Introduction
Esophageal cancer (EC) is the ninth most common type
of cancer and the sixth most common cause of cancer-related
mortality globally (1). EC is
associated with considerable morbidity and carries a poor prognosis
in its later stages. This disease requires a multidisciplinary
approach, such as neoadjuvant chemotherapy or chemoradiotherapy
(CRT) plus surgery for locally advanced disease. Definitive CRT is
also recognized as a curative treatment option (1–3),
particularly in patients who are not surgical candidates.
A number of clinical studies of radical CRT for EC
have been conducted since the 1980s, and radiotherapy (50–60 Gy)
with cisplatin and fluorouracil (CF) has become the standard of
care (4,5). However, CF is associated with a number
of problems. It has been reported that local failure and
toxicity-related deaths/other life-threatening toxicities were
observed in 46 and 20% of patients with EC, respectively, who
receive this CRT, and that 41% patients with EC could not complete
the CRT as planned (6). CRT with CF
may also cause thrombosis, sudden death, or other toxicities, and
it requires hospital admission due to the requirement for prolonged
intravenous hydration for cisplatin, and 5 days continuous infusion
for 5FU.
Therefore, a safer and more useful regimen of CRT
for EC is urgently needed. A phase II/III clinical study
(PRODIGE5/ACCORD17) was conducted to investigate the superiority of
leucovorin-fluorouracil-oxaliplatin (FOLFOX) to CF as the
chemotherapy component of CRT, replacing cisplatin with
oxaliplatin, which rarely induces kidney toxicity and does not
require intravenous hydration. Although the results of the study
did not meet the endpoint hypothesized, namely that FOLFOX is
superior to CF, the researchers demonstrated its lower toxicity and
non-inferior survival to CF; thus, CRT with FOLFOX for EC is
recognized as one of the standard treatment options in Europe
(7–9).
In Japan, oxaliplatin along with several other drugs
have not yet been approved for EC treatment, and CF is commonly
used as the chemotherapeutic component of CRT (10–12).
FOLFOX has been approved only for patients with colorectal or
gastric cancer. Thus, clinical data on the feasibility of this
treatment for EC in Japan are rare. We herein report the cases of 4
patients with esophageal squamous cell cancer (ESCC) and rectal
cancer (RC) who were treated with CRT with FOLFOX in our
hospital.
Patients and methods
Patients
Between January 2007 and December 2016, 740 patients
were registered in our hospital's database of chemotherapy for EC;
that database was searched for patients who received CRT with
FOLFOX, and their clinical data were investigated (Table I).
 | Table I.Patient characteristics. |
Table I.
Patient characteristics.
| Case no. | Sex/age (years) | Histology | TNM (UICC-7th) | Setting | Multiple primary
cancers |
|---|
| 1 | M/68 | SCC | T3/N1/M0 | dCRT | RC: cT4aN1H2 |
| 2 | M/68 | SCC | T3/N1/M0 | Neo-CRT | RC: cT3N1M0 |
| 3 | M/65 | SCC | T3/N2/M0 | Neo→dCRT | RC: cT3N0H0 |
| 4 | M/77 | SCC | T2/N1/M0 | dCRT | RC: preceding surgery
(pStage IIIB) |
Treatment
FOLFOX was administered every 2 weeks for 3–6
cycles, with the first 3 cycles administered concurrently with
radiotherapy. During each cycle, oxaliplatin (85 mg/m2)
was administered as a 2 h intravenous infusion in 250–500 ml of 5%
glucose on day 1, concurrently with leucovorin (200
mg/m2) as a 2 h intravenous infusion. Fluorouracil (400
mg/m2) was administered as a 10 min intravenous bolus
dose on day 1, followed by continuous intravenous infusion of
fluorouracil (1,600 mg/m2) over 2 days, which was based
on the PRODIGE5/ACCORD17 trial (8).
Radiation therapy was delivered with megavoltage
equipment (>6 MV) with anterior/posterior opposed or multi-field
irradiation and continuous bilateral oblique (off-cord) portals,
except for neoadjuvant cases. The patients were treated 5 days per
week at 1.8–2 Gy/day to a total dose of 41.4–60 Gy. The details of
the regimen for each patient are provided in the individual case
report descriptions and Table
II.
 | Table II.Summary of cases. |
Table II.
Summary of cases.
| Case no. | Chemotherapy regimen:
Dose (mg/m2) × cycles (Ox/bolus/civ FU) | Radiation dose
(Gy/fr) | Response | Later treatment | Status during
CRT | Outcome | Cause of death | Survival (days) |
|---|
| 1 | 85/400/1,600 × 3 | 60/30 | Non-CR/Non-PD | FOLFIRI +
cetuximab | Inpatient | Deceased | IP | 105 |
| 2 | 85/400/1,600 × 3 | 41.4/23 | Non-CR/Non-PD | Planned surgery | Inpatient | Deceased | Cause-specific | 194 |
| 3 | 85/400/1,600 × 3
(followed by 3 cycles) | 60/30 | PR (→CRu s/o) | Watchful waiting | Outpatient | No progression | – | 465 |
| 4 | 85/400/1,600 × 3
(followed by 3 cycles) | 60/30 | CR | Additional
capecitabine as adjuvant→surveillance | Outpatient | No progression | – | 450 |
Response to treatment
Response was assessed by esophageal endoscopy, and
neck-to-abdomen computed tomography (CT) 2–3 weeks after the
completion of radiotherapy or 6 cycles of FOLFOX for each case. The
response of primary tumors of the esophagus was assessed based on
the criteria of the Japanese Classification of Esophageal Cancer
(11th edition) (13). According to
these criteria, a CR required meeting all of the following: i) No
evidence of tumor except flat erosion, white exudate or a scar, ii)
a negative biopsy, iii) no new lesions and iv) confirmation of
i-iii with at least a 4-week interval. Progressive disease (PD)
required meeting any of the following criteria: i) Tumor growth and
ii) appearance of any new lesions or metastasis. If neither the
criteria of CR or PD were met, the response was categorized as
non-CR/non-PD. Overall response was assessed bases on RECIST
version 1.1. (14).
Toxicity was assessed according to Common
Terminology Criteria for Adverse Events (CTCAE) version 4.0
(15) during the treatment course.
The outcome and late toxicities were recorded until patient death
or the final visit on the chart. Survivors were followed up by
tri-monthly CT and endoscopy, and physical examination after the
confirmation of CR.
The analysis was performed in April 2017. All
patients provided written informed consent for treatment by CRT
with FOLFOX. Publication of this retrospective analysis was
approved by the Institutional Review Board of our hospital.
Results
Four patients with EC treated by CRT with FOLFOX
were selected from the database. All patients had synchronous ESCC
and RC. The patient characteristics and clinical courses are
summarized in Tables I and II. The details are described below.
Case 1
A 68-year-old male patient presented with dysphagia
and was diagnosed with type 2 ESCC (Lt, cT3N1) and RC (Rs, cT4aN1),
whereas liver metastases were synchronously diagnosed. Although
liver biopsy was not performed for histological analysis, the liver
metastases were clinically determined to be derived from the RC, as
progression of its primary and lymph node sites was observed, with
significant increases in the tumor marker carcinoembryonic antigen
(CEA) level to 31.2 ng/ml (normal, <5 ng/ml). As the patient had
synchronous stage IV metastatic RC, his ESCC did not fulfil the
indications for radical, invasive surgery; therefore, CRT was
performed. Due to the synchronous RC, FOLFOX was selected as the
concurrent chemotherapy. Radiotherapy (60 Gy/30 Fr/46 days,
omitting elective nodes due to the incurability of this condition)
was performed with 3 cycles of FOLFOX, as described in Methods. In
the following evaluation, although the response of the primary site
was assessed as non-CR due to persistent severe esophagitis, the
primary ESCC had regressed. The lymph nodes had also decreased in
size. The overall response was assessed as non-CR/non-PD in
accordance with RECIST. As regards RC, the outcome was determined
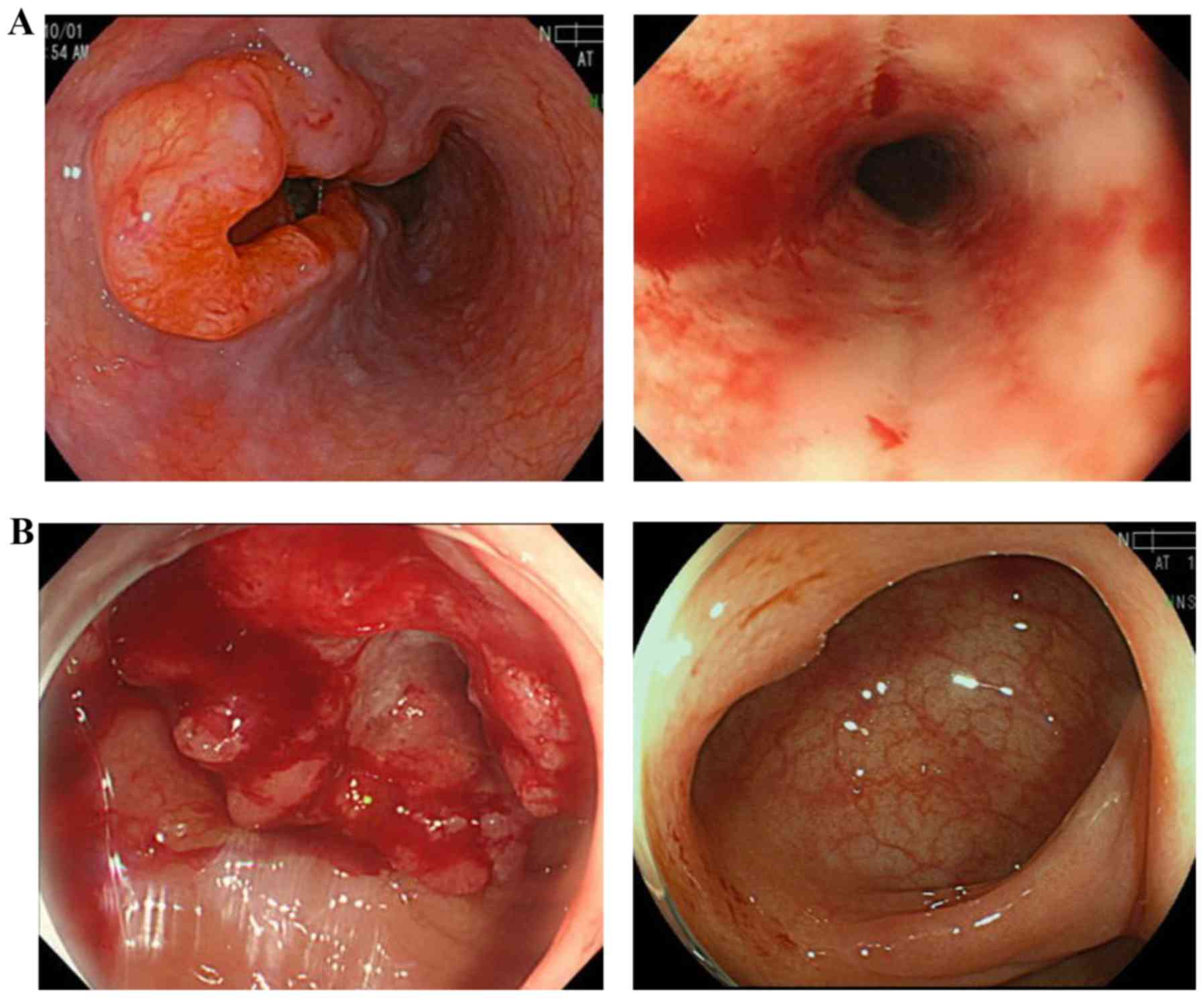
as stable disease (Fig. 1).
Chemotherapy was continued focusing on the
metastatic RC. Although FOLFOX was planned to continue for 6 cycles
after completing radiotherapy based on the PRODIGE5/ACCORD17 trial,
the subsequent treatment was changed to
fluorouracil-leucovorin-irinotecan (FOLFIRI)-cetuximab, as the
therapeutic efficacy of FOLFOX in RC was considered to be limited,
and the RAS status of the RC was wild-type. The patient developed a
fever (38–39°C) 10 days after the initiation of FOLFIRI-cetuximab.
As grade 3 neutropenia was also observed (960/µl), the patient was
hospitalized and treated with antibiotics. Although the neutrophil
count had recovered to 10,910 µl by day 12, the patient had
persistent fever and developed hypoxemia on day 19. Chest computed
tomography was performed and revealed interstitial pneumonia due to
cetuximab. The patient did not respond to pulse steroid therapy and
succumbed to aggravated interstitial pneumonia 46 days after the
start of FOLFIRI-cetuximab.
Case 2
A 68-year-old male patient presented with dysphagia
and was diagnosed with type 2 ESCC (Mt, cT3N1) and RC (Rs, cT3N1).
As both tumors were resectable, radical surgery was planned at the
same time after neoadjuvant therapy for the ESCC. For the RC, the
neoadjuvant therapy adopted was CRT with FOLFOX. As this was a
planned surgery, the dose of radiotherapy was adjusted to 41.4
Gy/23 Fr, which was generally administered as neoadjuvant CRT, with
3 cycles of FOLFOX administered concurrently. The response of the
ESCC was evaluated as non-CR/non-PD, with slight regression of the
lymph node metastases and tumor shrinkage, but prolonged
esophagitis due to the radiation. For the RC, the outcome was also
determined to be non-CR/non-PD (Fig.
2). During the 3rd cycle of FOLFOX, the patient developed
5-FU-induced encephalitis with hyperammonemia (252 µg/dl). Although
he rapidly recovered after administration of branched-chain amino
acids, continuation of the remaining FOLFOX cycle, similar to
PRODIGE5/ACCORD17, was considered to be harmful. At that time, both
cancers were assessed as resectable, so simultaneous radical
surgeries were performed.
Unexpectedly, the resection of the ESCC was
classified as R2. As the patient did not achieve recovery of his
performance status, additional treatment for ESCC was not feasible,
so close follow-up was planned. The patient developed recurrence of
mediastinal lymph node metastasis 84 days after surgery. His
performance status at the time was poor, and he succumbed to ESCC
112 days after surgery.
Case 3
A 65-year-old male patient presented with dysphagia
and was diagnosed with type 2 ESCC (Mt, cT3N2) and RC (Rs, cT3N0).
Due to mediastinal lymph node metastases (no. 112) invading the
aorta, they were deemed to be unresectable. Therefore, the
treatment selected for the ESCC was definitive CRT with FOLFOX,
followed by radical surgery for the RC. Three cycles of FOLFOX were
administered with definitive radiotherapy (60 Gy/30 Fr/46 days),
followed by another 3 cycles of FOLFOX. The response of the ESCC
was evaluated after all 6 cycles of chemotherapy as non-CR/non-PD,
with regression of lymph node metastases and prolonged radiation
esophagitis, but the primary tumor had shrank notably. On the other
hand, the outcome of FOLFOX for RC was CR (Fig. 3). Considering a delayed CR of the
ESCC, the patient was closely followed up to avoid invasive salvage
surgery. To date, neither the ESCC nor the RC have progressed. The
progression-free survival (PFS) for both cancers has been >2
years since CRT, without surgery.
Case 4
A 77-year-old male patient presented with defecation
problems. Type 2 circumferential RC (Rsa, cT3N0) and ESCC (Lt,
cT2N1) were diagnosed. The RC caused severe stenosis, and there was
a risk of ileus, so radical surgery was performed. Subsequently, it
was decided to treat the ESCC with definitive CRT due to an
anatomic anomaly (a right aortic arch) that would make the surgery
for the ESCC difficult. As the pathological stage of the RC after
resection was pStage IIIB (pT3N1), adjuvant chemotherapy with
FOLFOX was scheduled for 6 months. Therefore, CRT with FOLFOX was
selected for this ESCC case according to the PRODIGE5/ACCORD17
trial, as described in Methods. After completion of 6 cycles of
FOLFOX with definitive radiation (60 Gy/30 fr/46 days), the patient
developed grade 2 peripheral sensory neuropathy due to treatment
with oxaliplatin. Therefore, his chemotherapy was switched to
capecitabine monotherapy for the remaining 3 months of adjuvant
chemotherapy for the RC. The outcome for the ESCC was determined as
CR (Fig. 4). The patient has
survived without progression for >1 year and 9 months since the
completion of FOLFOX chemotherapy.
The encephalitis in patient 2 was the only grade ≥3
adverse event observed among the 4 patients, whereas the other AEs
were common events caused by CRT with CF (Table III).
 | Table III.Adverse events (n=4). |
Table III.
Adverse events (n=4).
|
| Grade, n (%) |
|---|
|
|
|
|---|
| Adverse
eventsa | All | ≥ 3 |
|---|
| Hematological |
|
|
|
Neutropenia | 3 (75) | 0 |
|
Anemia | 3 (75) | 0 |
|
Thrombocytopenia | 2 (50) | 0 |
| Febrile
neutropenia | 0 | 0 |
|
Anorexia | 3 (75) | 0 |
|
Esophagitis | 3 (75) | 0 |
|
Constipation | 3 (75) | 0 |
|
Dysphagia | 3 (75) | 0 |
|
Non-hematological |
|
|
|
Erythema | 2 (50) | 0 |
|
Diarrhea | 2 (50) | 0 |
|
Hypokalemia | 2 (50) | 0 |
|
Nausea | 2 (50) | 0 |
|
Paresthesia | 2 (50) | 0 |
|
Encephalopathy | 1 (25) | 1 (25) |
|
Vomiting | 1 (25) | 0 |
|
Hiccups | 1 (25) | 0 |
|
Pneumonia | 1 (25) | 0 |
|
Mucositis | 0 | 0 |
|
Thrombocytopenia | 0 | 0 |
| Renal
insufficiency | 0 | 0 |
Discussion
In the RTOG 85-01 study, CRT was found to be notably
superior to radiotherapy alone in terms of survival prolongation
for esophageal cancer. In that study, CF was concurrently
administered (1,000 mg/m2 5-FU on days 1–4; 75
mg/m2 cisplatin every 28 days) (4). In the RTOG 94-05 study, which was a
randomized controlled trial comparing radiation doses of 50.4 vs.
64.8 Gy, with concurrent CF, no survival benefit was achieved with
the increased radiation dose (5).
Therefore, in the US and Europe, CF in combination with a radiation
dose of 50.4 Gy is commonly used for EC, as in the RTOG 85-01
study. However, CF is associated with renal and gastrointestinal
toxicity, thrombosis, and other AEs linked to cisplatin, and
hospital admission is required for continuous 5-FU infusion.
FOLFOX is one of the global standard treatments for
colorectal cancer (16,17). In the US and Europe, oxaliplatin may
be used for treatment of EC (2).
FOLFOX is expected to contribute to a reduction in toxicity and to
increase outpatient treatment through replacement of cisplatin with
oxaliplatin in CRT for EC. The results of phase I and II clinical
studies of CRT with FOLFOX for EC have been promising (18–20).
Conroy et al conducted phase I and II clinical trials of
FOLFOX with CRT for EC and obtained favorable results (7). Consequently, randomized phase II and
III studies comparing FOLFOX with CF have been conducted, including
PRODIGE5/ACCORD17 (8). The results
of the phase II study were promising but, unexpectedly, the primary
endpoint was not met in the phase III study, as FOLFOX was not
found to be superior in terms of PFS with statistical significance
[9.7 months for the FOLFOX group vs. 9.4 months for the CF group;
hazard ratio (HR)=0.93; 95% confidence interval (CI): 0.7–1.24;
P=0.64). However, due to the lower rates of nephrotoxicity,
treatment-related death/sudden death, and other toxicities, and due
to the fact that it can be administered on an outpatient basis, CRT
with FOLFOX is commonly used in clinical practice in western
countries.
In Japan and worldwide, cisplatin has been replaced
with oxaliplatin for gastric cancer treatment, as it reduces
gastrointestinal toxicity and enables outpatient treatment
(3,21–24).
However, the use of oxaliplatin for EC has not been approved.
Therefore, clinical data of FOLFOX for EC in Japan were available
only from patients with multiple primary cancers, for which
oxaliplatin is approved. Watanabe et al administered CRT
with FOLFOX to a patient who had both locally advanced ESCC and
synchronous metastatic colon cancer (25). Unlike the ACCORD17 study, an 80% dose
of 5-FU-oxaliplatin-leucovorin (mFOLFOX6) (26), which is commonly used for colorectal
cancer, was used. Although a tracheoesophageal fistula developed at
the end of treatment, the patient was able to complete CRT with
FOLFOX, and the tumor size was reduced. The only other grade 3
adverse event was leukocytopenia (25). However, such cases are usually
excluded from clinical trials, and little is known on the
feasibility of this treatment for Japanese EC patients.
The 4 patients in the present study had both ESCC
and advanced RC. As patients 3 and 4 were able to ingest food and
did not require hydration, they were treated as outpatients and
they achieved a CR for ESCC. Moreover, patient 3 also achieved a CR
for RC and was able to avoid surgery. Patient 4 underwent CRT with
FOLFOX as postoperative adjuvant chemotherapy for RC and achieved a
satisfactory progression-free period. Although neither patient 1
nor patient 2 achieved a CR for ESCC, the primary lesions were
reduced in size, and progression of RC was arrested. In other
words, both ESCC and RC were well controlled, and the patients were
able to complete CRT with FOLFOX for ESCC. These experiences
suggest that CRT with FOLFOX may be a useful therapeutic option for
such patients, at least in Japan.
We should be careful regarding the extrapolation of
this regimen to ESCC in our country due to the differences in
clinical practice between Japan and other countries. First, the
major histological type of EC is adenocarcinoma in western
countries and SCC in Asia. However, although evidence of this
regimen has been verified in US and Europe, several trials include
a non-negligible ESCC population. Chiarion et al reported
that their study included 85% cases of ESCC, and PRODIGE5/ACCORD17
consists of 85–86% of cases of ESCC (8,19).
Therefore, CRT with FOLFOX for ESCC may be acceptable. Another
point is the difference in the radiation dose. Although a radiation
dose of 50.4 Gy was used in an overseas phase III clinical study
(5), a dose of 60 Gy/30 Fr is the
standard in Japan (10,11). Following a discussion, we decided to
use full-dose radiation as the domestic standard in cases 1, 3 and
4, as salvage surgery was unlikely, in an attempt to avoid non-CR.
Despite the high-dose radiation, neither acute nor late toxicity
was observed. Thus, 50.4 Gy will be adopted as it is estimated to
be an acceptable dose for ESCC in Japan.
Patient 2 was scheduled to receive neoadjuvant CRT;
therefore, an exposure dose of 41.4 Gy was used, and he also did
not experience any clinically significant toxicity. He developed an
AE of grade ≥3 (5-FU-induced encephalitis), but this toxicity was
predictable and it was quickly treated as reported in the
literature (27).
Unlike CRT with CF, CRT with FOLFOX did not cause
any specific or serious AEs in the 4 reported cases. Although
attention should be paid to variations in chemotherapy cycles or
irradiation dose and fields in each case, which is the limitation
of retrospective case studies, the safety and efficacy of radiation
with 3 cycles of FOLFOX may be discussed in approximation.
In conclusion, the present study suggests that CRT
with FOLFOX may be well tolerated and feasible in patients with
ESCC and synchronous RC, although the retrospective nature of the
study and the small number of patients in Japan constitute major
limitations. As the antitumor activity of this treatment was found
to be highly promising, further investigation with more subjects is
required.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
TY, HH, MA, YK, SM, NT, TM and SS and YS contributed
to the treatment and follow-up examinations, and TY wrote the
manuscript. All the authors have read and approved the final
version of this manuscript for publication.
Ethics approval and consent to
participate
The present study was approved by the Ethics
Committee of Saitama Cancer Center.
Patient consent for publication
The requirement for the patients' written consent
was waived with removal of all identifying information in this
retrospective study under the approval of the Ethics Committee of
Saitama Cancer Center.
Competing interests
Hiroki Hara received grants from the following
companies: Astrazeneca, Daiichi Sankyo, Dainippon Sumitomo Pharma,
Lilly, Merck Serono, MSD, Taiho, Chugai, Eisai, LSK BioPharma,
Incyte, Pfizer, Boehringer-Ingelheim, Beigene, ONO and BMS. These
grants did not directly support this submitted study. All the other
authors declare that they have no competing interests to
disclose.
References
|
1
|
Global Burden of Disease Cancer
Collaboration, ; Fitzmaurice C, Dicker D, Pain A, Hamavid H,
Moradi-Lakeh M, MacIntyre MF, Allen C, Hansen G, Woodbrook R, et
al: The global burden of cancer 2013. JAMA Oncol. 1:505–527. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
National Comprehensive Cancer Network, .
Clinical practice oncology: Esophageal and esophagogastric junction
cancers (version 2. 2017).
|
|
3
|
Stahl M, Mariette C, Haustermans K,
Cervantes A and Arnold D; ESMO Guidelines Working Group, :
Oesophageal cancer: ESMO clinical practice guidelines for
diagnosis, treatment and follow-up. Ann Oncol. 24 (Suppl
6):vi51–vi56. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Cooper JS, Guo MD, Herskovic A, Macdonald
JS, Martenson JA Jr, Al-Sarraf M, Byhardt R, Russell AH, Beitler
JJ, Spencer S, et al: Chemoradiotherapy of locally advanced
esophageal cancer: Long-term follow-up of a prospective randomized
trial (RTOG 85-01). Radiation therapy oncology group. JAMA.
281:1623–1627. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Minsky BD, Pajak TF, Ginsberg RJ, Pisansky
TM, Martenson J, Komaki R, Okawara G, Rosenthal SA and Kelsen DP:
INT 0123 (radiation therapy oncology group 94-05) phase III trial
of combined-modality therapy for esophageal cancer: High-dose
versus standard-dose radiation therapy. J Clin Oncol. 20:1167–1174.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
al-Sarraf M, Martz K, Herskovic A,
Leichman L, Brindle JS, Vaitkevicius VK, Cooper J, Byhardt R, Davis
L and Emami B: Progress report of combined chemoradiotherapy versus
radiotherapy alone in patients with esophageal cancer: An
intergroup study. J Clin Oncol. 15:277–284. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Conroy T, Yataghene Y, Etienne PL, Michel
P, Senellart H, Raoul JL, Mineur L, Rives M, Mirabel X, Lamezec B,
et al: Phase II randomised trial of chemoradiotherapy with FOLFOX4
or cisplatin plus fluorouracil in oesophageal cancer. Br J Cancer.
103:1349–1355. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Conroy T, Galais MP, Raoul JL, Bouche O,
Gourgou-Bourgade S, Douillard JY, Etienne PL, Boige V, Martel-Lafay
I, Michel P, et al: Definitive chemoradiotherapy with FOLFOX versus
fluorouracil and cisplatin in patients with oesophageal cancer
(PRODIGE5/ACCORD17): Final results of a randomised, phase 2/3
trial. Lancet Oncol. 15:305–314. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Messager M, Mirabel X, Tresch E, Paumier
A, Vendrely V, Dahan L, Glehen O, Vasseur F, Lacornerie T, Piessen
G, et al: Preoperative chemoradiation with paclitaxel-carboplatin
or with fluorouracil-oxaliplatin-folinic acid (FOLFOX) for
resectable esophageal and junctional cancer: The PROTECT-1402,
randomized phase 2 trial. BMC Cancer. 16:3182016. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Shinoda M, Ando N, Kato K, Ishikura S,
Kato H, Tsubosa Y, Minashi K, Okabe H, Kimura Y, Kawano T, et al:
Randomized study of low-dose versus standard-dose chemoradiotherapy
for unresectable esophageal squamous cell carcinoma (JCOG0303).
Cancer Sci. 106:407–412. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Kato K, Muro K, Minashi K, Ohtsu A,
Ishikura S, Boku N, Takiuchi H, Komatsu Y, Miyata Y, Fukuda H, et
al: Phase II study of chemoradiotherapy with 5-fluorouracil and
cisplatin for stage II–III esophageal squamous cell carcinoma: JCOG
trial (JCOG 9906). Int J Radiat Oncol Biol Phys. 81:684–690. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Kato H, Sato A, Fukuda H, Kagami Y,
Udagawa H, Togo A, Ando N, Tanaka O, Shinoda M, Yamana H and
Ishikura S: A phase II trial of chemoradiotherapy for stage I
esophageal squamous cell carcinoma: Japan clinical oncology group
study (JCOG9708). Jpn J Clin Oncol. 39:638–643. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Japan Esophageal Society, . Japanase
classification of esophageal cancer, 11th edition: Part II and III.
Esophagus. 14:37–65. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Eisenhauera EA, Therasseb P, Bogaertsc J,
Schwartzd H, Sargente D, Ford R, Dancey J, Gwyther S, Mooney M,
Rubinstein L, et al: New response evaluation criteria in solid
tumours: Revised RECIST guideline (version 1.1). Eur J Cancer.
45:228–247. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
National Institutes of Health Common
Terminology Criteria for Adverse Events (CTCAE) Version 4.0US
Department of Health and Human Services. National Institutes of
Health, National Cancer Center, National Cancer Center; Washington
DC: 2009
|
|
16
|
de Gramont A, Figer A, Seymour M, Homerin
M, Hmissi A, Cassidy J, Boni C, Cortes-Funes H, Cervantes A, Freyer
G, et al: Leucovorin and fluorouracil with or without oxaliplatin
as first-line treatment in advanced colorectal cancer. J Clin
Oncol. 18:2938–2947. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Andre T, Boni C, Mounedji-Boudiaf L,
Navarro M, Tabernero J, Hickish T, Topham C, Zaninelli M, Clingan
P, Bridgewater J, et al: Oxaliplatin, fluorouracil, and leucovorin
as adjuvant treatment for colon cancer. N Engl J Med.
350:2343–2351. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Khushalani NI, Leichman CG, Proulx G, Nava
H, Bodnar L, Klippenstein D, Litwin A, Smith J, Nava E, Pendyala L,
et al: Oxaliplatin in combination with protracted-infusion
fluorouracil and radiation: Report of a clinical trial for patients
with esophageal cancer. J Clin Oncol. 20:2844–2850. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Chiarion-Sileni V, Innocente R, Cavina R,
Ruol A, Corti L, Pigozzo J, Del Bianco P, Fumagalli U, Santoro A
and Ancona E: Multi-center phase II trial of chemo-radiotherapy
with 5-fluorouracil, leucovorin and oxaliplatin in locally advanced
esophageal cancer. Cancer Chemother Pharmacol. 63:1111–1119. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
O'Connor BM, Chadha MK, Pande A, Lombardo
JC, Nwogu CE, Nava HR, Yang G and Javle MM: Concurrent oxaliplatin,
5-fluorouracil, and radiotherapy in the treatment of locally
advanced esophageal carcinoma. Cancer J. 13:119–124. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Cunningham D, Allum WH, Stenning SP,
Thompson JN, Van de Velde CJ, Nicolson M, Scarffe JH, Lofts FJ,
Falk SJ, Iveson TJ, et al: Perioperative chemotherapy versus
surgery alone for resectable gastroesophageal cancer. N Engl J Med.
355:11–20. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Bang YJ, Kim YW, Yang HK, Chung HC, Park
YK, Lee KH, Lee KW, Kim YH, Noh SI, Cho JY, et al: Adjuvant
capecitabine and oxaliplatin for gastric cancer after D2
gastrectomy (CLASSIC): A phase 3 open-label, randomised controlled
trial. Lancet. 379:315–321. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Yamada Y, Higuchi K, Nishikawa K, Gotoh M,
Fuse N, Sugimoto N, Nishina T, Amagai K, Chin K, Niwa Y, et al:
Phase III study comparing oxaliplatin plus S-1 with cisplatin plus
S-1 in chemotherapy-naive patients with advanced gastric cancer.
Ann Oncol. 26:141–148. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Masuishi T, Kadowaki S, Kondo M, Komori A,
Sugiyama K, Mitani S, Honda K, Narita Y, Taniguchi H, Ura T, et al:
FOLFOX as first-line therapy for gastric cancer with severe
peritoneal metastasis. Anticancer Res. 37:7037–7042.
2017.PubMed/NCBI
|
|
25
|
Watanabe Y, Tsutsui M, Takeda S, Yoshino S
and Oka M: A case of esophageal cancer associated with colon cancer
successfully treated with combination chemotherapy of FOLFOX and
concurrent radiotherapy. Gan To Kagaku Ryoho. 36:2439–2441.
2009.(In Japanese). PubMed/NCBI
|
|
26
|
Allegra CJ, Yothers G, O'Connell MJ,
Sharif S, Colangelo LH, Lopa SH, Petrelli NJ, Goldberg RM, Atkins
JN, Seay TE, et al: Initial safety report of NSABP C-08: A
randomized phase III study of modified FOLFOX6 with or without
bevacizumab for the adjuvant treatment of patients with stage II or
III colon cancer. J Clin Oncol. 27:3385–3390. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Mitani S, Kadowaki S, Kato K, Masuishi T
and Muro K: Combination of oxaliplatin and
5-fluorouracil/leucovorin for advanced esophageal squamous cell
carcinoma refractory or intolerant to standard therapies. Case Rep
Oncol. 12:304–310. 2019. View Article : Google Scholar : PubMed/NCBI
|