPC
prior to the administration of new hormonal agents. In addition,
elderly patients and those with comorbidities are often treated
with alternative anti-androgens instead of new hormonal agents in
the actual clinical setting, and patients also often select
alternative anti-androgens, considering the treatment cost as well
as the efficacy, in developing countries (4).
The objective of this retrospective study was to
re-evaluate alternative anti-androgen therapies for CRPC and to
identify subgroups in whom this therapy may be beneficial.
Patients and methods
Patients
Eighty-eight patients with histologically confirmed
advanced prostate cancer who were treated with alternative
anti-androgen therapy following the failure of initial maximum
androgen blockade (MAB) at Kobe University Hospital in Japan
between January 2012 and September 2017 were included in this
retrospective study. The study design was approved by the Research
Ethics Committee of our institution (no. 180301), and was conducted
in accordance with the Declaration of Helsinki. All patients gave
their informed consent, and patient anonymity was preserved.
Evaluation of prostate cancer
The pathological findings were determined by
systematic ultrasonography-guided needle biopsy, and the Gleason
score was calculated according to the 2005 ISUP classification
(19). The disease was clinically
staged according to the 2010 TNM classification using magnetic
resonance imaging, computed tomography, and bone scintigraphy.
Diseases progression was defined as a ≥25% increase and an absolute
increase of ≥2 ng/ml from the nadir in the serum prostate-specific
antigen (PSA) value despite effective suppression of serum
testosterone, according to the Prostate Cancer Working Group 2
criteria (20). The PSA decline was
evaluated at ≥4 weeks after the initiation of treatment. The serum
PSA level was examined at least once every 12 weeks using a
chemiluminescent enzyme immunoassay.
Treatment
All patients were treated with medical castration
using a LH-RH analogue, Leuprorelin Acetate (3.75 mg/4 weeks;
Takeda Pharmaceutical Company, Ltd.) or Goserelin acetate (3.6 mg/4
weeks; AstraZeneca), plus bicalutamide (80 mg/day) in the initial
MAB (18). If failure of the initial
MAB was confirmed based on the definition or criteria shown above,
patients were diagnosed with CRPC, and bicalutamide was
discontinued. Of the 88 patients, 68 (77.3%) patients were
evaluated for the presence of anti-androgen withdrawal syndrome
(AWS), and alternative anti-androgen therapy, using a LH-RH
analogue plus flutamide (375 mg/day), was then started (18). The remaining 20 (22.7%) patients were
administered alternative anti-androgen therapy, as mentioned above
without being evaluated for AWS.
In the clinical strategy used since January 2016,
alternative anti-androgens were used only by the patients, who may
not immediately have to be treated with docetaxel: Those with a
≥12-month response to initial MAB, who are asymptomatic or have
minimally symptomatic disease or have no visceral disease (21). However, there were no clear
indications for this treatment prior to January 2016.
Statistical analysis
The PSA decline, PSA progression-free survival
(PSA-PFS) and OS of alternative anti-androgen therapy were
investigated in the present study. To determine the factors for
predicting the PSA decline, Student's t-test and Fisher's exact
test were performed in Table II.
For multiple comparisons in Table
III, Fisher's exact test followed by Bonferroni post hoc test
was used. The PSA-PFS and OS were estimated using the Kaplan-Meier
method, and several potential factors, including age, initial PSA
level, TNM classification, history of radical treatment and
response to initial MAB, for predicting a longer PSA-PFS with
alternative anti-androgen therapy were assessed using the Cox
proportional hazards model.
 | Table II.Association between several
parameters and for PSA decline of alternative anti-androgen
therapy. |
Table II.
Association between several
parameters and for PSA decline of alternative anti-androgen
therapy.
|
| Any PSA
decline | ≥50% PSA
decline |
|---|
|
|
|
|
|---|
| N=88 | Yes (n=61) | No (n=27) | P-value | Yes (n=45) | No (n=43) | P-value |
|---|
| Mean age (range),
years | 74.5 (52–88) | 74.1 (56–90) | 0.844 | 73.5 (52–88) | 75.3 (56–90) | 0.311 |
| Mean PSA at the
induction of | 950.3 | 1,430.9 | 0.548 | 1,252.0 | 936.3 | 0.669 |
| initial MAB
(range), ng/ml | (0.218–22,412) | (0.718–21,439) |
| (0.218–22,412) | (0.438–21,439) |
|
| T stage, n (%) |
|
| 0.782 |
|
| 0.607 |
|
≤T3 | 47 (77.0) | 22 (81.5) |
| 34 (75.6) | 35 (81.4) |
|
|
≥T4 | 14 (23.0) | 5 (18.5) |
| 11 (24.4) | 8 (18.6) |
|
| Gleason score, n
(%) |
|
| 0.568 |
|
| 0.793 |
| ≤7 | 14 (23.0) | 4 (14.8) |
| 10 (22.2) | 8 (18.6) |
|
| ≥8 | 47 (77.0) | 23 (85.2) |
| 35 (77.8) | 35 (81.4) |
|
| Lymph node
metastasis, n (%) |
|
| 0.648 |
|
| 0.677 |
| No | 30 (49.2) | 15 (55.6) |
| 22 (48.9) | 23 (53.5) |
|
|
Yes | 31 (50.8) | 12 (44.4) |
| 23 (51.1) | 20 (46.5) |
|
| Bone metastasis, n
(%) |
|
| 0.648 |
|
| 0.649 |
| No | 20 (32.8) | 8 (29.6) |
| 13 (28.9) | 15 (34.9) |
|
|
Yes | 41 (67.2) | 19 (70.4) |
| 32 (71.1) | 28 (65.1) |
|
| Lesions of bone
metastasis, n (%) |
|
| 0.106 |
|
| 0.831 |
| ≤2 | 35 (57.4) | 10 (37.0) |
| 24 (53.3) | 21 (48.8) |
|
| ≥3 | 26 (42.6) | 17 (63.0) |
| 21 (46.7) | 22 (51.2) |
|
| Visceral
metastasis, n (%) |
|
| 0.431 |
|
| 1.000 |
| No | 55 (90.2) | 26 (96.3) |
| 41 (91.1) | 40 (93.0) |
|
|
Yes | 6 (9.8) | 1 (3.7) |
| 4 (8.9) | 3 (7.0) |
|
| Prior prostatectomy
or primary radiation, n (%) |
|
| 0.747 |
|
| 0.770 |
| No | 51 (83.6) | 24 (88.9) |
| 39 (86.7) | 36 (83.7) |
|
|
Yes | 10 (16.4) | 3 (11.1) |
| 6 (13.3) | 7 (16.3) |
|
| Mean duration of
initial MAB (range), months | 27.9
(3.3–128.7) | 22.4
(2.3–99.7) | 0.360 | 25.5
(3.3–128.7) | 26.9
(2.3–106.9) | 0.799 |
| Anti-androgen
withdrawal, n (%) |
|
| 0.782 |
|
| 0.607 |
| No or
not assessed | 47 (77.0) | 22 (81.5) |
| 34 (75.6) | 35 (81.4) |
|
|
Yes | 14 (23.0) | 5 (18.5) |
| 11 (24.4) | 8 (18.6) |
|
 | Table III.Univariate and multivariate analyses
of several parameters for predicting PSA-PFS. |
Table III.
Univariate and multivariate analyses
of several parameters for predicting PSA-PFS.
|
| Univariate
analysis | Multivariate
analysis |
|---|
|
|
|
|
|---|
| N=88 | HR (95% CI) | P-value | HR (95% CI) | P-value |
|---|
| Age (<75 vs.
≥75), years | 1.19
(0.72–1.92) | 0.493 | – | – |
| PSA at the
induction of initial MAB, (≥100 vs. <100), ng/ml | 1.08
(0.66–1.76) | 0.765 | – | – |
| T stage (≥T4 vs.
≤T3) | 1.23
(0.69–2.21) | 0.480 | – | – |
| Gleason score (≥8
vs. ≤7) | 1.95
(1.01–3.75) | 0.047 | 1.81
(0.89–3.67) | 0.104 |
| Lymph node
metastasis (yes vs. no) | 1.25
(0.76–2.04) | 0.375 | – | – |
| Bone metastasis
(yes vs. no) | 1.97
(1.11–3.48) | 0.020 | 1.03
(0.46–2.33) | 0.945 |
| Lesions of bone
metastasis (≥3 vs. ≤2) | 2.53
(1.52–4.20) | <0.001 | 2.11
(1.23–3.62) | 0.007 |
| Visceral metastasis
(yes or no) | 1.08
(0.43–2.70) | 0.877 | – | – |
| Prior prostatectomy
or primary radiation (no or yes) | 2.08
(1.00–4.35) | 0.051 | – | – |
| Duration of initial
MAB (<12 vs. ≥12), months | 2.63
(1.56–4.35) | <0.001 | 2.08
(1.20–3.57) | 0.008 |
| Anti-androgen
withdrawal (no vs. yes) | 1.59
(0.85–2.94) | 0.141 | – | – |
For all statistical analyses, EZR was used
(http://www.jichi.ac.jp/saitama-sct/SaitamaHP.files/statmedEN.html;
Saitama Medical Center, Jichi Medical University), which is a
graphical user interface for R (22). It is a modified version of R
commander designed to add statistical functions frequently used in
biostatistics. Each test was 2-sided and P<0.05 was considered
to indicate a statistically significant difference.
Results
Patients characteristics
The characteristics of the 88 patients are shown in
Table I. The median PSA value at the
induction of initial MAB was 96.1 ng/ml (range, 0.218–22,412).
Thirty-two (36.3%) patients showed clinical T4 stage, and a ≥8
Gleason score was found in 70 (79.6%) patients. Prior prostatectomy
and primary radiation therapy were performed in 9 (10.2%) and 4
(4.5%) patients, respectively. The median duration of the initial
MAB was 15.7 months (range, 2.3–128.7), and the initial MAB was
effective for over 12 months in 60 (68.2%) patients. In addition,
no one showed any adverse events related to flutamide.
 | Table I.Patient characteristics. |
Table I.
Patient characteristics.
| Period of
observation, median (range), months | 24.7 (0.4–122.3) |
|---|
| Age, median (range),
years | 74 (52–90) |
| PSA at the induction
of initial MAB, median (range), ng/ml | 96.1
(0.218–22412) |
| T stage, n (%) |
|
| ≤T2 | 13 (14.8) |
| T3 | 43 (48.9) |
| T4 | 32 (36.3) |
| Gleason score, n
(%) |
|
| ≤7 | 18 (20.4) |
| 8 | 38 (43.2) |
| 9 | 27 (30.7) |
| 10 | 5 (5.7) |
| Site of disease, n
(%) |
|
| None | 16 (18.1) |
| Bone |
|
|
1 or 2
lesions | 18 (20.5) |
|
≥3 lesions | 43 (14.3) |
| Lymph
node | 43 (14.3) |
|
Visceral | 7 (8.0) |
| Prior prostatectomy
or primary radiation, n (%) |
|
|
Prostatectomy | 9 (10.2) |
| Primary
radiation | 4 (4.5) |
| PSA at baseline,
median (range), ng/ml | 3.40 (0.09–955) |
| Duration of initial
MAB, median (range), months | 15.7 (2.3–128.7) |
| Anti-androgen
withdrawal, n (%) |
|
| Yes | 19 (21.6) |
| No | 49 (55.7) |
| Not
assessed | 20 (22.7) |
PSA decline and PSA-PFS of alternative
antiandrogen therapy
Firstly, the PSA decline of an alternative
anti-androgen therapy was evaluated. The waterfall plots of the PSA
decline showed that 61 (69.3%) patients had some amount of decline,
and 45 (51.1%) had ≥50% PSA decline (Fig. 1A). The associations between several
potential risk factors and the PSA decline was investigated next,
but no significant factor for predicting any amount of PSA decline
or ≥50% PSA decline was identified (Table II).
Next, the PSA-PFS of the alternative anti-androgen
therapy was analyzed. As shown in Fig.
1B, the median PSA-PFS was 7.5 months [95% confidence interval
(CI), 5.7–10.3]. Notably, alternative anti-androgen therapy was
effective for over 2 years in 15 (17.0%) patients. A multivariate
analysis showed that ≥3 bone metastatic lesions and a duration
<12 months of initial MAB were significant factors for
predicting a shorter PSA-PFS, with hazard ratios of 2.11 (95% CI,
1.23–3.62; P=0.007) and 2.08 (95% CI, 1.20–3.57; P=0.008; Table III), respectively. PSA-PFS curves
based on these two factors are shown in Fig. 2A and B. The median PFS of patients
with ≤2 and ≥3 bone metastatic lesions was 17.9 months and 5.1
months (Fig. 2A), and that with a
duration <12 months and ≥12 months of initial MAB was 4.3 months
and 11.3 months, respectively (Fig.
2B). The association between the PSA-PFS and the number of each
of these two risk factors (≥3 bone metastatic lesions and a
duration <12 months of initial MAB) was further investigated.
The number of the above risk factors had no impact on the PSA
decline (Fig. 3A), but the PSA-PFS
of patients without these factors was markedly longer than that of
patients with 1 or 2 factors (Fig.
3B, the median PSA-PFS was 22.8 months vs. 6.7 months or 3.5
months, respectively; P<0.001), which suggests that these
factors had an additive impact on PSA-PFS.
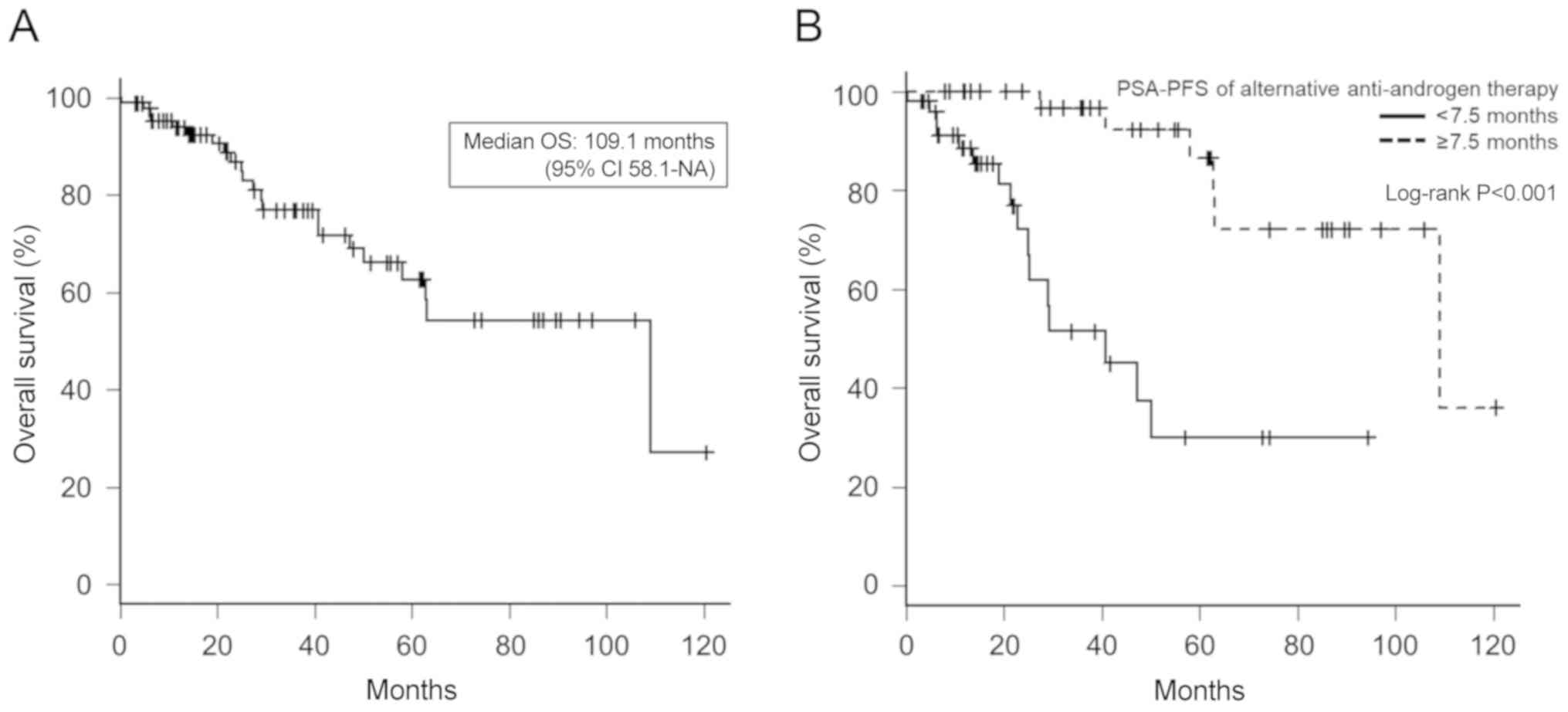
OS of alternative antiandrogen
therapy
As shown in Fig. 4,
the median OS was 109.1 months (95% CI, 58.1-NA), and the OS of
patients with ≥7.5 months of PSA-PFS of alternative anti-androgen
therapy was significantly longer than that of patients with <7.5
months of therapy (Fig. 4B; median
OS, 109.1 months vs. 40.8 months, respectively; P<0.001).
Discussion
Alternative anti-androgens are widely used as
secondary hormonal therapy to treat CRPC, but none have
demonstrated a survival advantage in previous studies (3). In Asian countries, including Japan,
several retrospective studies have shown that alternative
anti-androgens were effective for some patients (13–18), and
hormonal therapy may be a viable treatment, especially for Japanese
patients, not only with respect to effectiveness on cancer control
but adverse events as well (23,24).
Furthermore, alternative anti-androgen has shown significant
efficacy in certain clinical settings. Therefore, alternative
anti-androgens may be beneficial for selected patients with CRPC,
especially those in Asian countries, including Japan, prior to
treatment with docetaxel.
Several reports have evaluated the effectiveness of
alternative anti-androgens. Yokomizo et al (14) found a PSA decrease ≥50% in 40% of
patients who immediately switched from bicalutamide to flutamide as
a second-line MAB, and Okihara et al (25) reported that 38% of patients achieved
a ≥50% PSA decline on changing from bicalutamide to flutamide, with
a median PSA-PFS of 5.1 months. Yasui et al (15) showed that the median duration of
PSA-PFS was 4.6 months in patients with metastatic CRPC treated
with alternative anti-androgens. In the present study, it was found
that 45 of 88 (51.1%) patients had a ≥50% PSA decline, and the
median duration of PSA-PFS was 7.5 months, which was relatively
better than that in the aforementioned studies. This is probably
due to the present treatment strategy, as it was decided to use
this therapy only for patients who may not immediately have to be
treated with docetaxel (such as those with a ≥12-month response to
initial MAB, who are asymptomatic or have minimally symptomatic
disease or have no visceral disease), as mentioned in a previous
report (21). Thus, this favorable
result may reflect our selective use of alternative
anti-androgens.
However, despite these findings, the PSA response to
alternative anti-androgens of the present study was inferior to
that of enzalutamide and abiraterone acetate shown in a randomized
clinical study. In the PREVAIL study, 78% of patients treated with
enzalutamide had a ≥50% PSA decline, and the median PSA-PFS was
11.2 months (10). In the COU-AA-302
trial, the PSA decreased ≥50% in 62% of patients treated with
abiraterone acetate plus prednisone, and the median PSA-PFS was
11.1 months (26). However, while
previous reviews have indicated that switching to docetaxel
immediately after the failure of initial ADT is preferred for
patients who are suspected of having androgen receptor
axis-targeted treatment resistance (21,27),
whether or not new hormonal agents, such as enzalutamide or
abiraterone acetate, should be considered immediately over
alternative anti-androgen therapy following prior ADT remains
unclear. Randomized controlled trials comparing the efficacy of
flutamide and enzalutamide (Trial registration: ClinicalTrials.gov no. NCT02918968) or abiraterone
acetate (Trial registration: UMIN-CTR no. UMIN000016617) are
currently being carried out, and the results of these trials may
resolve this issue.
For predictive factors of second-line
anti-androgens, CRPC patients with a better clinical response, such
as a better PSA response, longer duration of response and shorter
time to PSA nadir, in the first MAB or without severe metastasis
offered a better PSA response to alternative anti-androgen therapy
(13–15,18). In
the present study, it was shown that patients with ≤2 bone
metastatic lesions and a duration ≥12 months of initial MAB had a
median PSA-PFS of 22.8 months. These findings suggest that the
effectiveness of the initial MAB and the severity of metastasis may
strongly influence the therapeutic efficacy of the subsequent
alternative anti-androgen therapy, and these results may be useful
for determining the selective introduction of alternative
anti-androgens. In addition, it was also shown that patients with a
longer PSA-PFS with alternative anti-androgens had a prolonged OS,
suggesting that alternative anti-androgen therapy may be beneficial
for these selected patients.
One concern is whether or not alternative
anti-androgen therapy will affect the therapeutic response of newly
developed hormonal agents. Zhao et al (4) reported that switching to flutamide did
not influence the OS of abiraterone acetate in patients with
metastatic CRPC, which suggests that the OS of patients with CRPC
may be prolonged based on the response duration of alternative
anti-androgen therapy. Nakai et al (28) showed that patients for whom
second-line flutamide was effective had a better response to
subsequent abiraterone acetate than those for who the treatment
proved to be ineffective. In a preclinical setting, Prekovic et
al (29) showed that the
combination of the androgen receptor (AR) F877L mutation, which
leads to enzalutamide resistance, and the AR T878A mutation, which
is often found in flutamide-treated patients, causes enzalutamide
to have strong agonistic activity, but that only the F877L mutation
has a weak effect on enzalutamide binding, which suggests that the
prior administration of flutamide may enhance enzalutamide
resistance. These findings indicate that the influence of
alternative anti-androgen therapy on new hormonal agents is still
controversial.
Even though no study has demonstrated a definitive
survival benefit of alternative anti-androgen therapy, the present
data suggest that there may be some subgroup for which alternative
anti-androgens may be beneficial, even in this era of new agents.
However, the present study had several limitations. Because the
present study was a retrospective and single-arm study, caution
should be given when interpreting the present results, considering
the possibility that the new hormonal agents may be much more
beneficial than alternative anti-androgens if they are used for
CRPC patients with a longer duration of initial MAB response and
the absence of severe metastasis. Therefore, further investigation,
such as the randomized controlled trials that are currently being
carried out, are necessary in order to decide whether or not to
include alternative anti-androgen therapy in the treatment strategy
of CRPC. In addition, not all patients were assessed for AWS, and
some received localized therapy. Furthermore, disease progression
was defined as an increased PSA level, but the metastasis-free
survival and radiographic progression, which is the standard
endpoint for new agents, were not assessed. Finally, because new
hormonal and cytotoxic agents became available during the study
period, the subsequent therapies applied after alternative
anti-androgens varied. Therefore, the clinical outcome of
subsequent therapies could not be evaluated in this retrospective
analysis.
In conclusion, it was found that a longer duration
of initial MAB and a lack of severe bone metastasis may predict a
favorable response to alternative anti-androgens in CRPC patients.
Furthermore, those with a favorable response to alternative
anti-androgens had a longer OS than those with a poor response.
Alternative anti-androgen therapy may still be beneficial for
selected patients, especially in Asian countries, including Japan,
and may be a viable choice for treating such patients, even in the
era of novel agents.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and analyzed in the present study
are available from corresponding author on reasonable request.
Authors' contributions
KSu, TT, NH, YN and MF designed the study. KSu, TT,
KSh, JF, KH acquired and analyzed the data. KSu, TT and KSh drafted
the manuscript, and KH, JF, NH, YN and MF revised it critically for
important intellectual content. All authors gave final approval of
the version to be published.
Ethics approval and consent to
participate
The study design was approved by the Research Ethics
Committee of Kobe University Hospital (no. 180301), which was
conducted in accordance with the Declaration of Helsinki. All
patients gave informed consent, and patient anonymity was
preserved.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no conflicts of
interest.
References
|
1
|
Lorente D, Mateo J, Zafeiriou Z, Smith AD,
Sandhu S, Ferraldeschi R and de Bono JS: Switching and withdrawing
hormonal agents for castration-resistant prostate cancer. Nat Rev
Urol. 12:37–47. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Laufer M, Denmeade SR, Sinibaldi VJ,
Carducci MA and Eisenberger MA: Complete androgen blockade for
prostate cancer: What went wrong? J Urol. 164:3–9. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Heidenreich A, Bastian PJ, Bellmunt J,
Bolla M, Joniau S, van der Kwast T, Mason M, Matveev V, Wiegel T,
Zattoni F, et al: EAU guidelines on prostate cancer. Part II:
Treatment of advanced, relapsing, and castration-resistant prostate
cancer. Eur Urol. 65:467–479. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Zhao JG, Liu JD, Shen PF, Tang X, Sun GX,
Zhang XM, Chen JR, Shu KP, Shi M and Zeng H: Prior switching to a
second-line nonsteroidal antiandrogen does not impact the
therapeutic efficacy of abiraterone acetate in patients with
metastatic castration-resistant prostate cancer: A real-world
retrospective study. Asian J Androl. 20:545–550. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
de Bono JS, Oudard S, Ozguroglu M, Hansen
S, Machiels JP, Kocak I, Gravis G, Bodrogi I, Mackenzie MJ, Shen L,
et al: Prednisone plus cabazitaxel or mitoxantrone for metastatic
castration-resistant prostate cancer progressing after docetaxel
treatment: A randomised open-label trial. Lancet. 376:1147–1154.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Kantoff PW, Higano CS, Shore ND, Berger
ER, Small EJ, Penson DF, Redfern CH, Ferrari AC, Dreicer R, Sims
RB, et al: Sipuleucel-T immunotherapy for castration-resistant
prostate cancer. N Engl J Med. 363:411–422. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Fizazi K, Scher HI, Molina A, Logothetis
CJ, Chi KN, Jones RJ, Staffurth JN, North S, Vogelzang NJ, Saad F,
et al: Abiraterone acetate for treatment of metastatic
castration-resistant prostate cancer: Final overall survival
analysis of the COU-AA-301 randomised, double-blind,
placebo-controlled phase 3 study. Lancet Oncol. 13:983–992. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Scher HI, Fizazi K, Saad F, Taplin ME,
Sternberg CN, Miller K, de Wit R, Mulders P, Chi KN, Shore ND, et
al: Increased survival with enzalutamide in prostate cancer after
chemotherapy. N Engl J Med. 367:1187–1197. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Parker C, Nilsson S, Heinrich D, Helle SI,
O'Sullivan JM, Fosså SD, Chodacki A, Wiechno P, Logue J, Seke M, et
al: Alpha emitter radium-223 and survival in metastatic prostate
cancer. N Engl J Med. 369:213–223. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Beer TM, Armstrong AJ, Rathkopf DE, Loriot
Y, Sternberg CN, Higano CS, Iversen P, Bhattacharya S, Carles J,
Chowdhury S, et al: Enzalutamide in metastatic prostate cancer
before chemotherapy. N Engl J Med. 371:424–433. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Ryan CJ, Smith MR, Fizazi K, Saad F,
Mulders PF, Sternberg CN, Miller K, Logothetis CJ, Shore ND, Small
EJ, et al: Abiraterone acetate plus prednisone versus placebo plus
prednisone in chemotherapy-naive men with metastatic
castration-resistant prostate cancer (COU-AA-302): Final overall
survival analysis of a randomised, double-blind, placebo-controlled
phase 3 study. Lancet Oncol. 16:152–160. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Cornford P, Bellmunt J, Bolla M, Briers E,
De Santis M, Gross T, Henry AM, Joniau S, Lam TB, Mason MD, et al:
EAU-ESTRO-SIOG guidelines on prostate cancer. Part II: Treatment of
relapsing, metastatic, and castration-resistant prostate cancer.
Eur Urol. 71:630–642. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Momozono H, Miyake H, Tei H, Harada KI and
Fujisawa M: Clinical outcomes of anti-androgen withdrawal and
subsequent alternative anti-androgen therapy for advanced prostate
cancer following failure of initial maximum androgen blockade. Mol
Clin Oncol. 4:839–844. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Yokomizo Y, Kawahara T, Miyoshi Y, Otani
M, Yamanaka S, Teranishi J, Noguchi K, Yao M and Uemura H: Efficacy
of immediate switching from bicalutamide to flutamide as
second-line combined androgen blockade. Biomed Res Int.
2016:40831832016. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Yasui M, Uemura K, Yoneyama S, Kawahara T,
Hattori Y, Teranishi JI, Inoue M, Ohta JI, Yokomizo Y, Yao M, et
al: Predictors of poor response to secondary alternative
antiandrogen therapy with flutamide in metastatic
castration-resistant prostate cancer. Jpn J Clin Oncol. 2016.(Epub
ahead of print). View Article : Google Scholar
|
|
16
|
Choi JI, Kim YB, Yang SO, Lee JK and Jung
TY: Efficacy of alternative antiandrogen therapy for prostate
cancer that relapsed after initial maximum androgen blockade.
Korean J Urol. 52:461–465. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Okegawa T, Nutahara K and Higashihara E:
Alternative antiandrogen therapy in patients with
castration-resistant prostate cancer: A single-center experience.
Int J Urol. 17:950–955. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Suzuki H, Okihara K, Miyake H, Fujisawa M,
Miyoshi S, Matsumoto T, Fujii M, Takihana Y, Usui T, Matsuda T, et
al: Alternative nonsteroidal antiandrogen therapy for advanced
prostate cancer that relapsed after initial maximum androgen
blockade. J Urol. 180:921–927. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Epstein JI, Allsbrook WC Jr, Amin MB and
Egevad LL; ISUP Grading Committee, : The 2005 international society
of urological pathology (ISUP) consensus conference on gleason
grading of prostatic carcinoma. Am J Surg Pathol. 29:1228–1242.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Scher HI, Halabi S, Tannock I, Morris M,
Sternberg CN, Carducci MA, Eisenberger MA, Higano C, Bubley GJ,
Dreicer R, et al: Design and end points of clinical trials for
patients with progressive prostate cancer and castrate levels of
testosterone: Recommendations of the prostate cancer clinical
trials working group. J Clin Oncol. 26:1148–1159. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Chi K, Hotte SJ, Joshua AM, North S, Wyatt
AW, Collins LL and Saad F: Treatment of mCRPC in the
AR-axis-targeted therapy-resistant state. Ann Oncol. 26:2044–2056.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Kanda Y: Investigation of the freely
available easy-to-use software ‘EZR’ for medical statistics. Bone
Marrow Tranfsplant. 48:452–458. 2013. View Article : Google Scholar
|
|
23
|
Fukagai T, Namiki TS, Carlile RG, Yoshida
H and Namiki M: Comparison of the clinical outcome after hormonal
therapy for prostate cancer between Japanese and Caucasian men. BJU
Int. 97:1190–1193. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Namiki M, Ueno S, Kitagawa Y, Fukagai T
and Akaza H: Effectiveness and adverse effects of hormonal therapy
for prostate cancer: Japanese experience and perspective. Asian J
Androl. 14:451–457. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Okihara K, Ukimura O, Kanemitsu N,
Mizutani Y, Kawauchi A and Miki T; Kyoto Prefectural University of
Medicine Prostate Cancer Research Group, : Clinical efficacy of
alternative antiandrogen therapy in Japanese men with relapsed
prostate cancer after first-line hormonal therapy. Int J Urol.
14:128–132. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Ryan CJ, Smith MR, de Bono JS, Molina A,
Logothetis CJ, de Souza P, Fizazi K, Mainwaring P, Piulats JM, Ng
S, et al: Abiraterone in metastatic prostate cancer without
previous chemotherapy. N Engl J Med. 368:138–148. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Fitzpatrick JM, Bellmunt J, Fizazi K,
Heidenreich A, Sternberg CN, Tombal B, Alcaraz A, Bahl A, Bracarda
S, Di Lorenzo G, et al: Optimal management of metastatic
castration-resistant prostate cancer: Highlights from a European
expert consensus panel. Eur J Cancer. 50:1617–1627. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Nakai Y, Tanaka N, Miyake M, Inoue T, Anai
S and Fujimoto K: Response to flutamide, as second-line therapy
after bicalutamide, predicts efficacy of abiraterone, not that of
enzalutamide. BMC Res Notes. 11:3422018. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Prekovic S, van Royen ME, Voet AR, Geverts
B, Houtman R, Melchers D, Zhang KY, Van den Broeck T, Smeets E,
Spans L, et al: The effect of F877L and T878A mutations on androgen
receptor response to enzalutamide. Mol Cancer Ther. 15:1702–1712.
2016. View Article : Google Scholar : PubMed/NCBI
|