Introduction
Elental® is an elemental diet (ED) with a
high L-glutamine content that has been used in Japan as a treatment
for individuals who are malnourished, patients with cancer, and
patients with inflammatory bowel disease (1-5).
This diet has an easily digestible nutritional formula that
combines 18 amino acids, carbohydrates, vitamins and minerals, with
minimal fat content (1,5). Elental® has been reported to
be useful in the treatment of Crohn's disease, as it can suppress
bowel inflammation and allow the bowel to rest (6-9).
It has also been suggested that Elental® consumption may
lower the incidence of chemotherapy-related hematological toxicity
in patients with gastric cancer, and may decrease pancreatic pain
in patients with chronic pancreatitis (10,11). It
was also reported that Elental® may be useful for the
management of chemotherapy-induced mucositis in patients with
various types of cancer (12-16).
Miki et al reported that the amino acid components of
Elental® increased the survival rate of rats with acute
liver injury, by lowering the expression of nitric oxide synthase
and tumor necrosis factor (TNF)-α; it was also found to inhibit the
expression of inflammatory mediators and nuclear factor (NF)-κB
activation in cultured hepatocytes in vitro (17).
Elental® has been used for the treatment
of malnutrition in patients with oral squamous cell carcinoma
(OSCC) in recent years, and our clinical studies have revealed its
healing effects on oral mucositis and dermatitis in patients with
OSCC who were receiving chemotherapy and radiotherapy (18,19).
Recently, it was also reported that Elental® reduced
chemotherapy-induced oral mucositis and dermatitis in hamster and
mouse models (20,21). In addition, our in vitro
studies revealed that Elental® may accelerate the
recovery from 5-fluorouracil (5-FU)-induced oral mucositis and
dermatitis through the induction of fibroblast growth factor
(20). Moreover, it was demonstrated
that Elental® suppresses the expression of
pro-inflammatory cytokines, such as TNF-α, interleukin (IL)-1β and
IL-6, in keratinocytes by inhibiting NF-κB (21). However, the detailed mechanisms of
its action in healing wounded regions, mucositis and dermatitis
remain unclear.
Interestingly, in our clinical studies it was
observed that Elental® tends to reduce
chemotherapy-induced oral mucositis more effectively by oral
administration (OA) rather than by nasal administration. Therefore,
it was hypothesized that Elental® may affect
chemotherapy-induced oral mucositis directly, as well as indirectly
through gut immunity in the intestine/stomach. However, it remains
unclear whether the oral intake of ED can act directly on
chemotherapy-induced oral mucositis or dermatitis.
The aim of the present study was to investigate
whether Elental® directly affects chemotherapy-induced
and mechanically induced dermatitis or raw wound areas in a mouse
model, and to elucidate the possible mechanism underlying the
action of Elental® in the wound healing process.
Therefore, the effects of Elental® on the growth and
migration of healthy and 5-FU-treated keratinocytes were examined
in vitro.
Materials and methods
Animals
A total of 24 female athymic nude mice, aged 4 weeks
and weighing 20-25 g, with a CAnN.Cg-Foxnlnu/CrlCrlj genetic
background, were purchased from CLEA Japan, Inc. The mice were
housed in a pathogen-free, sterile and temperature-controlled
environment under a 12 h light/dark cycle, and they were provided
access to water and food ad libitum. All procedures
including animal handling and the experimental protocols were
conducted according to the guidelines approved by the Ethical
Committee for Animal Experimentation of Yamaguchi University
(Yamaguchi, Japan).
Induction of experimental dermatitis
and raw wound areas
A total of 12 nude mice were used for the dermatitis
model. Dermatitis was induced by two intraperitoneal (i.p.)
administrations of 60 mg/kg 5-FU (Wako Pure Chemical Industries,
Ltd.) on the first and third days of the experiment, together with
superficial scratching of the dorsal skin with a metal brush under
anesthesia (pentobarbital sodium, 30 mg/kg, i.p.;
Somnopentyl®, Kyoritsu Seiyaku Co., Ltd.), until
erythematous changes in the skin were observed (Fig. 1A), on the second and third days of
the experiment. Similarly, raw wound areas were also induced in 12
nude mice by two i.p. administrations of 60 mg/kg 5-FU on the first
and third days of the experiment, followed by dermabrasion of the
dorsal skin with a surgical knife (no. 15) under
Somnopentyl® anesthesia on the second day of the
experiment (Fig. 1B).
 | Figure 1Induction of dermatitis or raw wound
areas in a mouse model. (A) Dermatitis on nude mouse dorsal skin
was induced by the intraperitoneal (i.p.) injection of 5-FU (60
mg/kg, i.p.) on days 1 and 3, along with mechanical trauma on days
2 and 3 of the experiment. There were three experimental groups as
follows: The 5-FU + abrasion group received saline only (1
ml/body/day; n=4); the Elental® TA group received
Elental® by TA (18 kcal/100 g body weight/day; n=4); and
the Elental® OA group received Elental® by OA
(18 kcal/100 g body weight/day; n=4); all administrations were
performed daily, starting from day 3 of the experiment until the
mice were sacrificed. (B) Raw wound areas on nude mouse dorsal skin
were induced by i.p. injection of 5-FU (60 mg/kg, i.p.) on days 1
and 3 of the experiment, followed by dermabrasion with a surgical
knife on day 2 of the experiment. There were three experimental
groups as follows: The 5-FU + dermabrasion group received saline
only (1 ml/body/day; n=4); the Elental® TA group
received Elental® by TA (18 kcal/100 g body weight/day;
n=4), and the Elental® OA group received
Elental® by OA (18 kcal/100 g body weight/day; n=4); all
administrations were performed daily, starting from day 3 of the
experiment until the mice were sacrificed. 5-FU, 5-fluorouracil;
OA, oral administration; TA, topical application. |
In vivo experimental groups
The experimental design of our in vivo study
is outlined in Fig. 1. A total of 6
groups of nude mice were set up (n=4/group), as described below,
with dermatitis induced by 5-FU + abrasion, or raw wound areas
induced by 5-FU + dermabrasion. Briefly, the 5-FU + abrasion and
the 5-FU + dermabrasion groups served as the untreated controls in
this experiment, and they received only topical application of
saline (1 ml/body/day) from the third day of the experiment
onwards. Elental® was purchased from EA Pharma Co., Ltd.
The OA group and the topical application (TA) group received
Elental® (18 kcal/100 g body weight/day), which was
administered daily, either orally or topically, from the third day
of experiment onwards until the wounded area was almost completely
healed. All mice were sacrificed using an overdose of
Somnopentyl® (sodium pentobarbital, 200 mg/kg; Merck
& Co., Inc.) at the end of the experiment. The healing of the
dermatitis or raw areas of each mouse was assessed daily and the
affected area was measured. The area of each lesion was calculated
by multiplying the length (mm) x width (mm).
Cell lines and cell culture
The human oral keratinocyte cell line, HOK, was
purchased from ScienCell Research Laboratories. The cells were
cultured in Oral Keratinocyte Medium (OKM)-New Zealand Origin BPE
medium (OKM-NZ; cat. no. 2611-NZ; ScienCell Research Laboratories)
at 37˚C in a humidified atmosphere containing 5% CO2.
OKM-NZ complete medium consists of OKM basal medium supplemented
with 1% oral keratinocyte growth supplement-New Zealand Origin BPE
(OKGS-NZ, cat. no. 2652NZ; ScienCell Research Laboratories) and 1%
penicillin/streptomycin solution (cat. no. 0503; ScienCell Research
Laboratories).
Cell proliferation assay
Cells (5x103 cells per well) were seeded
on 96-well plates (Becton Dickinson Labware) in OKM-NZ complete
medium. After 24 h, the medium was exchanged for OKM-NZ medium
containing 0 or 1% OKGS-NZ growth supplement, or OKM-NZ medium
containing 1% OKGS-NZ and 5-FU (final concentration, 2 µg/ml).
After 24 h, the cells were treated with different concentrations of
Elental® (0, 0.1, 0.5, 1, 5, 10, 50 or 100 µg/ml)
dissolved in OKM-NZ medium containing 0 or 1% OKGS-NZ growth
supplement (Fig. 2). After a further
24 h, MTT (25 µl/well) was added to the 96-well plate and incubated
for 4 h at 37˚C. Next, the culture medium was removed and replaced
with dimethyl sulfoxide (100 µl/well), and the absorbance was
measured with a spectrophotometer (BioRad Laboratories, Inc.) at
490 nm. Each treatment group was examined for its cell
proliferation ability. All assays were performed in triplicate.
Cell migration assay
The cell migration assay was performed using a
Boyden chamber, according to the manufacturer's instructions (Neuro
Probe). Briefly, 25 µl OKM-NZ medium containing 0 or 1% OKGS-NZ
growth supplement plus different concentrations of
Elental® (0, 0.1, 0.5, 1, 5, 10, 50 or 100 µg/ml) was
added as chemoattractant in the lower chamber. Next,
5x103 cells in 50 µl OKM-NZ medium containing 0% OKGS-NZ
were seeded on a gelatin-coated polycarbonate membrane in the upper
chamber. After the cells had been incubated for 24 h at 37˚C, the
polycarbonate membrane was washed three times at room temperature
(2 min/wash) with phosphate-buffered saline, and any cells on the
top surface of the polycarbonate membrane were removed using a
cotton swab. Cells adhering to the lower surface were fixed with
methanol for 15 min at room temperature, stained with hematoxylin
solution for 20 min at room temperature, and counted under an
optical microscope (BX51; Olympus Corporation) in five
predetermined fields (magnification, x200). All assays were
independently repeated at least three times.
Statistical analysis
All data are expressed as means ± standard deviation
(SD). The significance of the experimental results was determined
using one-way analysis of variance (ANOVA) and Tukey-Kramer
multiple comparisons tests. The differences were considered to be
statistically significant when P<0.05.
Results
Effect of Elental® on
dermatitis of mouse dorsal skin
To induce dermatitis on the dorsal skin in mice,
5-FU administration and mechanical trauma were used. Ulcerated skin
tissue was observed after the second mechanical irritation (on day
3). As shown in Fig. 3A, the
Elental® TA and OA groups exhibited better healing rates
compared with the untreated control group (5-FU + abrasion). After
7 days of treatment (10th day of the experiment), the affected
dorsal area had completely healed in the Elental® OA
group, whereas the wound persisted in the Elental® TA
group.
Effect of Elental® on raw
wound areas of mouse dorsal skin
The administration of 5-FU and dermabrasion with a
surgical knife was used to induce raw wound areas on mouse dorsal
skin. Compared with healing in the 5-FU + dermabrasion group
(untreated control), the Elental® TA and OA groups
exhibited better healing of the wounded areas (Fig. 3B). After 10 days of treatment (12th
day of the experiment), Elental® OA treatment had
completely healed the raw wound area, whereas Elental®
TA treatment had not.
Effect of Elental® on human
oral keratinocyte cell morphology and proliferation
The morphology of HOKs cultured in OKM-NZ complete
medium with or without Elental® was examined. No
differences in morphology were detected between untreated and
Elental®-treated HOKs (Fig.
4). An MTT assay was used to measure the growth rate of the
Elental®-treated and untreated HOKs. As shown in
Fig. 5, the growth rate of
Elental® (0.1-100 µg/ml)-treated HOKs was higher
compared with that of untreated HOKs after 24 h of culture, under
both nutritionally poor conditions (medium with 0% OKGS-NZ growth
supplement) and nutrient-rich or substantial conditions (medium for
the optimal growth of cells, with 1% OKGS-NZ). Moreover, a higher
concentration of Elental® (5-100 µg/ml) was able to
stimulate the proliferation of 5-FU-pretreated (2 µg/ml) damaged
HOKs, under both nutritionally poor and substantial conditions
after 24 h of treatment. Among the concentrations tested, 5 µg/ml
Elental® exerted the most prominent proliferative
effect, both in healthy and 5-FU-damaged HOKs. Briefly,
Elental® exerted a growth-promoting effect on all cells,
even on damaged cells or cells cultured under nutritionally poor
conditions.
Effect of Elental® on
migration ability
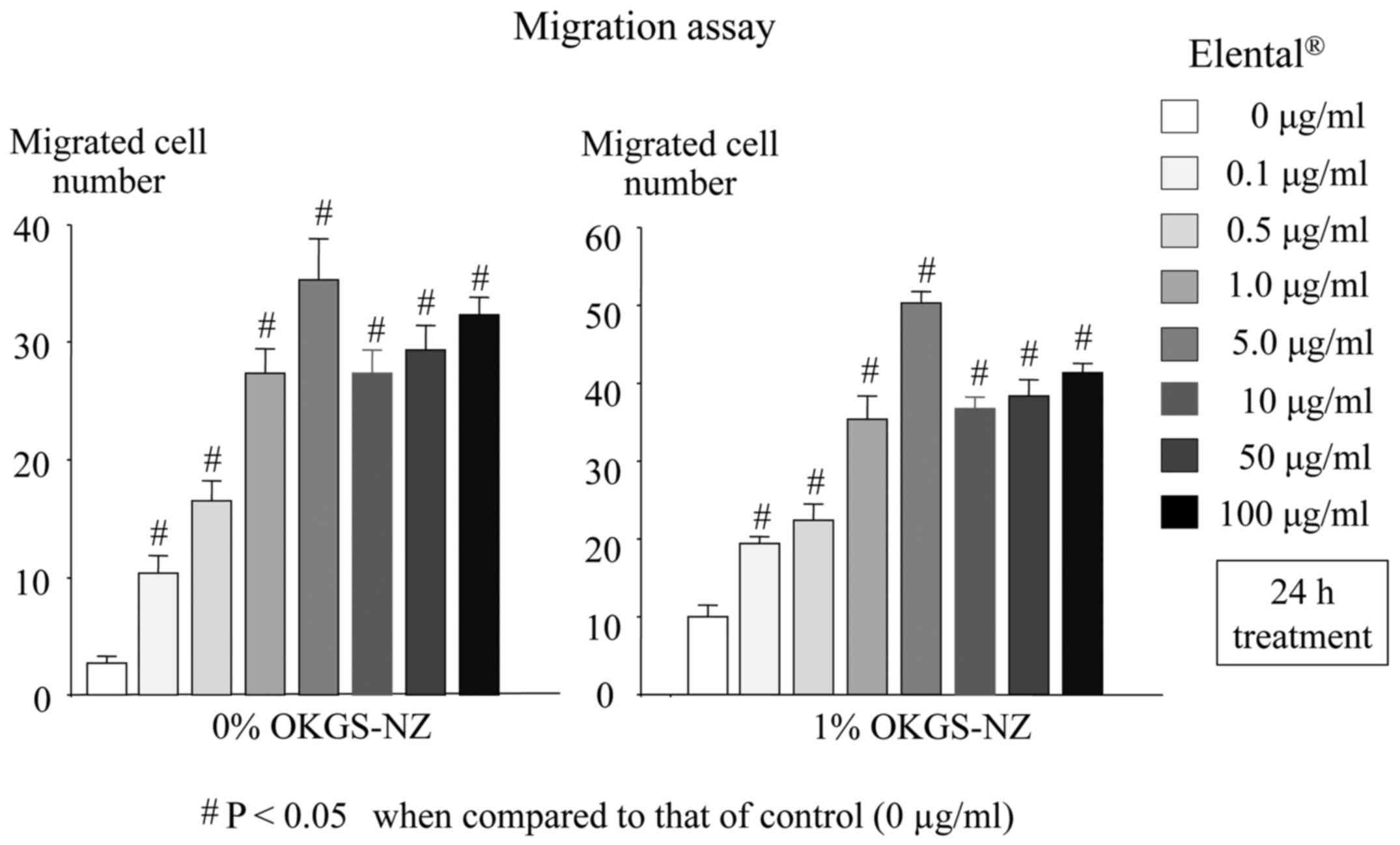
The migration activity of
Elental®-treated HOKs was measured using a Boyden
chamber. As shown in Fig. 6,
Elental®-treated HOKs exhibited a significantly higher
migration ability compared with that of untreated HOKs, under both
nutritionally poor conditions (medium with 0% OKGS-NZ growth
supplement), and nutrient-rich or substantial conditions (medium
with 1% OKGS-NZ medium), while 5 µg/ml Elental® exerted
a more noticeable effect on cell migration compared with other
concentrations.
Discussion
Elental® is a good source of nitrogen and
amino acids; it is an agent that is calorically dense, but with a
low fat content (Table SI), which
is suitable for enteral feeding, and its easily digestible
nutrition formula does not require a fully functional digestive
system (1,3). It has been reported that early
postoperative feeding with Elental® is beneficial in
patients who have undergone surgery, as it provides a higher amount
of nutrients that ultimately results in decreased weight loss
(2-4).
This ED is cost-effective, safe, and has been approved and covered
by public insurance in Japan as an appropriate prescription
treatment for malnutrition. Elental® has been reported
to be effective in reducing the severity of chemotherapy-induced
mucositis and dermatitis in patients with colorectal and esophageal
cancer, as well as in acute Crohn's disease (1,22-24).
Elental® contains a large quantity of L-glutamine (2.4
gm/100 g), which helps in the treatment of cellular injuries,
chemotherapy-induced cell toxicity and mucositis (1,17,25-30).
In our previous clinical study, Elental® was found to be
effective for the treatment of chemotherapy-induced oral mucositis
and dermatitis, without causing any adverse effects (18,19).
In the present clinical study, Elental®
exerted strong healing effects against chemotherapy-induced oral
mucositis when administered orally compared with its effects
following nasal administration, which indicates that
Elental® may not only act indirectly through gut
immunity, but may also have a direct healing effect on wounded
areas. However, there are currently no reports available on the
direct effects of any ED, including Elental®.
Therefore, it was investigated whether direct TA of
Elental® could also accelerate recovery from oral
mucositis or dermatitis in an animal model. The hamster cheek pouch
model is one potential model for examining the effect of ED on oral
mucositis. However, topically administering an ED inside a
hamster's mouth on a daily basis is quite difficult; additionally,
to assess any effects on a hamster's cheek pouch we would either
have to use general anesthesia or sacrifice the animal. Therefore,
numerous hamsters would be required for a single set of
experiments. Instead, in the present study, a mouse model of
dermatitis was used to assess the effect of Elental® on
chemotherapy-induced dermatitis or raw wound areas. This mouse
model of dermatitis may be considered as a suitable alternative
model for oral mucositis. As expected, TA and OA of
Elental® both exerted better healing effects on
dermatitis and raw wound areas compared with those observed in the
untreated control (Fig. 3A and
B), although the wounded areas
healed more rapidly following Elental® OA compared with
Elental® TA. MTT and migration assays in vitro
using HOKs were performed to further elucidate the effect of
Elental® on cell growth and migration, which is
important for the wound healing process. Although these in
vitro assays cannot explain the differences between OA and TA,
they may explain the mechanism of action of the possible ʻdirect
effectʼ of Elental® that was observed in our mouse
model. Elental® also exerted a growth-promoting effect
on all cells, even damaged cells treated with 5-FU, as well as
cells cultured under nutritionally poor conditions (0% OKGS-NZ
medium) (Fig. 5). Furthermore,
Elental® enhanced the migration ability of HOKs,
irrespective of the nutritional conditions (Fig. 6). Therefore, it may be inferred that
this cell growth-promoting property of Elental® may
contribute to its direct healing effect on chemotherapy-induced
dermatitis and mucositis. In the present study, no significant
differences in body weight were observed between the groups with or
without Elental® administration (data not shown). It was
hypothesized that this was due to the fact that the mice had free
access to food during these experiments. Whether
Elental® TA and OA exert synergistic effects against
5-FU-induced dermatitis or mucositis and in the healing of raw
wound areas has yet to be investigated. We aim to evaluate the
sequential or combined effects of Elental® TA and OA
using in vivo dermatitis and mucositis models in the
future.
In conclusion, the present study demonstrated that
both oral and topical Elental® may act directly on
chemotherapy-induced dermatitis and promote the healing of raw
wounds in vivo; therefore, it is possible that oral or
topical ED may also directly affect oral mucositis. This may
explain why Elental® was able to reduce
chemotherapy-induced oral mucositis more effectively when
administered orally compared with when it was administered nasally,
as observed in our previous clinical study.
Supplementary Material
Table SI. Composition of
Elental® (one package=80 g).
Acknowledgements
Not applicable.
Funding
The present study was supported in part by a
Grant-in-Aid from the Japanese Ministry of Education, Science and
Culture (grant no. 15K11292).
Availability of data and materials
All data generated or analyzed during the present
study are included in this published article.
Authors' contributions
KH and TT designed the study. TF performed the
experiments. KH and TF analyzed the data, wrote and revised the
manuscript. YM and KM revised the manuscript critically for
important intellectual content and provided valuable suggestions
during the study. All the authors have read and approved the final
version of the manuscript and are fully responsible for its
content.
Ethics approval and consent to
participate
All procedures including animal handling and the
experimental protocols were conducted according to the guidelines
approved by the Ethical Committee for Animal Experimentation of
Yamaguchi University (Yamaguchi, Japan).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Online EA Pharma Co., Ltd Products
Information, Elental®. http://www.eapharma.co.jp/medicalexpert/product/elental/elentalinformation.html,
Webpage in Japanese. Accessed online: 25 July 2019.
|
|
2
|
Kawada J, Nishino M, Hata T, Tanizaki K,
Ogino T, Hoshino H, Okano M, Nagai K, Kim Y, Okuyama M, et al:
Analysis of patients who received nutritional support and the
enhanced recovery after surgery (ERAS) protocol after
esophagectomy. Gan To Kagaku Ryoho. 45:1524–1526. 2018.(In
Japanese). PubMed/NCBI
|
|
3
|
Ohkura Y, Haruta S, Tanaka T, Ueno M and
Udagawa H: Effectiveness of postoperative elemental diet
(Elental®) in elderly patients after gastrectomy. World
J Surg Oncol. 14(268)2016.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Imamura H, Nishikawa K, Kishi K, Inoue K,
Matsuyama J, Akamaru Y, Kimura Y, Tamura S, Kawabata R, Kawada J,
et al: Effects of an oral elemental nutritional supplement on
post-gastrectomy body weight loss in gastric cancer patients: A
randomized controlled clinical trial. Ann Surg Oncol. 23:2928–2935.
2016.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Yamamoto T, Nakahigashi M, Umegae S,
Kitagawa T and Matsumoto K: Impact of elemental diet on mucosal
inflammation in patients with active Crohn's disease: Cytokine
production and endoscopic and histological findings. Inflamm Bowel
Dis. 11:580–588. 2005.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Hanai H, Iida T, Takeuchi K, Arai H, Arai
O, Abe J, Tanaka T, Maruyama Y, Ikeya K, Sugimoto K, et al:
Nutritional therapy versus 6-mercaptopurine as maintenance therapy
in patients with Crohn's disease. Dig Liver Dis. 44:649–54.
2012.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Johtatsu T, Andoh A, Kurihara M, Iwakawa
H, Tsujikawa T, Kashiwagi A, Fujiyama Y and Sasaki M: Serum
concentrations of trace elements in patients with Crohn's disease
receiving enteral nutrition. J Clin Biochem Nutr. 4:197–201.
2007.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Yamamoto T, Nakahigashi M, Saniabadi AR,
Iwata T, Maruyama Y, Umegae S and Matsumoto K: Impacts of long-term
enteral nutrition on clinical and endoscopic disease activities and
mucosal cytokines during remission in patients with Crohn's
disease: A prospective study. Inflamm Bowel Dis. 13:1493–1501.
2007.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Yamamoto T, Nakahigashi M, Umegae S,
Kitagawa T and Matsumoto K: Impact of long-term enteral nutrition
on clinical and endoscopic recurrence after resection for Crohn's
disease: A prospective, non-randomized, parallel, controlled study.
Aliment Pharmacol Ther. 25:67–72. 2007.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Kawada J, Nishino M, Hata T, Ogino T,
Hoshino H, Okano M, Nagai K, Kim Y, Okuyama M and Tsujinaka T:
Analysis of patients who received enteral nutrition in the course
of chemotherapy. Gan To Kagaku Ryoho. 44:900–902. 2017.(In
Japanese). PubMed/NCBI
|
|
11
|
Kawaguchi Y, Lin JC, Kawashima Y, Maruno
A, Ito H, Ogawa M and Mine T: Relationship between pain and plasma
amino acid levels in chronic pancreatitis. JOP. 16:53–57.
2015.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Ogata Y, Ishibashi N, Yamaguchi K, Uchida
S, Kamei H, Nakayama G, Hirakawa H, Tanigawa M and Akagi Y:
Preventive effects of amino-acid-rich elemental diet Elental® on
chemotherapy-induced oral mucositis in patients with colorectal
cancer: A prospective pilot study. Support Care Cancer. 24:783–789.
2016.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Ogata Y, Takeuchi M, Ishibashi N, Kibe S,
Takahashi K, Uchida S, Murakami N, Yahara T and Shirouzu K:
Efficacy of Elental on prevention for chemotherapy-induced oral
mucositis in colorectal cancer patients. Gan To Kagaku Ryoho.
39:583–587. 2012.(In Japanese). PubMed/NCBI
|
|
14
|
Toyomasu Y, Mochiki E, Yanai M, Suzuki M,
Yanoma T, Kimura A, Kogure N, Ogata K and Kuwano H: A prospective
pilot study of an elemental nutritional supplement for prevention
of oral mucositisduring S-1 adjuvant chemotherapy for gastric
cancer. Surg Oncol. 29:97–101. 2019.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Ishikawa T, Yasuda T, Doi T, Okayama T,
Sakamoto N, Gen Y, Dohi O, Yoshida N, Kamada K, Uchiyama K, et al:
The amino acid-rich elemental diet Elental® preserves
lean body mass during chemo- or chemoradiotherapy for esophageal
cancer. Oncol Rep. 36:1093–1100. 2016.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Fukui T, Itoh Y, Orihara M, Yoshizawa K,
Takeda H, Kawada S and Yoshioka T: Elental prevented and reduced
oral mucositis during chemotherapy in patients esophageal cancer.
Gan To Kagaku Ryoho. 38:2597–2601. 2011.(In Japanese). PubMed/NCBI
|
|
17
|
Miki H, Tokuhara K, Oishi M, Tanaka Y,
Nakatake R, Ueyama Y, Kaibori M, Nishizawa M, Okumura T and Kon M:
Elental® amino acid component has protective effects on
primary cultured hepatocytes and a rat model of acute liver injury.
Nutr Res. 42:71–84. 2017.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Harada K, Ferdous T, Horinaga D, Uchida K,
Mano T, Mishima K, Park S, Hanazawa H, Takahashi S, Okita A, et al:
Efficacy of elemental diet on prevention for
chemoradiotherapy-induced oral mucositis in patients with oral
squamous cell carcinoma. Support Care Cancer. 24:953–959.
2016.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Harada K, Minami H, Ferdous T, Kato Y,
Umeda H, Horinaga D, Uchida K, Park SC, Hanazawa H, Takahashi S, et
al: The Elental® elemental diet for
chemoradiotherapy-induced oral mucositis: A prospective study in
patients with oral squamous cell carcinoma. Mol Clin Oncol.
10:159–167. 2019.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Harada K, Ferdous T, Kobayashi H and
Ueyama Y: Elemental diet accelerates the recovery from oral
mucositis and dermatitis induced by 5-Fluorouracil through the
induction of fibroblast growth factor 2. Integr Cancer Ther.
17:423–430. 2018.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Harada K, Ferdous T, Mizukami Y and
Mishima K: Elemental diet inhibits pro-inflammatory cytokine
production in keratinocytes through the suppression of NF-κB
activation. Oncol Rep. 40:361–368. 2018.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Svanberg A, Ohrn K and Birgegard G: Oral
cryotherapy reduces mucositis and improves nutrition- A randomised
controlled trial. J Clin Nurs. 19:2146–2151. 2010.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Scully C, Epstein J and Sonis S: Oral
mucositis: A challenging complication of radiotherapy,
chemotherapy, and radiochemotherapy. Part 2: Diagnosis and
management of mucositis. Head Neck. 26:77–84. 2004.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Cowen D, Tardieu C, Schubert M, Peterson
D, Resbeut M, Faucher C and Franquin JC: Low energy helium-neon
laser in the prevention of oral mucositis in patients undergoing
bone marrow transplant: Results of a double blind randomized trial.
Int J Radiat Oncol Biol Phys. 38:697–703. 1997.PubMed/NCBI View Article : Google Scholar
|
|
25
|
O´Dwyer ST, Scott T, Smith RJ and Wilmore
DW: 5-fluorouracil toxicity on small intestinal mucosa but not
white blood cells is decreased by glutamine. Clin Res.
35(367A)1987.
|
|
26
|
Carneiro-Filho BA, Oriá RB, Wood Rea K,
Brito GA, Fujii J, Obrig T, Lima AA and Guerrant RL:
Alanyl-glutamine hastens morphologic recovery from
5-fluorouracil-induced mucositis in mice. Nutrition. 20:934–941.
2004.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Kandil HM, Argenzio RA, Chen W,
Berschneider HM, Stiles AD, Westwick JK, Rippe RA, Brenner DA and
Rhoads JM: L-glutamine and l-asparagine stimulate ODC activity and
proliferation in a porcine jejunal enterocyte line. Am J Physiol.
269:G591–G599. 1995.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Rhoads JM, Argenzio RA, Chen W, Rippe RA,
Westwick JK, Cox AD, Berschneider HM and Brenner DA: L-glutamine
stimulates intestinal cell proliferation and activates
mitogen-activated protein kinases. Am J Physiol. 272:G943–G953.
1997.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Hong RW, Rounds JD, Helton WS, Robinson MK
and Wilmore DW: Glutamine preserves liver glutathione after lethal
hepatic injury. Ann Surg. 215:114–119. 1992.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Denno R, Rounds JD, Faris R, Holejko LB
and Wilmore DW: Glutamine-enriched total parenteral nutrition
enhances plasma glutathione in the resting state. J Surg Res.
61:35–38. 1996.PubMed/NCBI View Article : Google Scholar
|