Introduction
Teratomas may occur in various parts of the body.
They may originate from embryonic and primordial germ cells,
embryonic mesenchymal stem cells or embryonic carcinoma cells, and
usually contain three embryonic components, which have evolved from
the inner, middle and outer primitive embryonic layers (1-3).
Retroperitoneal teratomas are located behind the
peritoneum and as such may be obscured by various organs in the
abdomen. The majority of patients present with digestive tract
symptoms and are easily misdiagnosed. Therefore, retroperitoneal
teratoma may be included in the differential diagnosis of gastric
stromal tumors and this can be used as a systematic and
comprehensive study (4).
The most common method of treatment for
retroperitoneal teratomas is surgical. In the present case,
surgical resection was performed and the pathological examination
revealed a retroperitoneal mature cystic teratoma. Retroperitoneal
teratomas are mostly benign, but a few are malignant; therefore,
regular re-examination is required in order to optimize
postoperative recovery and avoid tumor recurrence (5).
The follow-up results of the present case indicate
that the current condition was stable and there was no evidence of
recurrence; however, it is necessary to determine whether follow-up
should be continued for a long period of time according to the
patient's recovery. Therefore, it is crucial for physicians to
report and share their experience with the treatment of difficult
and/or rare cases (6,7).
Case report
A 39-year-old female patient was admitted to the
Department of Gastrointestinal Surgery of The First Affiliated
Hospital of the University of South China with abdominal distension
as the main symptom. A laboratory examination revealed mildly
elevated levels of α-fetoprotein (AFP; 8.230 ng/ml; normal range,
0.0-7.0 ng/ml), while other routine examinations did not reveal any
significant abnormalities. No abdominal abnormalities were observed
on physical examination by the abdominal specialist.
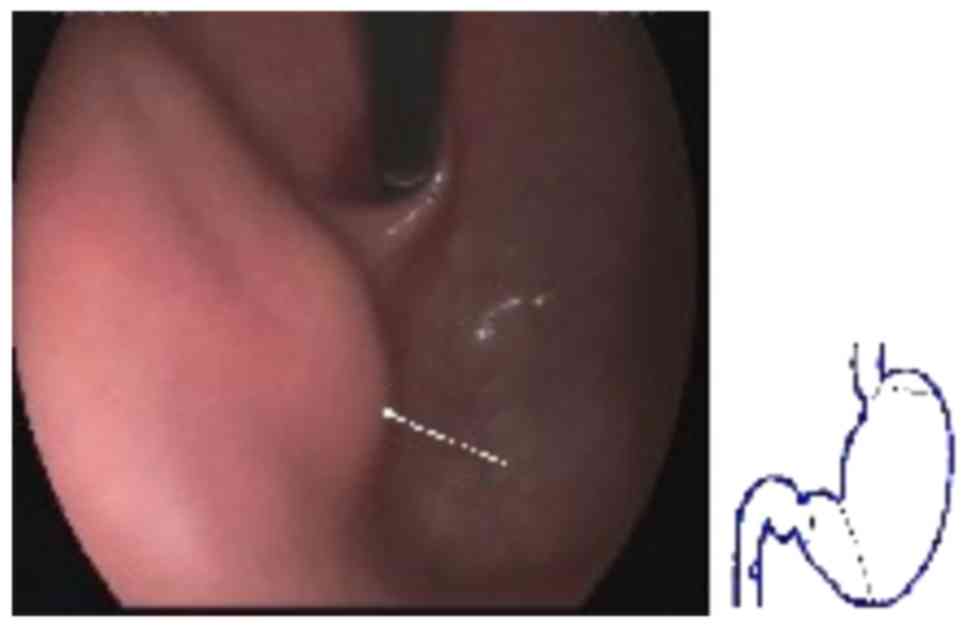
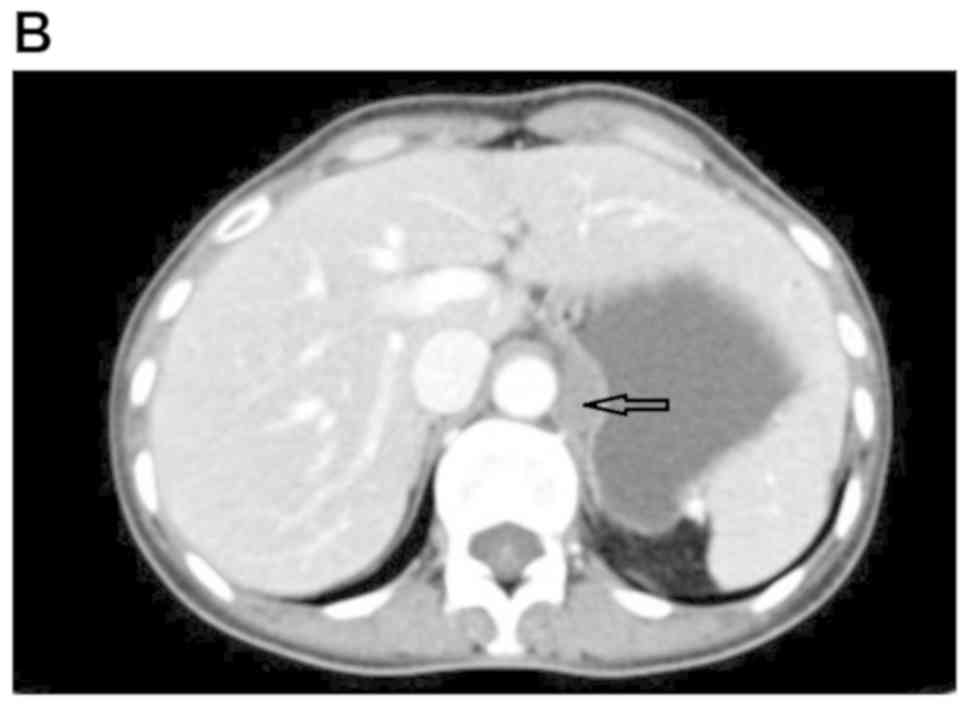
An auxiliary examination raised the suspicion of a
gastric stromal tumor (GST) by electronic gastroscopy (Fig. 1) and by dual-phase enhanced computed
tomography (CT) of the small intestine (Fig. 2).
According to the patient's medical history and
preoperative examination, the proximal tumor and lateral protrusion
of the lesser curvature of the stomach were considered to be close
to the abdominal aorta; thus, endoscopic submucosal dissection was
considered as a high-risk surgical procedure. Following completion
of the preoperative preparation, laparoscopy-assisted gastric tumor
resection was performed. A 2x2-cm large cystic mass was identified
intraoperatively, located close to the abdominal aorta and behind
the peritoneum of the lesser curvature of the stomach (Fig. 3). The gastric fundus and a bulge of
the gastric body were visible during the intraoperative endoscopic
examination, and the bulge disappeared when the stomach was lifted;
the cystic mass was then completely removed.
Fast frozen biopsy examination revealed that the
cyst was 2x1.5x0.3 cm in size, it was filled with mucus, it had a
smooth, pale and soft surface, and the cyst wall had a thickness of
0.1-0.2 cm. Thus, this was suspected to be a mature cystic
teratoma. No other mass or protuberance was identified on
gastroscopy. The results of the postoperative pathological
examination confirmed the diagnosis of retroperitoneal mature
cystic teratoma (Fig. 4). Mature
cystic teratomas are usually benign and contain several components,
including bone, cartilage, teeth, adipose tissue, nerve tissue and
other components; in the present case, calcification was also
observed (Figs. 2 and 4). The patient was discharged from the
hospital without obvious abdominal distension. There was no
abdominal distension or other signs or symptoms on the 6-month
postoperative follow-up.
Discussion
A teratoma is a tumor derived from germ cells that
may contain multiple types of tissue (1-3). It
may develop anywhere in the body, but is most commonly encountered
as sacrococcygeal, retroperitoneal, ovarian and testicular
teratoma. Teratomas may be classified into three main types, namely
immature, mature and monoembryonic. Mature retroperitoneal
teratomas are mostly benign and malignant, accounting for 26%
(8,9). The early symptoms of retroperitoneal
teratoma may not be readily evident and are non-specific; thus,
they are not easily identifiable and the tumor is often
misdiagnosed (10,11). Typically, symptoms become more
pronounced when the tumor grows to a large size and compresses
adjacent tissues or organs (12,13).
However, the tumor in the present case was located in the lesser
curvature of the stomach and was compressed, resulting in
herniation of the lesser curvature of the stomach near the fundus.
Preoperative CT examination and ultrasound gastroscopy did not
reveal the presence of a tumor, and the abdominal examination
revealed no obvious signs. In addition, such cases are rarely
reported clinically. For all these reasons, retroperitoneal
teratoma may be easily misdiagnosed as a GST (14). In the present case, the abdominal
distension had been present for >10 years, and there was no
recurrence on the 6-month postoperative follow-up. The cause of
abdominal distension in this patient was considered to be the
compression of the lesser gastric curvature by the tumor.
At its early stages, retroperitoneal teratoma often
lacks characteristic clinical manifestations (5). CT and magnetic resonance imaging (MRI)
are currently the main imaging examinations for the diagnosis of
teratomas. CT scans can determine the size, location, integrity,
invasion of adjacent tissues and organs, and the presence of lymph
node metastasis. An MRI scan not only clearly indicates the
structure of the tumors and surrounding soft tissues and blood
vessels, but can also reveal multiple organ structures. MRI is
often superior to other examinations for preoperative staging of
tumors such as rectal cancer, and the localization and qualitative
diagnosis of severe abdominal lesions, as it can provide a
favorable preoperative basis for selecting the specific surgical
treatment plan for the follow-up (15). Moreover, due to the lack of CT
three-dimensional images in the present case, the associations of
the tumor with the surrounding organs and structures could not be
observed from different angles and levels, and the accurate
location of the tumor could not be stereoscopically demonstrated,
which may also adversely affect the accuracy of the preoperative
diagnosis. For intraperitoneal tumors, when a CT scan is not
available, it is recommended that an MRI examination be performed
to reach a definitive diagnosis. However, in the present case, the
tumor was located behind the peritoneum, and was deeply seated and
complex. In such cases, a preoperative MRI may not be able to make
a definitive diagnosis due to the tumor being concealed by the
stomach and other organs situated in front of it. Furthermore, an
MRI scan is also associated with certain limitations: A relatively
long examination time, a poor dynamic scanning effect of organ
enhancement compared with CT, relatively high requirements
regarding the patient's cooperation and the poor diagnosis of
dehydrated organs and lesions; in addition, some patients, such as
those with pacemakers, cannot be subjected to an MRI. Endoscopic
ultrasound (EUS)-fine-needle aspiration (FNA) is a method of
fine-needle puncture biopsy guided by endoscopic ultrasonography
for the extraction of cells and tissue fragments for pathological
examination, in order to help determine the nature of the lesion.
Using this method, the pathological characteristics may be
evaluated in detail, and pathological tissues may also be obtained
for cytological and immunohistochemical examinations, in order to
determine the nature, origin and classification of the lesion
(16,17). As a result, the initial treatment
regimens often change. For medical units that have developed
EUS-FNA technology, EUS-FNA can be improved according to the needs
of the patients to further select specific treatment plans.
Although EUS-FNA has a number of advantages, it may not be applied
in all cases. In the present case, the tumor was close to the
abdominal aorta, and it was deep-seated with limited operating
field; furthermore, the risk of puncture associated with cystic
lesions is higher compared with that for solid lesions. Therefore,
in our patient, the preoperative incomplete MRI scan and EUS-FNA
may have also contributed to the misdiagnosis.
Surgical resection remains the most effective
treatment for retroperitoneal teratomas (18). Laparoscopic teratoma resection is
safe and feasible due to its relatively advanced technology,
minimal associated trauma, rapid patient recovery and preservation
of capsule integrity of the mature cystic teratoma (19,20). The
complete resection of benign teratomas may prevent malignant
transformation and recurrence (21).
If the intraoperative fast frozen biopsy examination indicates
malignancy, the surgical resection scope should be expanded. If the
intraoperative tumor is found to invade the surrounding tissues or
organs, multiple departments can cooperate to carry out multiple
organ resection, supplemented by radiotherapy and chemotherapy to
improve treatment efficacy (22,23). In
the present case, the combined findings of CT and other
preoperative examinations could not rule out the possibility of an
adrenal tumor, although GST was clinically suspected and despite
the fact that there was no history of hypertension. Preoperative
and intraoperative blood pressure monitoring revealed stable blood
pressure, and an adrenal tumor was thus considered unlikely.
However, for patients with irregular preoperative blood pressure,
obvious intraoperative blood pressure changes and marked
fluctuations, adrenal tumors should be considered in the
differential diagnosis, and appropriate preoperative and
intraoperative measures should be taken.
A teratoma is a congenital neoplasm originating from
germ cells. Therefore, for patients diagnosed with teratoma before
or after surgery, an ultrasound examination of the reproductive
system should be performed to avoid misdiagnosis. During follow-up
in the present case, no reproductive teratoma was identified on
gynecological ultrasound examination, although a regular follow-up
was still recommended. Laboratory examinations of mature
retroperitoneal teratomas are mostly negative. However, the
increased levels of AFP, carcinoembryonic antigen (CEA) and human
chorionic gonadotropin (HCG) may be of value for the diagnosis and
prognosis of immature teratomas (24,25). The
AFP levels of this patient were mildly elevated, and the result of
the pathological examination revealed a mature cystic teratoma.
Although there were no obvious indications for radiotherapy and
chemotherapy at the time, an abdominal CT examination, and
measurement of AFP, CEA and hCG levels should be performed
regularly after surgery to prevent tumor recurrence (26,27).
In the present case, the tumors were similar to the
gastric fundus of the lesser gastric curvature, with no obvious
space between the tumor and the stomach as they are in close
proximity, and it was difficult to detect on preoperative
examination. Retroperitoneal tumors may be complex, and the tumor
in the present case was deep-seated and fixed. The front surface of
the tumor was also concealed by the gastric fundus, and there were
no obvious changes observed with respiration and change in body
position. Moreover, ultrasound gastroscopy, EUS-FNA, B-mode
ultrasonography of the abdomen and MRI were not performed prior to
surgery, and the histological type of the lesion could not be
determined. In addition, this type of clinical case is rare, and
the clinician's diagnostic experience was relatively insufficient,
which is one of the main reasons for the misdiagnosis.
In conclusion, retroperitoneal teratoma must be
included in the differential diagnoses of GST, and may improve
comprehensive learning. The aim of the present case report was to
improve our current understanding of the clinical presentation of
GSTs and retroperitoneal teratomas in order to reduce the
likelihood of clinical misdiagnosis.
Acknowledgements
Not applicable.
Funding
The present study was supported by the key guiding
project of Health and Health Committee of Hunan Province of China
(grant no. 20201919 ).
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
LG was responsible for the conception, content,
image collection and manuscript writing. QH was responsible for
providing guidance with manuscript writing, revision and
proofreading. Both authors contributed to the writing of the
manuscript. Both authors have read and approved the final version
of the manuscript.
Ethics approval and consent to
participate
This case study was approved by the Ethics Committee
of the First Affiliated Hospital, University of South China. The
patient provided consent for inclusion in this study.
Patient consent for publication
Written informed consent was obtained from the
patient for the publication the case details and any associated
images.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Pandit N, Awale L and Jaiswal LS: Giant
calcified retroperitoneal teratoma. Indian J Surg Oncol. 9:436–437.
2018.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Huang X, Liu B and Xie L: Giant primary
retroperitoneal teratoma in an adult female patient: A case report.
Oncol Lett. 6:460–462. 2013.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Skinovsky J, Tsumanuma FK, Sigwalt MF,
Panegalli-Filho F, Kawakubo AM, Rolim BG and Godoy LA: Thirty
kilograms giant retroperitoneal teratoma: Case report. Arq Bras Cir
Dig. 29:128–129. 2016.PubMed/NCBI View Article : Google Scholar : (In English).
|
|
4
|
Huľová S, Aziri R, Chovanec M, Mardiak J,
Mego M and Pinďák D: Clinical management and outcome in extreme
retroperitoneal growing teratoma syndrome of testicular
origin-clinical management and effect of the treatment. Klin Onkol.
32:129–132. 2019.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Richie JP: Re: Post-chemotherapy
retroperitoneal teratoma in nonseminomatous germ cell tumors: Do
predictive factors exist? Results from a national multicenter
study. J Urol. 199(894)2018.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Chalhoub K, Abou Zahr R, Mansour E, Aoun M
and Jabbour M: Primary mature cystic teratoma compressing the
prostate in a 28-year-old male: A case report and literature
review. Case Rep Urol. 2019(8970172)2019.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Morotti A, Busso M, Consiglio Barozzino M,
Cinardo P, Angelino V, Familiari U, Veltri A and Guerrasio A:
Detection and management of retroperitoneal cystic lesions: A case
report and review of the literature. Oncol Lett. 14:1602–1608.
2017.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Cvijanović R, Ivanov D and Zivojinov M:
Thoracoabdominal teratoma-rare location. Med Pregl. 64:89–92.
2011.PubMed/NCBI View Article : Google Scholar : (In Serbian).
|
|
9
|
Li S, Liu Z, Dong C, Long F, Liu Q, Sun D,
Gao Z and Wang L: Growing teratoma syndrome secondary to ovarian
giant immature teratoma in an adolescent girl: A case report and
literature review. Medicine (Baltimore). 95(e2647)2016.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Maenosono R, Saito K, Ibuki N, Takahara K,
Inamoto T, Nomi H and Azuma H: A case of retroperitoneal teratoma
difficult to distinguish from adrenal tumor. Hinyokika Kiyo.
63:525–528. 2017.PubMed/NCBI View Article : Google Scholar : (In Japanese).
|
|
11
|
Tong HX, Liu WS, Jiang Y, Liu JU, Zhou JJ,
Zhang Y and Lu WQ: Giant retroperitoneal bronchogenic cyst
mimicking a cystic teratoma: A case report. Oncol Lett.
9:2701–2705. 2015.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Ma Y, Zheng J, Feng J, Zhu H, Xiao X and
Chen L: Ectopic nephrogenic rests in children: A series of 13 cases
in a single institution. Pediatr Blood Cancer.
65(e26985)2018.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Lee KH, Song MJ, Jung IC, Lee YS and Park
EK: Autoamputation of an ovarian mature cystic teratoma: A case
report and a review of the literature. World J Surg Oncol.
14(217)2016.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Goel S, Aeron R, Goel A and Singhai A:
Retroperitoneal teratoma simulating giant adrenal myelolipoma: A
diagnostic puzzle. BMJ Case Rep 2017: pii: bcr-2017-221762,
2017.
|
|
15
|
Cheng W, Qi Y, Wang B, Tian L, Huang W and
Chen Y: Characteristics and computed tomography evaluation of
primary retroperitoneal tumours: Report of 113 cases. Ann R Coll
Surg Engl. 99:55–59. 2017.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Val-Bernal JF, Martino M, Terán A, Yllera
E and Castro-Senosiain B: Endoscopic ultrasound-guided fine-needle
aspiration cytology in the diagnosis of leiomyomas of the
gastrointestinal tract. Rev Esp Patol. 52:154–162. 2019.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Matsumoto K, Takeda Y, Onoyama T, Kawata
S, Kurumi H, Koda H, Yamashita T and Isomoto H: Endoscopic
ultrasound-guided fine-needle aspiration biopsy-Recent topics and
technical tips. World J Clin Cases. 7:1775–1783. 2019.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Kriger AG, Berelavichus SV, Son AI, Gorin
DS, Ikramov RZ and Kalinin DV: Robot-assisted operations for
non-organ retroperitoneal tumors. Khirurgiia (Mosk). 24–28.
2015.PubMed/NCBI View Article : Google Scholar : (In Russian).
|
|
19
|
Lv Z, Bai X, Sheng Q, Liu J and Wu Y: A
case report of a giant mature teratoma of the thyroid gland in a
young girl. Medicine (Baltimore). 98(e14703)2019.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Li H, Zhao T, Wei Q, Yuan H, Cao D, Shen
P, Liu L, Zeng H and Chen N: Laparoscopic resection of a huge
mature cystic teratoma of the right adrenal gland through
retroperitoneal approach: A case report and literature review.
World J Surg Oncol. 13(318)2015.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Jones VS and Burns CR: Operative
considerations in pediatric retroperitoneal teratomas-a review. Eur
J Pediatr Surg. 23:265–269. 2013.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Narasimhan V, Smith M, Claydon M, Wale R
and Warrier S: Incidental large retroperitoneal teratoma in a
patient with colorectal carcinoma. ANZ J Surg. 88:1350–1352.
2018.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Kim JH, Lee TS, Oh HK and Choi YS: A case
of mucinous adenocarcinoma arising from retroperitoneal teratoma
treated with chemoradiation. J Gynecol Oncol. 20:126–128.
2009.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Seike T, Kagaya T, Komura T, Nakai R,
Shimizu Y, Omura H, Ohta H, Ohyama S, Kawashima A and Unoura M: A
rapidly growing, large, mature retroperitoneal teratoma in an adult
male patient. Nihon Shokakibyo Gakkai Zasshi. 114:1008–1014.
2017.PubMed/NCBI View Article : Google Scholar : (In Japanese).
|
|
25
|
Sasi W, Ricchetti GA, Parvanta L and
Carpenter R: Giant mature primary retroperitoneal teratoma in a
young adult: Report of a rare case and literature review. Case Rep
Surg. 2014(930538)2014.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Hoang VT, Trinh CT, Le TB and Le TK:
Recurrence of retroperitoneal mature cystic teratoma in an adult: A
case report. Radiol Case Rep. 14:692–696. 2019.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Li J, Gong P, Liu F, Sun P and Wu C:
Retroperitoneal cystic immature teratoma: A case report. Oncol
Lett. 10:1023–1025. 2015.PubMed/NCBI View Article : Google Scholar
|