Introduction
The Check Mate 025 study recently showed that
second-line treatment with nivolumab extends the overall survival
(OS) of patients with metastatic renal cell carcinoma (mRCC) and
exhibits a higher objective response rate than everolimus (1). Moreover, as a second-line treatment,
the National Comprehensive Cancer Network has recommended
cabozantinib, nivolumab, axitinib, and lenvatinib plus everolimus
as category 1 after tyrosine kinase inhibitor (TKI) treatment for
patients (2). The European Society
of Medical Oncology considers axitinib as an optional treatment
(recommended degree II, B) after TKI treatment (3). In Japan, cabozantinib is not approved
for use at present, and nivolumab and axitinib have become key
drugs as the second-line treatment after TKI treatment. Nivolumab
is a humanized antibody against programmed cell death-1 (PD-1). It
inhibits the binding of PD-1 to its ligands PD-L1 and PD-L2 and
suppresses tumor growth by inducing the proliferation of cancer
antigen-specific T cells and enhancing cytotoxic activity (4). Meanwhile, axitinib is a potent,
selective, second-generation inhibitor of vascular endothelial
growth factor receptor (VEGFR)1, 2 and 3 that blocks VEGFR
signalling at sub-nanomolar concentrations (5).
Axitinib is a TKI that is similar to sunitinib and
pazopanib; thus, axitinib may not be effective after treatment
failure with first-line agents because of the similarity between
axitinib and first-line TKIs. In contrast, the mechanism of action
of nivolumab differs from that of TKIs, so there is a possibility
that it may be effective as second-line treatment for mRCC. To
date, there has been no study comparing the OS, treatment
continuation, and drug cost for nivolumab and axitinib as a
second-line treatment in patients with mRCC after TKI treatment.
The determination of these parameters will demonstrate the
effectiveness, safety, and medical and economic superiority of the
treatment regimen. Eventually, this can guide decision making for
treatment selection. In the present study, OS, treatment
continuation, and the cost of nivolumab and axitinib-the
second-line treatment agents for mRCC-were compared and
examined.
Patients and methods
Patient selection
The present study retrospectively surveyed 26
patients with pathologically confirmed mRCC, who were treated with
nivolumab or axitinib at Ogaki Municipal Hospital (Ogaki, Japan)
between January 2012 and May 2019. Patients who discontinued their
treatment due to financial reasons (1/26) were excluded. Patient
characteristics, adverse events (AEs), treatment period, reasons
for discontinuation or postponement, postponement period, OS, and
drug cost over the treatment duration were analyzed retrospectively
using the data collected from the electronic charts and pharmacy
service records. OS was defined as the interval between the
initiation of nivolumab or axitinib administration as the
second-line treatment and the date of death from any cause. AEs
were evaluated according to the Common Terminology Criteria for
Adverse Events, version 4.0(6), and
the most severe grades during chemotherapy were reported. The drug
cost for one year in clinical practice was calculated based on the
amount of the drug actually used and the dosing period. Personal
information was protected in the aggregated data. The Institutional
Review Board of Ogaki Municipal Hospital, Ogaki, Japan, approved
the present study under the approval no. 20190627-7.
Drug treatment
Before September 2016, axitinib was selected as the
second-line treatment for mRCC because nivolumab was not covered by
insurance. Axitinib was administered at a dose of 5 mg twice a day
for 2 consecutive weeks. If axitinib was tolerated, the dose was
increased as necessary to 7 mg once a day. If tolerability was
observed after two consecutive weeks of administration, the dose of
axitinib was increased as necessary to 10 mg twice a day.
After September 2016, nivolumab was selected as the
second-line treatment for mRCC because it was covered by insurance.
From September 2014 to July 2018, nivolumab was administered at a
dose of 3 mg/kg of body weight, through a 60 min intravenous
infusion every 2 weeks. From August 2018, 240 mg of nivolumab was
administered through a 60-min intravenous infusion every 2
weeks.
Dose modifications were permitted for axitinib but
not for nivolumab.
Statistical analysis
The Mann-Whitney's U test or the χ2 test
of independence (Fisher's exact probability test) was used to
analyze patient characteristics, AEs, and reasons for
discontinuation. The Kaplan-Meier log-rank test was used to compare
OS. P<0.05 was considered to indicate a statistically
significant difference. All statistical analyses were performed
using the EZR software (v1.30; Saitama Medical Center, Jichi
Medical University), which is a graphical user interface for R (The
R Foundation for Statistical Computing) (7).
Results
Patient characteristics
The patient characteristics are summarized in
Table I. The median ages of patients
receiving nivolumab and axitinib were 68 (range: 58-84) and 71
(range: 51-74) years, respectively. Patients were categorized into
the following risk groups (favourable/intermediate/poor) according
to the International mRCC Database Consortium (IMDC): 1/6/2 for
nivolumab-administered patients and 1/12/3 for
axitinib-administered patients (P=0.667, 0.876, 0.834).
 | Table IPatient characteristics. |
Table I
Patient characteristics.
| Characteristic | Nivolumab, n=9 | Axitinib, n=16 | P-value |
|---|
| Age, years | | | |
| Median (range) | 68 (58-84) | 71 (51-74) | 0.869a |
| Sex, n | | | |
|
Male | 8 | 13 | 0.617b |
|
Female | 1 | 3 | |
| Metastatic site,
n | | | 0.264b |
|
Lung | 3 | 10 | |
|
Liver | 3 | 4 | |
|
Bone | 2 | 3 | |
|
Lymph
node | 6 | 6 | |
|
Peritoneal | 3 | 0 | |
|
Recurrence | 2 | 2 | |
|
Left adrenal
gland | 2 | 0 | |
|
Pleura | 1 | 1 | |
| Organization group,
n | | | |
|
Clear
cell | 8 | 15 | 0.187 |
|
Papillary | 1 | 0 | |
| IMDC risk group,
n | | | 0.878b |
|
Favorable | 1 | 1 | |
|
Intermediate | 6 | 12 | |
|
Poor | 2 | 3 | |
| Body surface area,
m2 | | | |
| Median (range) | 1.64 (1.42-1.78) | 1.62 (1.41-2.01) | 0.655a |
| CrCl, mg/ml | | | |
| Median (range) | 41.2 (7.9-69.2) | 55.6
(30.0-123.3) | 0.165a |
| First-line treatment
drugs | | | 0.586b |
|
Sunitinib | 6 | 9 | |
|
Pazopanib | 3 | 4 | |
|
Sorafenib | 0 | 2 | |
|
Temsirolimus | 0 | 1 | |
| Drugs used after
third-line treatment | | | 0.426b |
|
None | 2 | 6 | |
|
Temsirolimus | 1 | 5 | |
|
Sunitinib | 1 | 2 | |
|
Axitinib | 4 | 3 | |
|
Pazopanib | 0 | 5 | |
|
Everolimus | 0 | 3 | |
|
Sorafenib | 0 | 1 | |
|
Nivolumab | 0 | 2 | |
| Treatment ongoing at
data collection (Nivolumab or axitinib) | 2 | 1 | 0.524b |
Treatment duration and reasons for
discontinuation during second-line treatment
Treatment duration and reasons for discontinuation
of second-line treatment are summarized in Table II. The treatment enforcement periods
for the nivolumab- and axitinib-administered groups were 5.4
(range: 1.4-21.3) and 3.4 (range: 0.3-28.1) months, respectively,
and the difference between the two groups was not statistically
significant (P=0.089). The number of nivolumab-administered
patients who discontinued treatment due to progressive disease
(PD), AEs, deterioration of condition, and deterioration in
performance status (PS) was 6, 0, 1 and 0, respectively.
Conversely, the number of axitinib-administered patients who
discontinued treatment due to PD, AEs, deterioration of condition,
and deterioration in PS was 6, 6, 1 and 2, respectively.
Discontinuation of treatment in axitinib-administered patients
owing to AEs included the following observations: Nausea, vomiting,
diarrhea, and cerebral hemorrhage.
 | Table IISecond-line treatment period and
reasons for discontinuation. |
Table II
Second-line treatment period and
reasons for discontinuation.
| Period and
reason | Nivolumab, n=9 | Axitinib, n=16 | P-value |
|---|
| Second-line treatment
period, months |
| Median (range) | 5.4
(1.4-21.3c) | 3.4
(0.3-28.1c) | 0.089a |
| Number of regimens
used after second-line treatment, n |
| Median (range) | 1 (0-2) | 1 (0-4) | 0.598a |
|
Reasons for
discontinuation | | | 0.140b |
|
Progressive
disease | 6 | 6 | |
|
Adverse
events | 0 | 6 | |
|
Deterioration
of condition | 1 | 1 | |
|
Deterioration
in performance status | 0 | 2 | |
|
Treatment
ongoing at data collection | 2 | 1 | |
Postponement period and reasons for
postponement during second-line treatment
The postponement period and reasons for postponement
are summarized in Table III. The
postponement period for the nivolumab- and axitinib-administered
groups were 7 (range: 0-186) and 0 (range: 0-262) days,
respectively, and the difference between the two groups was
statistically significant (P=0.008). The main reasons for
postponement for the nivolumab-administered group were holidays,
increase in blood sugar levels, disease condition deterioration
(ascites and pain control), and poor medical history
(hospitalization for acute myocardial infarction, acute cardiac
insufficieny and angina pectoris), among other reasons.
 | Table IIIPostponement period and reasons for
postponement during second-line treatment |
Table III
Postponement period and reasons for
postponement during second-line treatment
| Period and
reason | Nivolumab
(n=9) | Axitinib
(n=16) | P-value |
|---|
| Postponement
period, days |
| Median (range) | 7 (0-186) | 0 (0-262) | 0.008a |
| Rate of
postponement, % | 16.40 | 13.50 | 0.008b |
| Reasons for
postponement, n | | | 0.025b |
|
Holiday | 7 | 0 | |
|
Adverse
events | 1c | 0 | |
|
Deterioration
of condition | 1d | 1g | |
|
Palliative
surgery | 0 | 2 | |
|
Poor medical
history | 2e | 0 | |
|
Patient's
convenience | 2 | 0 | |
|
Other
reasons | 2f | 0 | |
Adverse events analysis
The major AEs for the nivolumab- and
axitinib-administered groups are summarized in Table IV. The AEs for the
nivolumab-administered group consisted of increased creatinine
levels (44.4%), anemia (33.3%), pruritus (33.3%), rash (33.3%), and
increased creatine kinase levels (33.3%). The AEs for the
axitinib-administered group consisted of anorexia (41.2%),
hoarseness (35.5%), diarrhea (29.4%), hand-foot syndrome (23.5%),
and nausea (23.5%). AEs of grade 3 or higher in the
nivolumab-administered group were AST/ALT increase (1) and creatinine (1), while those in the axitinib-administered
group were nausea (1) and diarrhea
(1).
 | Table IVTreatment-related adverse events
reported in 10% or more of treated patients in either group |
Table IV
Treatment-related adverse events
reported in 10% or more of treated patients in either group
| | Nivolumab, n=9 | Axitinib, n=16 |
|---|
| Event | Any grade number of
patients (%) | Any grade number of
patients (%) |
|---|
| Leucopenia | 1 (11.1) | 0 (0.0) |
| Neutropenia | 1 (11.1) | 1 (6.3) |
| Anemia | 3 (33.3) | 2 (12.5) |
| Increased AST/ALT
level | 2 (22.2) | 1 (5.9) |
| Increased
creatinine level | 4 (44.4) | 0 (0.0) |
| Fatigue | 2 (22.2) | 0 (0.0) |
| Anorexia | 0 (0.0) | 7 (43.8) |
| Nausea | 0 (0.0) | 4 (25.0) |
| Stomatitis | 0 (0.0) | 3 (18.8) |
| Diarrhea | 2 (22.2) | 5 (31.3) |
| Constipation | 1 (11.1) | 1 (6.3) |
| Pruritus | 3 (33.3) | 1 (6.3) |
| Rash | 3 (33.3) | 2 (12.5) |
| Hyperkalemia | 1 (11.1) | 1 (6.3) |
| Increased CPK
level | 3 (33.3) | 0 (0.0) |
| Hypothyroidism | 1 (11.1) | 3 (18.8) |
| HFS | 0 (0.0) | 4 (25.0) |
| Epistaxis | 0 (0.0) | 2 (12.5) |
| Hypertension | 0 (0.0) | 8 (37.5) |
| Hoarseness | 0 (0.0) | 6 (12.5) |
| Proteinuria | 0 (0.0) | 2 (11.8) |
| Hyperglycemia | 1 (11.1) | 0 (0.0) |
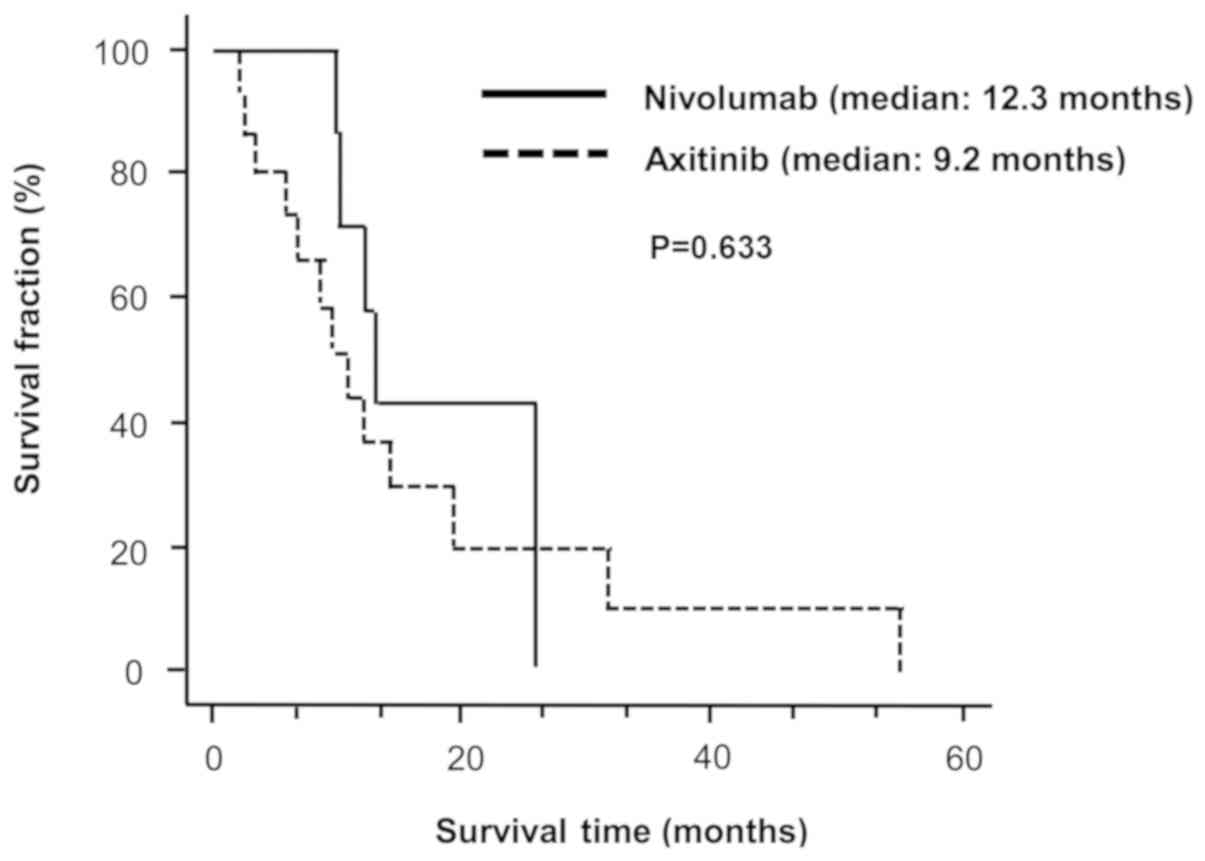
Overall survival
Fig. 1 shows the
Kaplan-Meier survival curves for the OS of patients administered
with nivolumab or axitinib as second-line treatment. The median OS
for nivolumab- (n=9) and axitinib-administered patients (n=16) was
12.3 (range: 1.5-25.5) and 9.2 (range: 2.2-55.0) months,
respectively (log-rank test, P=0.633).
Drug cost
The administration of axitinib and nivolumab to
patients at the dose indicated in the package insert resulted in a
2 week cost of $2,333.9 and $3,918.4, respectively. The one-year
cost estimates for axitinib and nivolumab in clinical practice were
$60,694.2 and $86,544.4, respectively (P=0.017) (Table V).
 | Table VAnnual drug cost |
Table V
Annual drug cost
| Regimens | Standard dose
(calculated at 50 kg) | Single dose drug
cost | One-year cost
estimate | One-year cost
estimate in clinical practice | P-value |
|---|
| Axitinib | 10 mg | $166.7 | $60,846.3 | $60,694.2 | 0.017a |
| Nivolumab | 240 mg | $3,918.4 | $101,877.2 | $86,544.4 | |
Discussion
In this study, we examined the characteristics of
nivolumab and axitinib in terms of overall survival, treatment
continuation, and cost for patients with mRCC. Our results
indicated that despite frequent interruptions in nivolumab
administration and a longer postponement period for the
nivolumab-administered group than for the axitinib-administered
group, both groups exhibit comparable treatment period and OS.
Moreover, the cost for nivolumab administration was higher than
that for axitinib administration.
In the Check Mate 025 study comparing the nivolumab-
and everolimus-administered groups, nivolumab did not extend PFS,
but it did extend OS. A previous study has suggested that the
so-called durable response may produce long effect duration
(1). Another study has revealed that
the effect lasts for approximately one year after the end of
treatment (8). The Japanese subgroup
analysis from the Check Mate 025 study showed that the OS of both
nivolumab- and axitinib-administered groups is higher than that of
the global population (9).
Similarly, in this study, no significant difference in the OS
between nivolumab- and axitinib-administered patients was observed.
This observation may explain the reason for the difference in the
treatment regimens between the Western countries and Japan. The
ESMO Guideline considers axitinib as an optional treatment agent.
However, the NCCN Guideline regards it as the standard drug for
second-line treatment (3,10). In Japan, axitinib, nivolumab and
sorafenib are recommended for both second- and third-line
treatments. In the event of a shortage of TKI and mammalian target
of rapamycin (mTOR) inhibitors, there is a possibility that there
is no difference in the priority between nivolumab and axitinib as
second-line treatment drugs. In this study, various TKI and mTOR
inhibitors, such as unitinib, pazopanib, and everolimus, were used
as third-line treatments. The administration of nivolumab to
patients with mRCC was postponed due to poor medical history or
holidays. However, treatment duration and OS were identical despite
the longer postponement period than that for the
axitinib-administered group. The so-called durable response might
have produced this effect. The sustained response of patients with
mRCC treated with nivolumab has also been observed in phase I and
phase II trials (8,11). In the case of molecularly targeted
drugs such as axitinib, postponement of treatment may lead to a
risk of disease progression.
Immune checkpoint inhibitors exert their clinical
effects by enhancing the antitumor effects of T cell, including
restoration of tumor immunity and development of autoimmune
diseases. Therefore, the expression level and pattern of the AEs is
largely different from those of the conventional molecular targeted
drugs. In this study, results suggested that AE profiles differ
between nivolumab and axitinib. The AE status of the
nivolumab-administered group in our study is similar to that of the
Check Mate 025 study and the study by De Giorgi et al
(1,12). The absence of subjective symptoms,
such as nausea, maintains the quality of life (QOL) of the
nivolumab-administered patients. Immune-related AEs that should be
particularly noted include thyroid dysfunction and type I diabetes,
both of which are also described in this study (8). The administration of axitinib to
patients with mRCC was often stopped due to symptoms such as
nausea, vomiting, and diarrhea. These patients may have exhibited
similar AEs if they used TKIs similar to axitinib as first-line
treatment drugs.
In the UK, the cost-effectiveness assessment of
expensive drugs is conducted by the National Institute for Health
and Clinical Excellence (NICE). NICE did not recommend using
market-authorized nivolumab within the Cancer Drugs Fund to treat
locally advanced, unresectable, or metastatic urothelial carcinoma
in adults who had previously received platinum-containing therapy
(13). The cost-effectiveness of
nivolumab for patients with recurrent/metastatic head and neck
squamous cell carcinoma and advanced non-flat non-small-cell lung
cancer is lower (14,15). In this study, the one-year estimate
of the cost of nivolumab was significantly higher than that of
axitinib in clinical practice (92,559,26 vs. 64,912,49 yen,
respectively). However, the dose of axitinib can be increased to 20
mg/day for patients that present a low blood level elevation, which
can increase the annual drug cost. The cost-effectiveness of using
nivolumab and axitinib in clinical practice is not available;
however, both drugs are expected to be less cost-effective
(13-15).
The results of this study will aid in the selection
of the appropriate second-line treatment drug after TKI treatment.
To guide decision making for the choice of second-line treatment
drug after TKI treatment, we suggest that nivolumab takes
precedence over axitinib for the treatment of mRCC patients with a
medical history, poor general condition, or severe AEs. Considering
that nivolumab is more expensive than axitinib, determining the
effects at an early stage and performing early transition of drug
treatment may reduce the overall drug cost.
For future studies, it will be necessary to
accumulate a considerable number of clinical cases to accurately
determine drug administration period. The number of patients was
limited in this study because it was reported as an initial
experience in a single-center clinical practice setting. In the
future, it is hoped that a positive randomized controlled trial
will be implemented.
These findings provide novel insights into the
characteristics of nivolumab and axitinib for the treatment of
patients with mRCC, and can guide decision making for the choice of
second-line treatment drug after TKI treatment.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
MK, EU, HT and TY conceived and designed this study.
MK acquired the data. MK, EU, HT and TY drafted the manuscript. All
authors read and approved the final manuscript.
Ethics approval and consent to
participate
The present study was approved by the Institutional
Review Board of Ogaki Municipal Hospital (approval no. 20190627-7).
The requirement of informed consent was waived by the Institutional
Review Board.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Motzer RJ, Escudier B, McDermott DF,
George S, Hammers HJ, Srinivas S, Tykodi SS, Sosman JA, Procopio G,
Plimack ER, et al: Nivolumab versus everolimus in advanced
renal-cell carcinoma. N Engl J Med. 373:1803–1813. 2015.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Motzer RJ, Jonasch E, Agarwal N, Bhayani
S, Bro WP, Chang SS, Choueiri TK, Costello BA, Derweesh IH, Fishman
M, et al: Kidney cancer, version 2.2017, NCCN clinical practice
guidelines in oncology. J Natl Compr Canc Netw. 15:804–834.
2017.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Escudier B, Porta C, Schmidinger M,
Riou-Leclercq N, Bex A, Khoo V, Gruenvald V and Horwich A: ESMO
Guidelines Committee. Renal cell carcinoma: ESMO Clinical Practice
Guidelines for diagnosis, treatment and follow-up. Ann Oncol.
27:v58–v68. 2016.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Wang C, Thudium KB, Han M, Wang XT, Huang
H, Feingersh D, Garcia C, Wu Y, Kuhne M, Srinivasan M, et al: In
vitro characterization of the anti-PD-1 antibody nivolumab,
BMS-936558, and in vivo toxicology in non-human primates. Cancer
Immunol Res. 2:846–856. 2014.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Sonpavde G, Hutson TE and Rini BI:
Axitinib for renal cell carcinoma. Expert Opin Investig Drugs.
17:741–748. 2008.PubMed/NCBI View Article : Google Scholar
|
|
6
|
US Department Of Health And Human
Services: Common terminology criteria for adverse events (CTCAE)
version 4.0. United States, National Cancer Institute, Sept 15,
2009. http://www.acrin.org/Portals/0/Administration/Regulatory/CTCAE_4.02_2009-09-15_QuickReference_5X7.pdf.
|
|
7
|
Kanda Y: Investigation of the freely
available easy-to-use software ‘EZR’ for medical statistics. Bone
Marrow Transplant. 48:452–458. 2013.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Motzer RJ, Rini BI, McDermott DF, Redman
BG, Kuzel TM, Harrison MR, Vaishampayan UN, Drabkin HA, George S,
Logan TF, et al: Nivolumab for metastatic renal cell carcinoma:
Results of a randomized phase II trial. J Clin Oncol. 33:1430–1407.
2015.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Tomita Y, Fukasawa S, Shinohara N,
Kitamura H, Oya M, Eto M, Tanabe K, Kimura G, Yonese J, Yao M, et
al: Nivolumab versus everolimus in advanced renal cell carcinoma:
Japanese subgroup analysis from the checkmate 025 study. Jpn J Clin
Oncol. 47:639–646. 2017.PubMed/NCBI View Article : Google Scholar
|
|
10
|
National Comprehensive Cancer Network:
Clinical practice guidelines in oncology: Kidney Cancer. version 2.
JNCCN · June 2017.
|
|
11
|
McDermott DF, Drake CG, Sznol M, Choueiri
TK, Powderly JD, Smith DC, Brahmer JR, Carvajal RD, Hammers HJ,
Puzanov I, et al: Survival, durable response, and long-term safety
in patients with previously treated advanced renal cell carcinoma
receiving nivolumab. J Clin Oncol. 33:2013–2020. 2015.PubMed/NCBI View Article : Google Scholar
|
|
12
|
De Giorgi U, Cartenì G, Giannarelli D,
Basso U, Galli L, Cortesi E, Caserta C, Pignata S, Sabbatini R,
Bearz A, et al: Safety and efficacy of nivolumab for metastatic
renal cell carcinoma: Real-World results from an expanded access
programme. BJU Int. 123:98–105. 2019.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Grimm SE, Armstrong N, Ramaekers BLT,
Pouwels X, Lang S, Petersohn S, Riemsma R, Worthy G, Stirk L, Ross
J, et al: Nivolumab for treating metastatic or unresectable
urothelial cancer: An evidence review group perspective of a NICE
single technology appraisal. Pharmacoeconomics. 37:655–667.
2019.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Zargar M, McFarlane T, Chan KKW and Wong
WWL: Cost-Effectiveness of nivolumab in recurrent metastatic head
and neck squamous cell carcinoma. Oncologist. 23:225–233.
2018.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Matter-Walstra K, Schwenkglenks M, Aebi S,
Dedes K, Diebold J, Pietrini M, Klingbiel D, von Moos R and
Gautschi O: Swiss Group for Clinical Cancer Research: A
cost-effectiveness analysis of nivolumab versus docetaxel for
advanced nonsquamous NSCLC including PD-L1 testing. J Thorac Oncol.
11:1846–1855. 2016.PubMed/NCBI View Article : Google Scholar
|