Introduction
Chronic obstructive pulmonary disease (COPD) is a
risk factor for lung cancer (1).
Patients with non-small cell lung cancer (NSCLC) with coexisting
COPD tend to have poor survival (2,3). They
also are at high risk of mortality after surgical resection
(4). Compared with surgery,
stereotactic body radiotherapy (SBRT) has a lower mortality risk
and results in higher survival in patients with severe COPD
(5). SBRT is now the treatment of
choice for non-operable early-stage NSCLC; however, radiation
pneumonitis (RP) may occur as an adverse event (6).
Dosimetric parameters, including mean lung dose and
percentage of lung volume irradiated with ≥20 Gy, can estimate the
risk of RP (7-9).
However, whether patients with pulmonary emphysema are at a higher
risk for RP is unclear.
Quantitative CT assessment of patients with
emphysema is now used to measure pulmonary function (10-14)
since Hayhurst et al (15)
reported a correlation between emphysema and lung CT values in low
attenuation areas. By quantitative assessment, we previously showed
that patients with low average Hounsfield units (HU) of the whole
lung had a low rate of RP after conventional low dose radiotherapy
(75 Gy administered in 30 fractions) (16). However, the major criticism of our
report was the use of a conventional low dose of SBRT rather than a
high dose.
In this study, to investigate whether quantitative
CT measurement and/or lung irradiated dose were associated with RP,
we used a high dose of SBRT and observed the rates of RP in
patients with and without emphysema.
Patients and methods
Patients
This retrospective study was approved by the
institutional review board of the National Center for Global Health
and Medicine Ethics Committee. Informed consent was waived by the
Ethics Committee due to the retrospective nature of the study.
According to the approval, informed consent from the patients was
not required. Inclusion criteria were histologically/cytologically
confirmed NSCLC or the tumor size enlarged more than 25% on
sequential CT examination, clinical stage I (T1/2N0M0) staged by CT
examination, age ≥20 years, Eastern Cooperative Oncology Group
performance status 0-2 and no history of radiation therapy.
Exclusion criteria were interstitial pneumonitis or huge bullae on
CT examination, centrally located tumor, history of chemotherapy,
sever psychologically disease and active infectious disease.
Between November 2003 and October 2015, patients with stage I NSCLC
receiving SBRT at a dose of 50 or 60 Gy over five fractions at our
hospital were studied. Eighty consecutive patients were identified.
The median age was 75.5 years (range, 29-95 years), 54 and 26
patients were male and female, respectively, and there were 49 and
31 T1 and T2 tumors, respectively (Table I). There were no patients with
interstitial pneumonitis. Patients with interstitial pneumonitis
did not undergo SBRT because of a risk of pneumonitis in our
treatment policy. Centrally located tumor was treated by a
different dose fraction. None underwent chemotherapy or molecular
targeting therapy during the observation period. Two experienced
radiologists diagnosed 33 patients with emphysema based on CT
examination before radiotherapy. RP was determined according to
common terminology criteria for adverse events (CTCAE v. 4.0). All
patients underwent physical examination, blood tests, and chest
X-rays at least every 4 months up to 3 years, and then every 6
months. CT examinations were performed at least every 6 months up
to 3 years and then every year or if tumor enlargement was
suspected upon chest X-ray.
 | Table IPatient characteristics. |
Table I
Patient characteristics.
| Variable | Value (n=80) |
|---|
| Median age, years
(range) | 75.5 (29-95) |
| Sex, n (%) | |
|
Male | 54 (67.5) |
|
Female | 26 (32.5) |
| T factor | |
|
T1 | 49 (61.3) |
|
T2 | 31 (38.8) |
| Tumor location in
lung, n (%) | |
|
Right
lung | 49 (61.3) |
|
Left
lung | 31 (38.8) |
| Tumor location in
lobe, n (%) | |
|
Upper
lobe | 31 (38.8) |
|
Middle or
lingual lobe | 11 (13.8) |
|
Lower
lobe | 38 (47.5) |
| Histological type, n
(%) | |
|
Adenocarcinoma | 43 (53.8) |
|
Squamous
cell carcinoma | 16(20) |
|
NSCLC | 4(5) |
|
LCNEC | 1(1) |
|
Unproven | 16(20) |
Radiotherapy technique and dose
evaluation
A planning CT was acquired with patients in the
supine position on a vacuum cushion with their arms raised. To
assess intra-fraction tumor movement, four-dimensional (4-D) CT
images were obtained using commercial software (Real-Time Position
Management System [RPM] system®, Varian Medical System,
Inc.). The respiratory cycle was controlled by a self-monitoring
device for respiratory movement (Abches®, APEX Medical,
Inc.) Ten equally spaced respiratory time bins from CT images were
reconstructed from 4-D CT. An inspiratory phase CT image was also
obtained using a self-monitoring device. Typically, three bins were
used to create the gross tumor volume (GTV) in each bin. Internal
target volume (ITV) was created from each GTV and contoured on an
inspiratory phase CT image. Planning target volume (PTV) was
generated by adding 5 mm to the ITV. Respiratory-gated irradiation
or breath-hold technique was used according to patients'
respiratory cycles and tumor movement. CT images were obtained
within at least 10 days before the initiation of radiotherapy. Dose
calculation was performed by a planning system (Eclipse ver.
10®, Varian Medical Systems). A dose of 50 or 60 Gy
given in five fractions was prescribed to the isocenter. Five to 10
(median, seven) ports to optimize the directions for non-coplanar
static beams or dynamic arcs using 6 MV energy were planned. Mean
lung dose and the percentage of normal lung volume receiving ≥20 Gy
(V20) and >5 Gy (V5) were evaluated in the inspiratory phase of
CT images. On-board cone beam CT was performed to reduce
inter-fraction error before every irradiation session.
CT assessment of emphysema
Parameters of the 64-detector CT (Aquilion
TSX-101HA, Toshiba Medical System, Inc.) were as follows: Auto
exposure control mA (tube current range, 40-200 mA); tube voltage,
120 KV; and gantry rotation time, 0.5 sec. CT data were
reconstructed in an axial CT image with 1.6-mm section thickness.
CT values varied with the CT scanner, and thus all patients
underwent CT using the same machine. The details of quantitative
assessment by CT have been reported elsewhere (16). CT images of the inspiratory phase
were obtained from a planning CT, and quantitative assessment of
the percentage of lung CT voxels below the threshold of -910 HU
were analyzed (Synapse Vincent® software, Fujifilm Co.).
The percentage of low attenuation areas (LAA%) in both whole lungs
and the average density value of both lungs were obtained.
Statistical analysis
Quantitative CT value and dosimetric parameters were
analyzed by Wilcoxon's rank-sum test. The time to RP development
was calculated from the first day of SBRT. The time to RP was
estimated by Kaplan-Meier analysis and compared with log-rank
tests. Cox's proportional hazard model was used to analyze risk
factors for RP. The CT values assessed for emphysema were evaluated
by a receiver operating characteristic (ROC) curve. Most cases of
radiation pneumonitis occurred within 1 year, and hence ROC
analysis is an acceptable approach to clarify radiation
pneumonitis. A two-sided probability value (P<0.05) was
considered statistically significant. Statistical analysis was
performed with commercial software (STATA v. 13®,
StataCorp).
Results
RP rate
During the median observation period of 18.8
(1.8-106.8) months, 26 (33%) and three (4%) patients experienced
Grade 1 and Grade 2 RP, respectively. Three patients with Grade 2
RP were successfully treated with steroids. No patients suffered
from ≥Grade 3 RP. The median time to development of RP was 14
months. Actuarial RP rates at 6, 12 and 18 months were 16.9% [95%
confidence interval (CI), 9.7-28.5%)], 49.0% (95% CI, 35.1-65.0%),
and 74.1% (95% CI, 51.6-92.0%), respectively.
The quantitative CT value of
emphysema
LAA% (<-910 HU) in patients with emphysema was
significantly higher than that in patients with non-emphysema
(P<0.001, Table II). The
average HU of the whole lung in patients with emphysema was
significantly lower than that in patients without emphysema
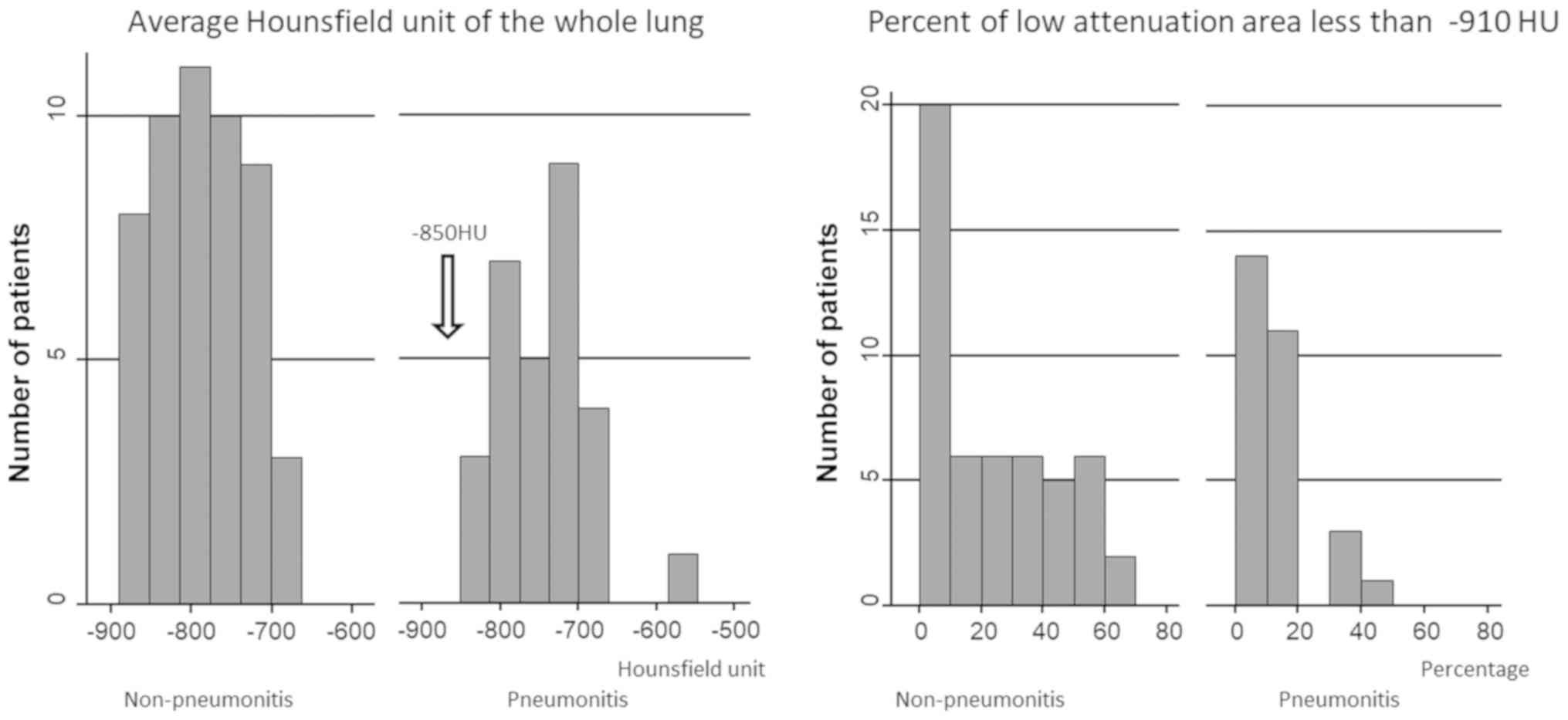
(P<0.001). Fig. 1 shows the
number of patients with an average HU of the whole lung and LAA% of
-910 HU and less. No patients who had an average HU of ≤-850 HU
experienced RP (Fig. 1).
 | Table IIQuantitative CT value for
non-emphysema and emphysema. |
Table II
Quantitative CT value for
non-emphysema and emphysema.
| Quantitative CT
value | Total (n=80) | Non-emphysema
(n=47) | Emphysema (n=33) | P-value |
|---|
| Percentage of low
attenuation area less than -910 HU, % | 29.5±2.1 | 6.7±1.3 | 36.2±2.8 | <0.001 |
| Average HU of whole
lung, HU | -772.4±59.3 | -737.2±6.4 | -822.5±6.7 | <0.001 |
In ROC analysis of patients with and without
emphysema, the AUC of LAA% (<-910 HU) and average HU were 0.948
and 0.942, respectively. Fig. 2
shows a ROC curve comparing patients with and without RP. The AUC
of average HU, mean lung dose, LAA% (<-910 HU), V20, and V5 were
0.692, 0.643, 0.641, 0.619, and 0.591, respectively.
RP and the quantitative CT value
Table III shows
the difference in the quantitative CT value and dosimetric
parameters between RP and non-RP. A LAA% (<-910 HU) in patients
without RP was significantly higher than that in patients with RP
(P=0.037). The average density of the whole lung in patients
without RP was significantly lower than that in patients with RP
(P=0.004). V20 (P=0.078) and V5 (P=0.178) were not significantly
different between subjects with and without RP. The mean lung dose
in patients with RP was higher than that in patients without RP
(P=0.034). Fig. 3 shows RP rates
analyzed by the Kaplan-Meier method. The RP rate with a mean lung
dose of <4 Gy was not significantly different compared with
doses ≥4 Gy (P=0.11). The RP rate in LAA% (<-910 HU) of ≤25% was
significantly higher compared with that of subjects with an LAA%
(<-910 HU) >25% (P=0.037). The RP rate in subjects with an
average HU >-790 HU was significantly higher compared with those
with ≤-790 HU (P=0.036). The RP rate at an average HU of >-850
HU was significantly higher compared with those with an average HU
of ≤-850 HU (P=0.045).
 | Table IIIQuantitative CT value and dosimetric
parameters for non-pneumonitis and pneumonitis. |
Table III
Quantitative CT value and dosimetric
parameters for non-pneumonitis and pneumonitis.
| Quantitative CT value
and dosimetric parameters | Non-pneumonitis
(n=51) | Pneumonitis
(n=29) | P-value |
|---|
| Percent of low
attenuation area <-910 HU, % | 22.9±2.1 | 11.8±1.2 | 0.037 |
| Average HU of whole
lung, HU | -787.5±7.9 | -745.8±10.2 | 0.004 |
| V20, % | 5.4±0.4 | 7.0±0.7 | 0.078 |
| V5, % | 17.8±0.8 | 20.4±1.4 | 0.178 |
| Mean lung dose,
Gy | 3.8±0.2 | 4.6±0.3 | 0.034 |
Table IV shows age
[hazard ratio (HR)=2.46, P=0.03] and average HU (HR=3.39, P=0.02)
were significant risk factors for RP by multivariate analysis. Sex,
T factor, and mean lung dose were not significant.
 | Table IVRisk factors for radiation pneumonitis
analyzed using a Cox proportional hazard model. |
Table IV
Risk factors for radiation pneumonitis
analyzed using a Cox proportional hazard model.
| Factor | Hazard ratio | 95% CI | P-value |
|---|
| Age (<75 years vs.
>75 years) | 2.46 | 1.07-5.67 | 0.03 |
| Sex (male vs.
female) | 0.43 | 0.17-1.07 | 0.07 |
| T factor (T1 vs.
T2) | 2.04 | 0.90-4.62 | 0.09 |
| Mean lung dose
(<4 Gy vs. >4 Gy) | 1.75 | 0.80-3.85 | 0.16 |
| Average CT value
(<-790 cs. >-790 HU) | 3.39 | 1.24-9.24 | 0.02 |
Discussion
Radiation oncologists are concerned about RP after
SBRT, especially in the case of emphysema. This study showed that
patients with emphysema can be safely treated with SBRT. In our
cohort, no patient suffered ≥Grade 3 RP and none had emphysema.
Takeda et al (17) reported
that patients with severe COPD could be treated with SBRT; however,
they did not specifically distinguish between emphysema and COPD.
Some authors also reported that patients with stage I NSCLC who had
emphysema had a low rate of RP after SBRT (18,19).
Based on these results, we believe that emphysema is not a
contraindication for SBRT.
In our previous study, the actuarial rates of RP at
6 and 12 months were 52 and 75%, respectively, when a conventional
dose of 75 Gy was administered in 30 fractions (16). In this study, the rate of RP at 12
and 18 months was 49 and 74%, respectively. The rate of RP was
similar in both studies, but RP events in SBRT were late compared
with the conventional dose. Recently, a prospective randomized
trial of an SBRT dose of 66 Gy given in three fractions compared
with a conventional dose of 70 Gy given in 35 fractions (20) showed that the rate of RP was not
significantly different between the two dose schedules, suggesting
that RP may not be prevented by hyperfractionation.
The effectiveness of the quantitative CT method
relating LAA% and anatomical emphysema has been proven. Gevenois
et al (21,22) described a cutoff value of -950 HU
corresponding closely with pathological emphysema. They noted a
cutoff value of >-950 HU overestimated emphysema whereas
<-950 HU underestimated emphysema. Müller et al (23) reported a <-910 HU threshold of
LAA was the best predictor for discrimination between normal lung
and emphysema. Therefore, we chose -910 HU as the cut-off value for
LAA. Reports on the correlation between pulmonary function tests
and LAA% have reported cut-off thresholds of LAA from -960 to -860
HU (10,11,13,14).
This study showed that quantitative CT is a good
predictor for RP risk. Another study suggested that quantitative CT
values correlated with RP after radiotherapy (24). Yamamoto et al (24) showed a quantitative CT value of LAA%
was associated with Grade 1 RP, but not with Grade 2 and 3 because
they were affected by other factors such as V20. These results were
similar to ours, where no patient had Grade 3 or higher RP in our
study, and thus we did not observe LAA% or average HU to be
associated with Grade 3 RP. The previous study showed that V20 was
associated with radiation pneumonitis; however, we did not include
interstitial disease in our study and used the average HU of the
whole lung, which we believe to be the reason for the different
results.
This study showed that the average HU of the whole
lung was associated with RP, with a higher AUC for average HU
compared with that of LAA%. The patients with an average HU of
<-850 HU (implying severe emphysema) did not develop RP.
Patients with severe emphysema had a low risk of RP, which is in
accordance with our previous studies (16,25).
We speculate that average whole lung HU is a convenient measurement
in quantitative CT assessment to estimate the risk RP.
This study had some limitations. This research was a
retrospective single institution study and included relatively
small numbers. Smoking history and histological grade information
were not available. However, in this study we showed that the
average HU value of the whole lung was associated with RP after
SBRT. A prospective multi-institution study is required to validate
the association between the quantitative CT value and RP after
SBRT.
In conclusion, pulmonary emphysema patients,
especially those with severe emphysema, can safely undergo SBRT.
The quantitative CT value was associated with RP after SBRT.
Acknowledgements
The authors would like to thank Dr Nikki March for
editing a draft of this manuscript.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
The present study was designed by HN and RM. Data of
CT values were analyzed by TT, YT and FU. Statistical analyses was
performed by HN and WW. The manuscript was written by HN with
contributions from FU, YU, WW and RM. All authors read and approved
the final manuscript.
Ethics approval and consent to
participate
The present retrospective study was approved by the
Institutional Review Board of the National Center for Global Health
and Medicine Ethics Committee (approval no. NCGM-G-002164-00). The
requirement for informed consent was waived by the Ethics Committee
due to the retrospective nature of the study.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
de-Torres JP, Wilson DO, Sanchez-Salcedo
P, Weissfeld JL, Berto J, Campo A, Alcaide AB, García-Granero M,
Celli BR and Zulueta JJ: Lung cancer in patients with chronic
obstructive pulmonary disease. Development and validation of the
COPD lung cancer screening score. Am J Respir Crit Care Med.
191:285–291. 2015.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Zhai R, Yu X, Shafer A, Wain JC and
Christiani DC: The impact of coexisting COPD on survival of
patients with early-stage non-small cell lung cancer undergoing
surgical resection. Chest. 145:346–353. 2014.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Putila J and Guo NL: Combining COPD with
clinical, pathological and demographic information refines
prognosis and treatment response prediction of non-small cell lung
cancer. PLoS One. 9(e100994)2014.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Brunelli A, Kim AW, Berger KI and
Addrizzo-Harris DJ: Physiologic evaluation of the patient with lung
cancer being considered for resectional surgery: Diagnosis and
management of lung cancer, 3rd ed: American College of Chest
Physicians evidence-based clinical practice guidelines. Chest. 143
(Suppl 5):e166S–e190S. 2013.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Palma D, Lagerwaard F, Rodrigues G,
Haasbeek C and Senan S: Curative treatment of stage I
non-small-cell lung cancer in patients with severe COPD:
Stereotactic radiotherapy outcomes and systematic review. Int J
Radiat Oncol Biol Phys. 82:1149–1156. 2012.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Chi A, Liao Z, Nguyen NP, Xu J, Stea B and
Komaki R: Systemic review of the patterns of failure following
stereotactic body radiation therapy in early-stage non-small-cell
lung cancer: Clinical implications. Radiother Oncol. 94:1–11.
2010.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Baker R, Han G, Sarangkasiri S, DeMarco M,
Turke C, Stevens CW and Dilling TJ: Clinical and dosimetric
predictors of radiation pneumonitis in a large series of patients
treated with stereotactic body radiation therapy to the lung. Int J
Radiat Oncol Biol Phys. 85:190–195. 2013.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Barriger RB, Forquer JA, Brabham JG,
Andolino DL, Shapiro RH, Henderson MA, Johnstone PA and Fakiris AJ:
A dose-volume analysis of radiation pneumonitis in non-small cell
lung cancer patients treated with stereotactic body radiation
therapy. Int J Radiat Oncol Biol Phys. 82:457–462. 2012.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Zhao J, Yorke ED, Li L, Kavanagh BD, Li
XA, Das S, Miften M, Rimner A, Campbell J, Xue J, et al: Simple
factors associated with radiation-induced lung toxicity after
stereotactic body radiation therapy of the thorax: A pooled
analysis of 88 studies. Int J Radiat Oncol Biol Phys. 95:1357–1366.
2016.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Ando K, Sekiya M, Tobino K and Takahashi
K: Relationship between quantitative CT metrics and pulmonary
function in combined pulmonary fibrosis and emphysema. Lung.
191:585–591. 2013.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Madani A, Van Muylem A and Gevenois PA:
Pulmonary emphysema: Effect of lung volume on objective
quantification at thin-section CT. Radiology. 257:260–268.
2010.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Wang G, Wang L, Ma Z, Zhang C and Deng K:
Quantitative emphysema assessment of pulmonary function impairment
by computed tomography in chronic obstructive pulmonary disease. J
Comput Assist Tomogr. 39:171–175. 2015.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Schroeder JD, McKenzie AS, Zach JA, Wilson
CG, Curran-Everett D, Stinson DS, Newell JD Jr and Lynch DA:
Relationships between airflow obstruction and quantitative CT
measurements of emphysema, air trapping, and airways in subjects
with and without chronic obstructive pulmonary disease. AJR Am J
Roentgenol. 201:W460–W470. 2013.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Washko GR, Hunninghake GM, Fernandez IE,
Nishino M, Okajima Y, Yamashiro T, Ross JC, Estépar RS, Lynch DA,
Brehm JM, et al: Lung volumes and emphysema in smokers with
interstitial lung abnormalities. N Engl J Med. 364:897–906.
2011.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Hayhurst MD, MacNee W, Flenley DC, Wright
D, McLean A, Lamb D, Wightman AJ and Best J: Diagnosis of pulmonary
emphysema by computerised tomography. Lancet. 2:320–322.
1984.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Saito T, Nakayama H, Yamada T, Shiraishi S
and Tokuuye K: Is severe emphysema, as defined by quantitative CT
measurement, a negative risk factor of radiation fibrosis? Br J
Radiol. 91(20170921)2018.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Takeda A, Kunieda E, Ohashi T, Aoki Y, Oku
Y, Enomoto T, Nomura K and Sugiura M: Severe COPD is correlated
with mild radiation pneumonitis following stereotactic body
radiotherapy. Chest. 141:858–866. 2012.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Trovo M, Linda A, El Naqa I, Javidan-Nejad
C and Bradley J: Early and late lung radiographic injury following
stereotactic body radiation therapy (SBRT). Lung Cancer. 69:77–85.
2010.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Kimura T, Matsuura K, Murakami Y,
Hashimoto Y, Kenjo M, Kaneyasu Y, Wadasaki K, Hirokawa Y, Ito K and
Okawa M: CT appearance of radiation injury of the lung and clinical
symptoms after stereotactic body radiation therapy (SBRT) for lung
cancers: Are patients with pulmonary emphysema also candidates for
SBRT for lung cancers? Int J Radiat Oncol Biol Phys. 66:483–491.
2006.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Nyman J, Hallqvist A, Lund JÅ, Brustugun
OT, Bergman B, Bergström P, Friesland S, Lewensohn R, Holmberg E
and Lax I: SPACE-A randomized study of SBRT vs. conventional
fractionated radiotherapy in medically inoperable stage I NSCLC.
Radiother Oncol. 121:1–8. 2016.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Gevenois PA, de Maertelaer V, De Vuyst P,
Zanen J and Yernault JC: Comparison of computed density and
macroscopic morphometry in pulmonary emphysema. Am J Respir Crit
Care Med. 152:653–657. 1995.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Gevenois PA, Koob MC, Jacobovitz D, De
Vuyst P, Yernault JC and Struyven J: Whole lung sections for
computed tomographic-pathologic correlations. Modified
Gough-Wentworth technique. Invest Radiol. 28:242–246.
1993.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Müller NL, Staples CA, Miller RR and
Abboud RT: ‘Density mask’. An objective method to quantitate
emphysema using computed tomography. Chest. 94:782–787.
1988.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Yamamoto T, Kadoya N, Sato Y, Matsushita
H, Umezawa R, Kubozono M, Ishikawa Y, Kozumi M, Takahashi N,
Morishita Y, et al: Prognostic value of radiation pneumonitis after
stereotactic body radiotherapy: Effect of pulmonary emphysema
quantitated using CT images. Clin Lung Cancer. 19:e85–e90.
2018.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Ishijima M, Nakayama H, Itonaga T, Tajima
Y, Shiraishi S, Okubo M, Mikami R and Tokuuye K: Patients with
severe emphysema have a low risk of radiation pneumonitis following
stereotactic body radiotherapy. Br J Radiol.
88(20140596)2015.PubMed/NCBI View Article : Google Scholar
|