Introduction
Pressurized intraperitoneal aerosol chemotherapy
(PIPAC) has been suggested as an innovative approach for the
application of intraperitoneal chemotherapy (IPC) (1). The drug-containing solution is
delivered into the abdominal cavity as microparticles using an
aerosol-producing device (2,3). Penetration depth of PIPAC has been
observed to be superior to liquid applications and research on this
field is ongoing (4–6). New combinations of drugs and carrier
molecules are currently being tested for intraperitoneal delivery
and especially for PIPAC applications (7).
Previous studies have been conducted to evaluate the
effects of new substances and other strategies to apply PIPAC with
the aim to enhance drug availability (8–10).
Previous studies focusing on the use of coated particles, such as
liposomal doxorubicin (LD) for IPC, have shown promising results
(11,12). However, previous studies on LD have
indicated that the pharmacology of liposomal particles is quite
complex, since, in contrast to regular doxorubicin, LD particles do
not release their drug content easily at the target site (7,13). A
recent study has investigated the effect of LD on the peritoneal
surface, describing that particles on the surface remain stable and
a small amount of doxorubicin is delivered to the local peritoneal
tissue (7). Similar observations
have been documented in the application of liquid IPC where LD is
at least partially detectable in the plasma (12).
However, particles applied locally or intravenously
accumulate in certain organs, including the kidneys and the heart,
causing local toxicity (14,15). These side effects limit the use of LD
for intraperitoneal applications. In some applications, high
intensity ultrasound (HIUS) has demonstrated to facilitate the
release of doxorubicin from LD particles (16). Notably, a clinical trial indicated
promising antitumoral effects (17).
However, it is unclear whether the content of these
liposomal-coated particles can be released on the peritoneal
surface using HIUS. Moreover, if the release of doxorubicin is
increased, it remains unclear whether this is relevant for the drug
absorption rate in the peritoneum. Therefore, HIUS may facilitate
the use of these particles for IPC treatments. The aim of the
present study was to evaluate the effect of HIUS combined with LD
administration on the peritoneal surface compared with LD
administration without HIUS using an established ex vivo
model.
Materials and methods
Ex vivo PIPAC model
Since the experiments were performed in an ex
vivo model using commercially available tissue samples (local
pork supplier, Zerniki Wielkie), no approval from the Local Board
on Animal Care was required. Fresh post mortem swine peritoneum
samples were cut into smaller pieces of 40×40×5 mm.
PIPAC procedure
A commercially available hermetically sealable
plastic box, representing the abdominal cavity, was used. The ex
vivo PIPAC model was established as previously described
(18,19). Fresh tissue samples from swine
peritoneum (size, 40×40×5 mm) were placed at the bottom of the box.
A trocar of 5 mm in diameter (Kii Balloon Blunt Tip System; Applied
Medical Resources Corporation) was placed in the center of the lid.
The nozzle of a microcatheter (MC; Olympus PW-205V; Olympus
Corporation) was introduced into the trocar. The ex vivo
model was kept at a constant room temperature of 27°C during the
entire procedure. The entire procedure lasted 30 min, including the
injection phase and the exposure phase after aerosolization.
The distance between the nozzle of the MC and the
bottom of the plastic box was 10 cm. The plastic box was tightly
sealed and a constant CO2 capnoperitoneum pressure of 12
mmHg (Olympus UHI-3; Olympus Corporation) was maintained during the
entire PIPAC procedure. In total, 3 mg LD (Caelyx®;
Janssen-Cilag Ltd.; Johnson and Johnson; cat. no. BHZ0V00) were
dissolved in 50 ml NaCl (0.9%) at 27°C and administered via MC.
Ultrasound of treated PIPAC
samples
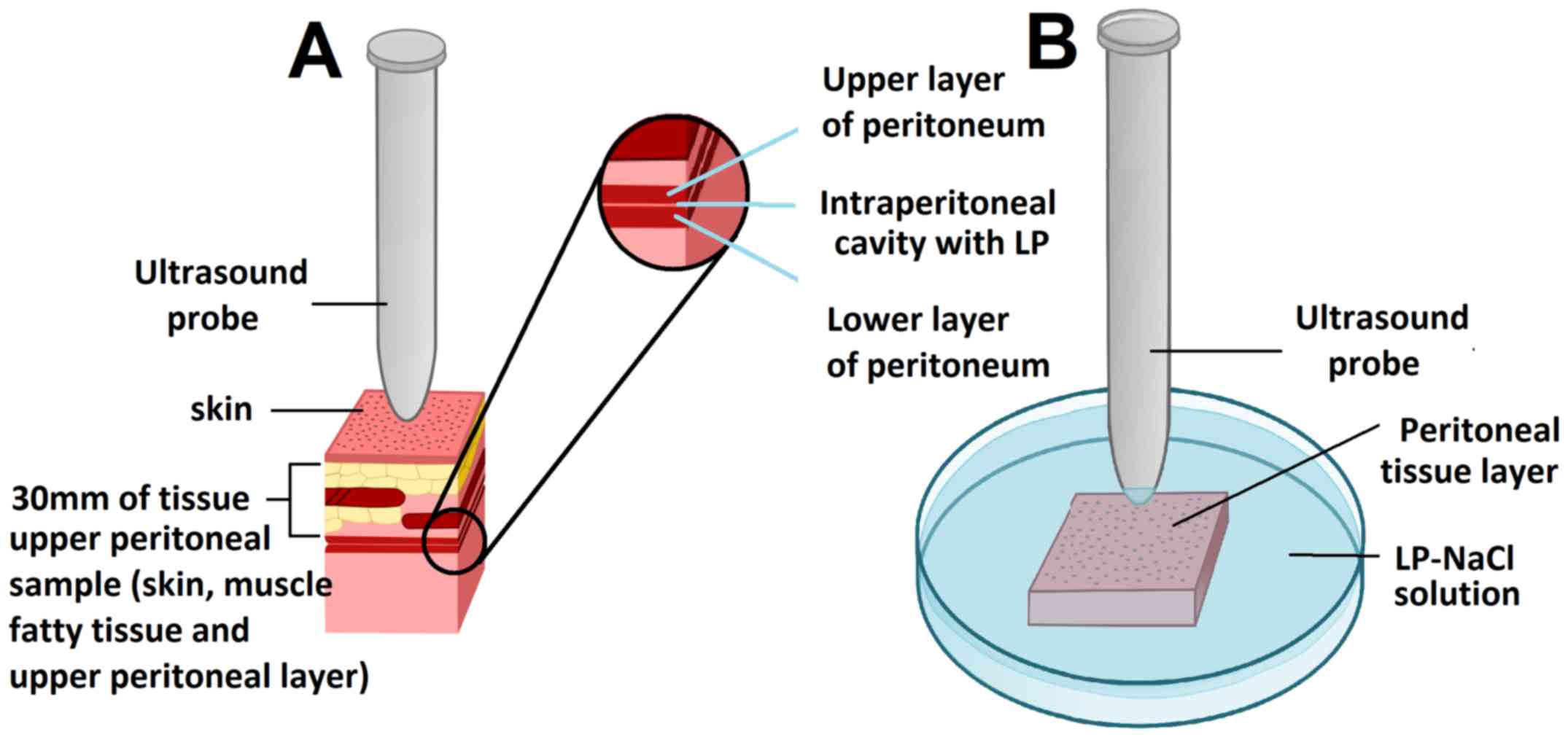
All tissue samples were removed from the ex
vivo model and covered with a 30-mm-thick tissue section of the
abdominal wall containing parietal peritoneum, muscle, adipose
tissue and abdominal skin. The samples for transcutaneous HIUS
consisted of two attached peritoneal tissue samples forming an
intraperitoneal cavity with two opposing peritoneal layers. Half of
the samples were treated with HIUS. During HIUS, the tip of the
probe was in direct contact with the abdominal wall (Fig. 1A). Sonication was applied for 30 sec
at a frequency of 20 kHz, an output power of 70 W and an amplitude
of 50%.
Ultrasound of samples treated by
lavage
Other peritoneal tissue samples were placed in a
Petri dish and covered by a solution containing 3 mg of LD
dissolved in 50 ml NaCl (0.9%). A HIUS probe was placed in the
Petri dish (Fig. 1). The tip of the
probe was positioned at a distance of 3 mm from the surface of the
peritoneum (Fig. 1B). Sonication was
applied for 30 sec at a frequency of 20 kHz, an output power of 70
W and an amplitude of 50%.
Microscopic analysis
After treatments, all tissue samples were rinsed
with sterile NaCl (0.9%) solution in order to eliminate superficial
drug particles and immediately frozen in liquid nitrogen.
Cryosections at −25°C (thickness, 10 µm) were prepared from
different areas of each specimen. Sections were mounted with
VectaShield mounting media containing 1.5 µg/ml DAPI to stain
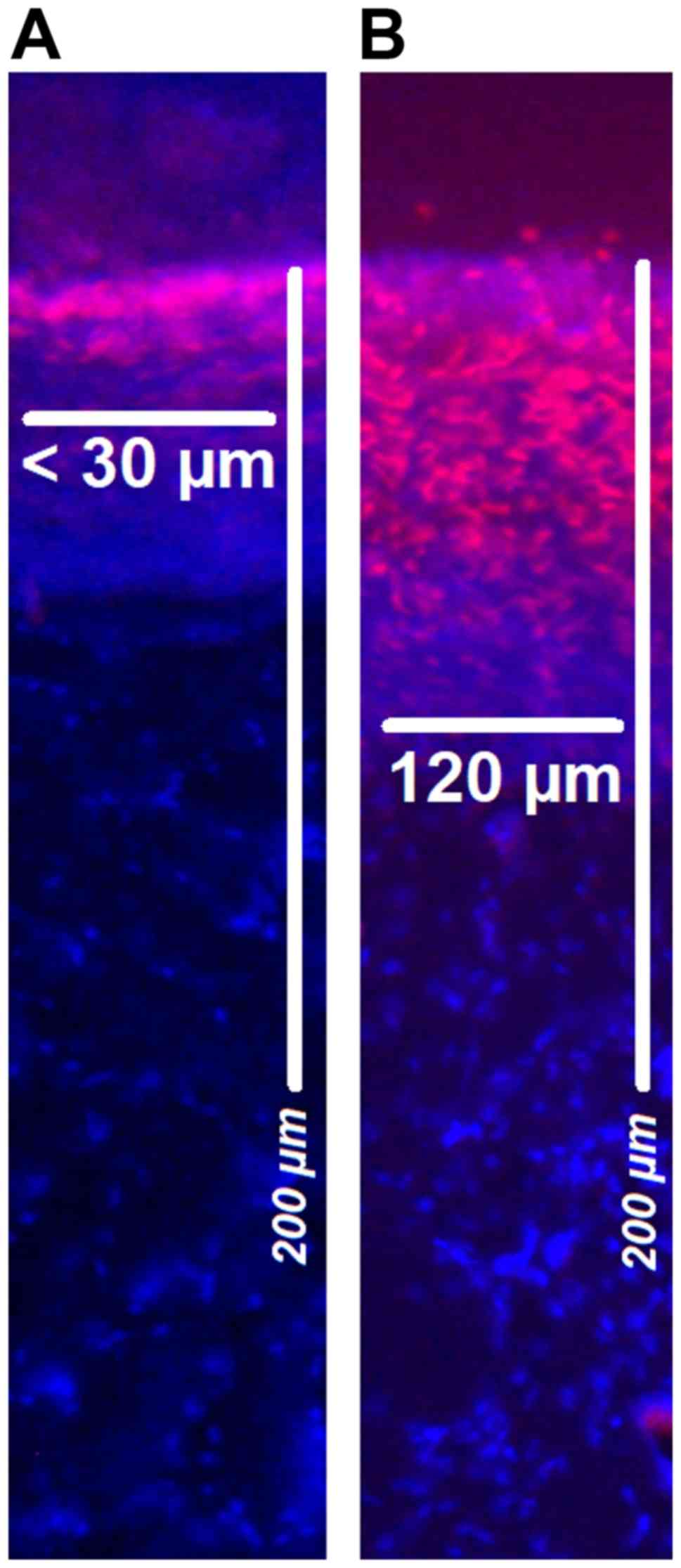
nuclei at room temperature (27°C). Penetration depth of doxorubicin
was monitored using a Nikon Eclipse 80i fluorescence microscope
(Nikon Corporation; magnification, ×10) to detect intrinsic
doxorubicin fluorescense. The distance between the luminal surface
and the innermost positive staining for doxorubicin accumulation
was measured and reported in µm (Figs.
2 and 3).
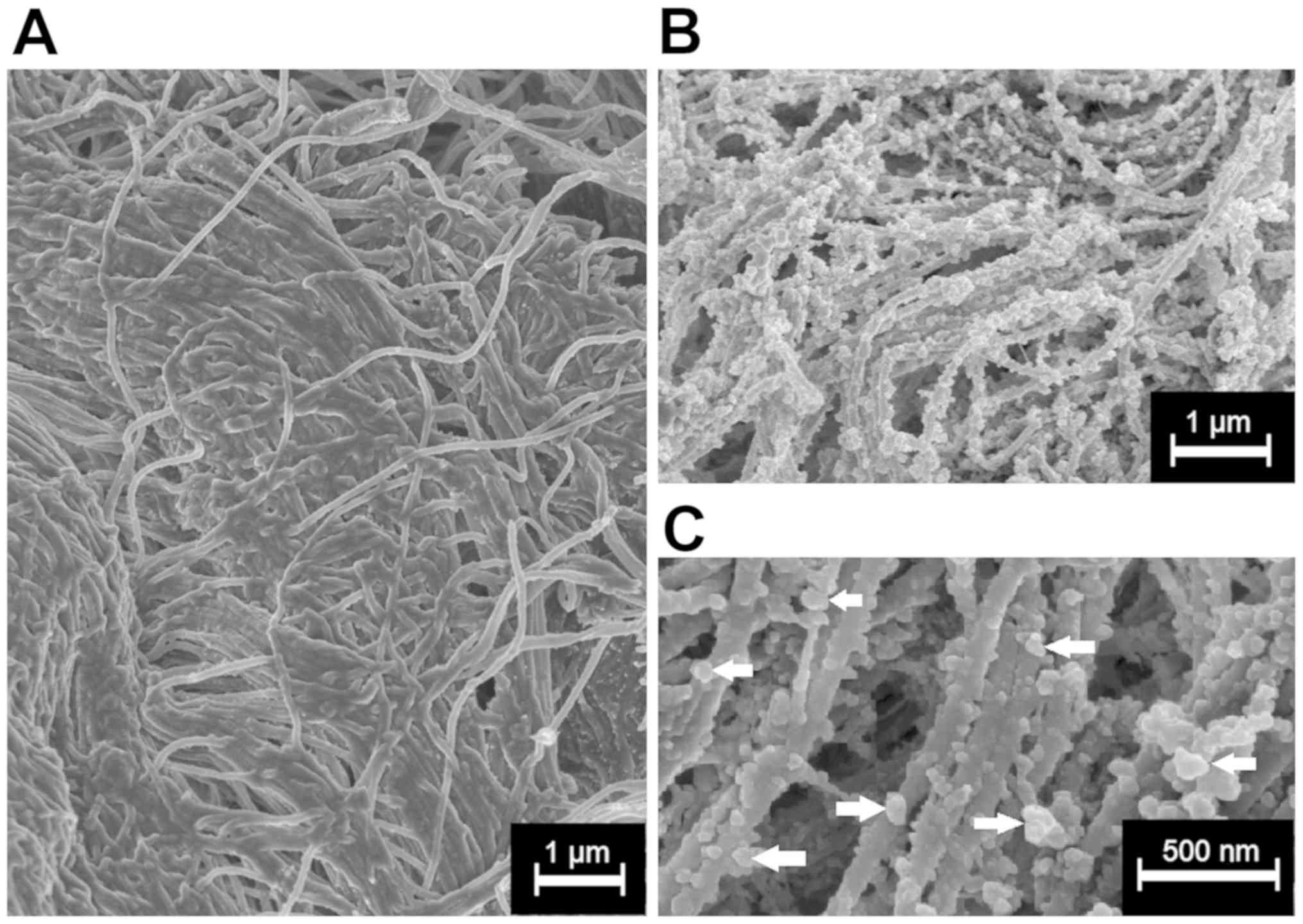
LD detection via electron microscopy
(EM)
The surface of the peritoneal tissue samples treated
with LD was analyzed and visualized via cryogenic scanning EM
(cryo-SEM) (Fig. 4). Tissue samples
were fixed overnight at −5°C in 2.5% glutaraldehyde solution in PBS
(pH 7.2). After fixation, samples were washed in PBS, rinsed in
ultrapure deionized water, which was filtered through syringe
filters (pore diameter, 0.1 µm), mounted on a cryoshuttle using a
mixture containing optimal cutting temperature compound and colloid
graphite, and immersed in liquid nitrogen. The frozen specimen was
then quickly transferred to a cryo-preparation chamber (Cryo Quorum
PP3010T; Quorum Technologies Ltd.), sputtered with a conductive
layer of platinum at −140°C, and transferred to the microscope
chamber maintaining the same temperature of −140°C (Auriga 60;
Zeiss AG). Samples were observed at 2 kV of acceleration voltage
using In-Lens and Type II secondary electron detectors.
Statistical analysis
Experiments were independently performed three
times. In total, 12 Cryosections per tissue sample were subject to
doxorubicin penetration measurements. Data are presented as the
mean ± SD. The statistical analyses were performed using Sigma Plot
(version 12; Systat Software Inc.). The Kruskal-Wallis test by
ranks was used for analyzing independent groups. P<0.01 was
considered to indicate a statistically significant difference.
Results
Ex vivo experiment
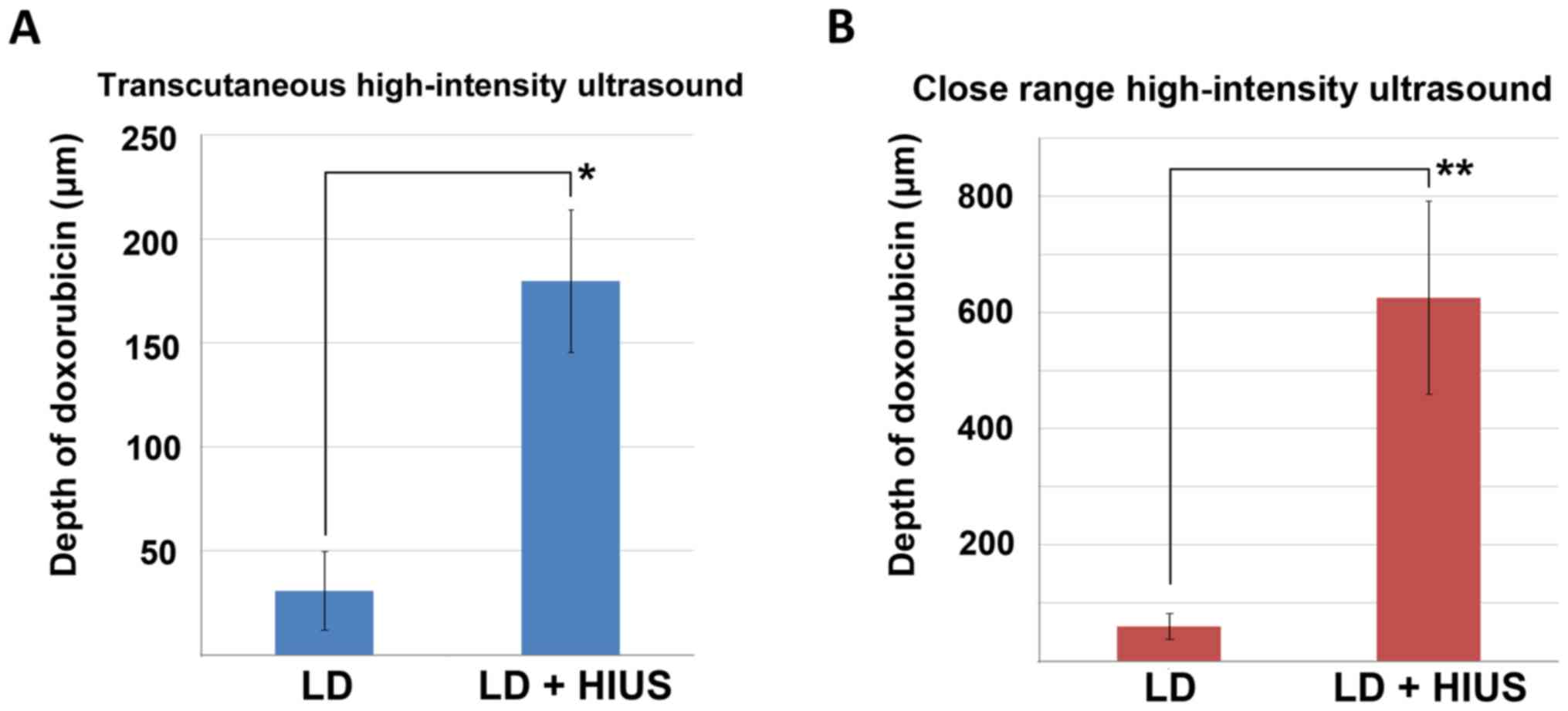
Mean penetration of doxorubicin following
PIPAC-mediated LD treatment was 31±19 µm. In some of these samples,
doxorubicin was undetectable by fluorescence microscopy. After
PIPAC and transcutaneous HIUS, the mean penetration rates were
significantly increased, and the mean was 180±35 µm (P<0.001;
Fig. 2A). No structural damage was
detected in the peritoneal tissue after PIPAC and HIUS. Mean
penetration of doxorubicin after lavage with LD was 60±22 µm. After
close-range HIUS, the penetration depth of doxorubicin was 625±166
µm. The levels of LD measured following close-range HIUS were
significantly higher (P<0.00001; Fig.
2B). Samples treated only via lavage did not show any
structural damage; however, lavage samples treated with HIUS
presented signs of structural alterations. Discrete structural
damage was observed in few samples in the form of partial
disruptions within the upper peritoneal layer. These defects were
observed also in the subperitoneal tissue (data not shown).
Doxorubicin was detected via fluorescence microscopy on the
peritoneal surface at different depths (Fig. 3).
EM of peritoneal tissue
LD was detected on the peritoneal surface of samples
that were not treated with HIUS via cryo-SEM (magnification,
×20,000; Fig. 4B). Most LD particles
had a spherical form and were <200 nm in diameter. LD particles
were detected throughout the entire surface. No LD particles were
detected on the surface of the samples treated with transcutaneous
HIUS (Fig. 4A).
Discussion
Despite progress in chemotherapeutic regimens and
new drug compositions, poor response to systemic and local
treatment is still observed in a considerable part of patients
suffering from peritoneal metastasis (PM), due to the occurrence of
molecular mechanisms causing drug resistance and limited drug
availability within the tumor tissue (20,21). New
forms of applications such as PIPAC, pressurized intravesical
aerosol therapy and others alongside new drugs for intraperitoneal
administration have been introduced and tested (7,22,23).
PIPAC has been demonstrated to be a possible novel
application method to administer new complex particles without
altering their structural integrity (24). Furthermore, in contrast with liquid
drugs, high local drug concentrations can be achieved with smaller
quantities of applied volume (5).
Coated particles, including LD, have been described
as good carriers for chemotherapeutics (25). In particular, LD has attracted high
interest because of its ability to carry high concentrations of
doxorubicin in proximity to malignant cells and release it on
contact or in proximity to the cell wall (26). However, the main problem of LD is
that the entire content of doxorubicin is not released in proximity
to the target sites (7,27).
In a previous study, HIUS was reported to be able to
destroy the liposomal wall of LD, facilitating the release of
doxorubicin (16). As assessed by
the present study via EM, the liposomal coating of LD appeared
damaged in tissues treated with HIUS compared with tissues that
were not subjected to HIUS. In line with previous studies, the
present results suggested that HIUS can mediate the release of
doxorubicin from its liposomal coating on the peritoneal surface,
thus significantly increasing drug penetration. This effect induces
a 5–10 time increase in tissue penetration at the target
destination. Further in vivo and clinical studies are
required to evaluate the relevance and efficacy of PIPAC with LD
and HIUS.
Recent clinical trials on PIPAC have shown promising
results with good overall drug tolerance for the application of
chemotherapy in standard dosages (28,29).
Future clinical dose-escalation trials may identify the limits of
standard liquid chemotherapy. In addition, thanks to these recent
developments, liposomal particles such as LD may play a significant
role in the future treatment of PM. These particles have been
better tolerated compared to conventional liquid chemotherapy
(30,31). Drug release can increase with time
and be enhanced by ultrasound, hyperthermia or other methods, as
previously described (32,33). Since the interaction of complex
particles with peritoneal tissue has not yet been fully
investigated, further studies are required to analyze benefits and
disadvantages of LD application with HIUS.
The present results indicated that doxorubicin
release may be limited in LD applications. This effect is probably
due to the coating of LD particles. Mechanical release of
doxorubicin via HIUS may be used to increase drug penetration into
metastatic tissue. Further studies are required to investigate the
impact and therapeutic possibilities of LD on tumor cells during
PIPAC or heated intraperitoneal chemotherapy applications and to
investigate the optimal conditions to use chemotherapeutic agents
in the treatment of PM.
Acknowledgements
Not applicable.
Funding
The present study was funded by institutional funds
of the Faculty of Veterinary Medicine, Wroclaw University of
Environmental and Life Sciences.
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
AM designed the present study, performed experiments
and analyzed the data. MA assisted in cryosectioning and performed
data analysis and interpretation. VK helped in writing the
manuscript, performed PIPAC procedures, and was involved in
designing the study and data interpretation. VK supervised the
study, drafted and critically revised the manuscript for important
intellectual content. JK performed experiments and constructed the
schematic diagram. KK and PM performed experiments. MA drafted and
critically revised the manuscript for important intellectual
content. TK designed the study, performed experiments and drafted
the manuscript.
Ethics approval and consent to
participate
The experiments were performed in an ex vivo
model using commercially available tissue samples. Therefore, no
approval from the Institutional Review Board and the Local Board on
Animal Care were required.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
EM
|
electron microscopy
|
|
HIUS
|
high intensity ultrasound
|
|
IPC
|
intraperitoneal chemotherapy
|
|
LD
|
liposomal doxorubicin
|
|
MC
|
microcatheter
|
|
PIPAC
|
pressurized intraperitoneal aerosol
chemotherapy
|
|
PM
|
peritoneal metastasis
|
References
|
1
|
Solass W, Kerb R, Mürdter T, Giger-Pabst
U, Strumberg D, Tempfer C, Zieren J, Schwab M and Reymond MA:
Intraperitoneal chemotherapy of peritoneal carcinomatosis using
pressurized aerosol as an alternative to liquid solution: First
evidence for efficacy. Ann Surg Oncol. 21:553–559. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Göhler D, Khosrawipour V, Khosrawipour T,
Diaz-Carballo D, Falkenstein TA, Zieren J, Stintz M and Giger-Pabst
U: Technical description of the microinjection pump
(MIP®) and granulometric characterization of the aerosol
applied for pressurized intraperitoneal aerosol chemotherapy
(PIPAC). Surg Endosc. 31:1778–1784. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Khosrawipour V, Khosrawipour T, Kern AJ,
Osma A, Kabakci B, Diaz-Carballo D, Förster E, Zieren J and
Fakhrian K: Distribution pattern and penetration depth of
doxorubicin after pressurized intraperitoneal aerosol chemotherapy
(PIPAC) in a postmortem swine model. J Cancer Res Clin Oncol.
142:2275–2280. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Khosrawipour V, Khosrawipour T,
Falkenstein TA, Diaz-Carballo D, Förster E, Osma A, Adamietz IA,
Zieren J and Fakhrian K: Evaluating the effect of micropump©
position, internal pressure and doxorubicin dosage on efficacy of
pressurized intra-peritoneal aerosol chemotherapy (PIPAC) in an ex
vivo model. Anticancer Res. 36:4595–4600. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Khosrawipour V, Khosrawipour T,
Diaz-Carballo D, Förster E, Zieren J and Giger-Pabst U: Exploring
the spatial drug distribution pattern during pressurized
intraperitoneal aerosol chemotherapy (PIPAC). Ann Surg Oncol.
23:1220–1224. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Khosrawipour T, Schubert J, Khosrawipour
V, Chaudhry H, Grzesiak J, Arafkas M and Mikolajczyk A: Particle
stability and structure on the peritoneal surface in pressurized
intra-peritoneal aerosol chemotherapy (PIPAC) analysed by electron
microscopy: First evidence of a new physical concept for PIPAC.
Oncol Lett. 17:4921–4927. 2019.PubMed/NCBI
|
|
7
|
Mikolajczyk A, Khosrawipour V, Schubert J,
Grzesiak J, Chaudhry H, Pigazzi A and Khosrawipour T: Effect of
liposomal doxorubicin pressurized intra-peritoneal aerosol
chemotherapy (PIPAC). J Cancer. 9:4301–4305. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Khosrawipour V, Khosrawipour T,
Hedayat-Pour Y, Diaz-Carballo D, Bellendorf A, Böse-Riberio H,
Mücke R, Mohanaraja N, Adamitz IA and Fakhrian K: Effect of whole
abdominal irradiation on penetration depth of doxorubicin in normal
tissue after pressurized intraperitoneal aerosol chemotherapy
(PIPAC) in a post-mortem swine model. Anticancer Res. 37:1677–1680.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Khosrawipour V, Bellendorf A, Khosrawipour
C, Hedayat-Pour Y, Diaz-Carballo D, Förster E, Mücke R, Kabakci B,
Adamietz IA and Fakhrian K: Irradiation does not increase the
penetration depth of doxorubicin in normal tissue after pressurized
intra-peritoneal aerosol chemotherapy (PIPAC) in an ex vivo model.
In Vivo. 30:593–597. 2016.PubMed/NCBI
|
|
10
|
Khosrawipour V, Giger-Pabst U,
Khosrawipour T, Pour YH, Diaz-Carballo D, Förster E, Böse-Ribeiro
H, Adamietz IA, Zieren J and Fakhrian K: Effect of irradiation on
tissue penetration depth of doxorubicin after pressurized
intra-peritoneal aerosol chemotherapy (PIPAC) in a novel ex-vivo
model. J Cancer. 7:910–914. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Harrison LE, Bryan M, Pliner L and
Saunders T: Phase I trial of pegylated liposomal doxorubicin with
hyperthermic intraperitoneal chemotherapy in patients undergoing
cytoreduction for advanced intra-abdominal malignancy. Ann Surg
Oncol. 15:1407–1413. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Salvatorelli E, De Tursi M, Menna P,
Carella C, Massari R, Colasante A, Iacobelli S and Minotti G:
Pharmacokinetics of pegylated liposomal doxorubicin administered by
intraoperative hyperthermic intraperitoneal chemotherapy to
patients with advanced ovarian cancer and peritoneal
carcinomatosis. Drug Metab Dispos. 40:2365–2373. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Hirano K and Hunt CA: Lymphatic transport
of liposome-encapsuled agents: Effect of liposome size following
intraperitoneal administration. J Pharm Sci. 74:915–921. 1985.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Carron PL, Padilla M and Maurizi Balzan J:
Nephrotic syndrome and acute renal failure during pegylated
liposomal doxorubicin treatment. Hemodial Int. 18:846–847. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Ansari L, Shiezadeh F, Taherzadeh Z,
Nikoofal-Sahlabadi S, Momtazi-Borojeni AA, Sahebkar A and Eslami S:
The most prevalent side effects of pegylated liposomal doxorubicin
monotherapy in women with metastatic breast cancer: A systemic
review of clinical trials. Cancer Gene Ther. 24:189–193. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Rizzitelli S, Guistetto P, Faletto D,
Delli Castelli D, Aime S and Terreno E: The release of Doxorubicin
from liposomes monitores by MRI and triggered by a combination of
US stimuli led to a complete tumor regression in a breast cancer
mouse model. J Control Release. 230:57–63. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Lyon PC, Griffiths LF, Lee J, Chung D,
Carlise R, Wu F, Middelton MR, Gleeson FV and Coussios CC: Clinical
trial protocol for Tardox: A phase I study to investigate the
feasibility of target release of lyso-thermosensitive liposomal
doxorubicin (ThermoDox®) using focused ultrasound in
patients with liver tumors. J Ther Ultrasound. 5:282017. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Khosrawipour V, Diaz-Carballo D, Acikelli
AH, Khosrawipour T, Falkenstein TA, Wu D, Zieren J and Giger-Pabst
U: Erratum to: Cytotoxic effect of different treatment parameters
in pressurized intraperitoneal aerosol chemotherapy (PIPAC) on the
in vitro proliferation of human colonic cancer cells. World J Surg
Oncol. 15:942017. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Khosrawipour V, Mikolajczyk A, Schubert J
and Khosrawipour T: Pressurized intra-peritoneal aerosol
chemotherapy (PIPAC) via endoscopical microcatheter system.
Anticancer Res. 38:3447–3452. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Flessner MF, Choi J, Credit K, Deverkadra
R and Henderson K: Resistance of tumor intestinal pressure to the
penetration of intraperitoneally delivered antibodies into
metastatic ovarian tumors. Clin Cancer Res. 11:3117–3125. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Holzer AK, Katano K, Klomp LW and Howell
SB: Cisplatin rapidly down-regulates its own influx transporter
hCTR1 in cultured human ovarian carcinoma cells. Clin Cancer Res.
10:6744–6749. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Mikolajczyk A, Khosrawipour V, Schubert J,
Plociennik M, Nowak K, Fahr C, Chaudhry H and Khosrawipour T:
Feasibility and characteristics of pressurized aerosol chemotherapy
(PAC) in the bladder as a therapeutical option in early-stage
urinary bladder cancer. In Vivo. 32:1369–1372. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Schubert J, Khosrawipour V, Chaudhry H,
Arafkas M, Knoefel WT, Pigazzi A and Khosrawipour T: Comparing the
cytotoxicity of taurolidine, mitomycin C, and oxaliplatin on the
proliferation of in vitro colon carcinoma cells following
pressurized intra-peritoneal aerosol chemotherapy (PIPAC). World J
Surg Oncol. 17:932019. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Mikolajczyk A, Khosrawipour V, Schubert J,
Chaudhry H, Pigazzi A and Khosrawipour T: Particle stability during
pressurized intra-peritoneal aerosol chemotherapy (PIPAC).
Anticancer Res. 38:4645–4649. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Sharma P, Mehta M, Dhanjal DS, Kaur S,
Gupta G, Singh H, Thangavelu L, Rajeshkumar S, Tambuwala M, Bakshi
HA, et al: Emerging trends in the novel drug delivery approaches
for the treatment of lung cancer. Chem Biol Interact.
309:1087202019. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Kai M, Ziemys A, Liu YT, Kojic M, Ferrari
M and Yokoi K: Tumor site-dependent transport properties determine
nanotherapeutics delivery and its efficacy. Transl Oncol.
12:1196–1205. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Lokerse WJM, Bolkestein M, Dalm SU,
Eggermont AMM, de Jong M, Grüll H and Koning GA: Comparing the
therapeutic potential of thermosensitive liposomes and hyperthermia
in two distinct subtypes of breast cancer. J Control Release.
258:34–42. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Falkenstein TA, Götze TO, Ouaissi M,
Tempfer CB, Giger-Pabst U and Demtröder C: First clinical data of
pressurized intraperitoneal aerosol chemotherapy (PIPAC) as salvage
therapy for peritoneal metastatic biliary tract cancer. Anticancer
Res. 38:373–378. 2018.PubMed/NCBI
|
|
29
|
Khosrawipour T, Khosrawipour V and
Giger-Pabst U: Pressurized intra peritoneal aerosol chemotherapy in
patients suffering from peritoneal carcinomatosis of pancreatic
adenocarcinoma. PLoS One. 12:e01867092017. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Armstrong DK, Fleming GF, Markman M and
Bailey HH: A phase I trial of intraperitoneal sustained-release
paclitaxel microspheres (Paclimer) in recurrent ovarian cancer: A
Gynecologic Oncology Group study. Gynecol Oncol. 103:391–396. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Sugiyama T, Kumagai S, Nishida T, Ushijima
K, Matuso T, Yakushiji M, Hyon SH and Ikada Y: Experimental and
clinical evaluation of cisplatin-containing microspheres as
intraperitoneal chemotherapy for ovarial cancer. Anticancer Res.
18:2837–2842. 1998.PubMed/NCBI
|
|
32
|
Lyon PC, Gray MD, Mannaris C, Folkes LK,
Stratford M, Campo L, Chung DYF, Scott S, Anderson M, Goldin R, et
al: Safety and feasibility of ultrasound-triggered targeted drug
delivery of doxorubicin from thermosensitive liposomes in liver
tumours (TARDOX): A single-centre, open-label, phase 1 trial.
Lancet Oncol. 19:1027–1039. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Besse HC, Bos C, Zandvliet MMJM, van der
Wulff-Jacobs K, Moonen CTW and Deckers R: Triggered radiosensitizer
delivery using thermosensitive liposomes and hyperthermia improves
efficacy of radiotherapy: An in vitro proof of concept study. PLoS
One. 13:e02040632018. View Article : Google Scholar : PubMed/NCBI
|